Abstract
24(S)-Hydroxycholesterol (24S-OHC) produced by cholesterol 24-hydroxylase expressed mainly in neurons plays an important physiological role in the brain. Conversely, it has been reported that 24S-OHC possesses potent cytotoxicity. The molecular mechanisms of 24S-OHC-induced cell death have not yet been fully elucidated. In this study, using human neuroblastoma SH-SY5Y cells and primary cortical neuronal cells derived from rat embryo, we characterized the form of cell death induced by 24S-OHC. SH-SY5Y cells treated with 24S-OHC exhibited neither fragmentation of the nucleus nor caspase activation, which are the typical characteristics of apoptosis. 24S-OHC-treated cells showed necrosis-like morphological changes but did not induce ATP depletion, one of the features of necrosis. When cells were treated with necrostatin-1, an inhibitor of receptor-interacting serine/threonine kinase 1 (RIPK1) required for necroptosis, 24S-OHC-induced cell death was significantly suppressed. The knockdown of RIPK1 by transfection of small interfering RNA of RIPK1 effectively attenuated 24S-OHC-induced cell death. It was found that neither SH-SY5Y cells nor primary cortical neuronal cells expressed caspase-8, which was regulated for RIPK1-dependent apoptosis. Collectively, these results suggest that 24S-OHC induces neuronal cell death by necroptosis, a form of programmed necrosis.
Keywords: Apoptosis, Caspase, Cell Death, Lipid Oxidation, Necrosis (Necrotic Death), 24(S)-Hydroxycholesterol, Necroptosis, Necrostatin-1, Oxysterol, Receptor-interacting Serine/Threonine Kinase 1
Introduction
Brain is the organ rich in cholesterol and contains about 25% of the total amounts of cholesterol in the body (1). Brain cholesterol is locally synthesized, and its levels are not influenced by the nutritional intake of cholesterol (2). The blood-brain barrier prevents cholesterol transport from the brain into the blood (1–3). Therefore, there is a need to eliminate excess brain cholesterol, which is derived from either increased cholesterol synthesis or neuronal cell death. Brain cholesterol hydroxylation catalyzed by a cytochrome P-450-dependent enzyme, cholesterol 24(S)-hydroxylase, results in the formation of 24(S)-hydroxycholesterol (24S-OHC),3 which can be transported across the blood-brain barrier (4). It has been reported that free 24S-OHC is present at high concentrations up to 30 μm in the brain (5). More than 90% of the 24S-OHC in plasma originates from the brain, and 24S-OHC is thought to be the main cholesterol elimination product of the brain (5, 6). Cholesterol metabolism is involved in the pathogenesis of Alzheimer disease; for example, the ϵ4 allele of the apolipoprotein E has been shown to be a genetic risk factor for late-onset Alzheimer disease (7). In addition, it has been reported that a polymorphism in the cholesterol 24S-hydroxylase gene is associated with Alzheimer disease (8).
Oxysterols are oxygenated derivatives of cholesterol and have been shown to possess diverse biological activities, including mediation of cell signaling, regulation of cellular cholesterol synthesis, and induction of cytotoxicity (9–11). It has been known that 24S-OHC binds to insulin-induced gene, blocking cholesterol synthesis (12). It has also been shown that 24S-OHC acts as a ligand of the nuclear receptor liver X receptor, which regulates the gene expression of proteins involved in cholesterol transport in cell membrane (13). 7-Ketocholesterol, nonenzymatic oxidation product of cholesterol, has been shown to induce cell death through inactivation of the phosphatidylinositol 3-kinase/Akt signaling pathway (14), which is considered to be highly specific to lipid raft domains (15). 24S-OHC has been shown to possess potent neurotoxicity (16); however, the molecular mechanisms of the induction of neurotoxicity, particularly in the form of cell death, are less clearly defined.
Classically, two forms of cell death, namely apoptosis and necrosis, are known. Apoptosis and necrosis are distinguished on the basis of their morphological and biochemical features (17). Apoptosis is thought to be a regulated and physiological type of death by which the organisms eliminate senescent, abnormal, and potentially harmful cells (18). The biological process of apoptosis has been well investigated, and many key molecules in apoptosis, including caspases, have been identified. In contrast, necrosis is described as a passive, nonphysiological type of death that is caused by accidental and acute damage to cells. Necrosis is characterized by cell swelling and disruption of the cell membrane, leading to the release of cellular contents (19, 20); however, the details of this process are still unknown. Recent evidence suggests that necrosis can be a programmed event (21). The term “necroptosis” has been used to designate one particular type of programmed necrosis that depends on the receptor-interacting serine/threonine kinase 1 (RIPK1) (21, 22). Several lines of evidence have revealed that the inhibitor of RIPK1, methylthiohydantoin-dl-tryptophan (necrostatin-1; Nec-1), can inhibit certain types of caspase-independent cell death with the features of necrosis in experimental paradigms such as cell cultures treated with a combination of an apoptosis inducer and a caspase inhibitor, cells treated with an activator of the receptor-interacting protein, and neurons in vivo after ischemic brain injury (21–24). The death-inhibiting effect of Nec-1 has been reported to be highly specific for this form of cell death, which has been called necroptosis. Caspase-8, a key mediator of the extrinsic apoptosis pathway, is considered to be one of the major molecular switches between apoptosis and necroptosis (25). It should be noted that cell death induced by autophagy is known as another type of caspase-independent cell death (26).
In this study, using human neuroblastoma SH-SY5Y cells and primary cortical neuronal cells, we characterized the form of cell death induced by 24S-OHC. We found that 24S-OHC induced necroptosis of neuronal cells in a cell type-dependent manner.
EXPERIMENTAL PROCEDURES
Materials
24S-OHC was obtained from Biomol International, Plymouth Meeting, PA; Dulbecco's modified Eagle's medium/nutrient mixture Ham's F-12 = 1:1 (DMEM/F-12) was from Invitrogen; fetal bovine serum was from Hyclone, Logan, UT; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Nacalai Tesque, Kyoto, Japan; staurosporine (STS) was from Wako Pure Chemical Industries, Tokyo, Japan; and necrostatin-1 (Nec-1), 3-methyladenine, LY294002, and wortmannin were obtained from Sigma. Anti-caspase-3 antibody (catalog no. 9662) and anti-caspase-9 antibody (catalog no. 9504) were purchased from Cell Signaling (Beverly, MA); anti-RIPK1 antibody (catalog no. 551041) was from BD Biosciences; and anti-caspase-8 antibody (catalog no. M058–3) was obtained from Medical and Biological Laboratories, Nagoya, Japan. Other chemicals were of the highest quality commercially available.
Cell Culture and Determination of Cell Viability
SH-SY5Y human neuroblastoma cells (American Type Culture Collection) were routinely maintained in DMEM/F-12 medium containing 10% heat-inactivated fetal bovine serum and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin; Invitrogen) at 37 °C under an atmosphere of 95% air and 5% CO2. To examine the toxicity of 24S-OHC, SH-SY5Y cells were grown on plates at a density of 2 × 105 cells/ml. After the cells were attached (16–18 h), they were treated with each oxysterol at different concentrations for 24 h.
In order to determine mitochondrial function, which is a measure of cell viability, an MTT assay was conducted for the indicated periods, as described previously (27). The cells were incubated with 0.5 mg/ml MTT at 37 °C for 4 h. Isopropyl alcohol containing 0.04 n HCl was added to the culture medium (3:2, by volume), and the mixture was agitated by pipette until the formazan was completely dissolved. The optical density of formazan was measured at 570 nm using a OptiMax tunable microplate reader (Molecular Devices, Sunnyvale, CA). For additional determination of cell viability, a lactate dehydrogenase (LDH) release assay was performed for the indicated periods, as described previously (27). LDH release was measured by using iodotetrazolium chloride in the culture medium. The maximum LDH release was determined by incubation of the cells with 1% Triton X-100. Data were expressed as a percentage of total LDH activity, after subtraction of background determined from the serum medium alone.
Primary Cortical Neurons
Cells were isolated from the cerebral cortex of rat fetuses (Sprague-Dawley rats, day 17 of gestation; SLC, Shizuoka, Japan) as described previously (28). Briefly, the cells were gently dissociated with a plastic pipette after digestion with papain (90 units/ml, Worthington) at 37 °C, and the cells were then plated at a final density of 8 × 104 cells/cm2 on polyethyleneimine-coated plates. Cells were grown in DMEM/F-12 supplemented with 10% heat-inactivated fetal bovine serum and antibiotics (100 units/ml penicillin G and 100 μg/ml streptomycin). Over 90% of the cells in the cultures were neurons as determined by immunostaining with the astrocyte-specific marker glial fibrillary acidic protein (Upstate Biotechnology, Inc., Lake Placid, NY) and the neuron-specific marker microtubule-associated protein-2 (Sigma). More than 6 days after plating the cultured neurons with serum medium, the cells were treated with 24S-OHC at different concentrations for 24 h. For the determination of cell viability, the LDH and MTT methods were used as described previously (28).
Cell Death Assay
To examine changes in nuclear morphology, cells were collected and stained with 1 μg/ml Hoechst 33258 (Sigma). Nuclear condensation of the cells was observed through an Olympus IX-71 epifluorescent microscope (Olympus, Tokyo, Japan). Phosphatidylserine (PS) exposure and caspase activity were analyzed as described previously (29). PS exposure was measured by the binding of annexin V-fluorescein isothiocyanate (FITC). Treated cells were also stained with propidium iodide (PI), which was followed by analysis with a FACSAriaTM II cell sorter (BD Biosciences) with a 488-nm argon laser. Caspase activity was measured by the cleavage of Asp-Glu-Val-Asp (DEVD) and Leu-Glu-His-Asp (LEHD) peptide-conjugated aminomethylcoumarin (AMC) according to the protocol outlined by the manufacturer in the EnzChek caspase assay kit (Invitrogen). Substrate cleavage and the release of AMC were measured using a FLUOstar Galaxy (BMG Labtechnologies, Offenburg, Germany) with excitation and emission wavelengths of 342 and 441 nm, respectively. The intensity of fluorescence was converted to picomoles of AMC by using a standard curve generated with free AMC.
Preparation of Cellular Samples and Western Blot Analysis
A total cell extract was prepared as described previously (29). Treated cells were suspended in lysis buffer (150 mm NaCl, 50 mm Tris-HCl (pH 7.5), 50 mm NaF, 5 mm EDTA, 1% Triton X-100, and 1 mm Na3VO4 with a protease inhibitor mixture tablet) at 4 °C for 30 min. Nuclei and unlysed cellular debris were removed by centrifugation at 15,000 × g for 10 min. The detection of specific proteins in the total cell extracts was performed by Western blot analysis, following the procedure described previously (29).
Measurement of Intracellular ATP
The cellular ATP content was determined using a bioluminescent somatic cell assay kit according to the manufacturer's instructions (Sigma). The ATP content was measured with the luciferin/luciferase method in a luminescence reader BLR-301 (ALOKA, Tokyo, Japan). The absolute values of ATP content were determined using an internal ATP standard.
Transfection of Small Interfering RNA
The human RIPK1-small interfering RNAs (siRNA) were designed and manufactured by Invitrogen, according to the current guidelines for effective knockdown by this method (30). The target sequences for RIPK1-siRNA (RIPK1-HSS112846, catalog numbers 10620318 and 10620319) were used. The siRNA were transfected into SH-SY5Y cells at a concentration of 100 pmol/105 cells by Lipofectamine RNAi MAX (Invitrogen) 3 days before further experiments.
Statistical Analysis
Data are reported as mean ± S.D. of at least three independent experiments. The statistical significance of the difference between the determinations was calculated by an analysis of variance using Tukey's test for multiple comparisons. The calculation method was described in each figure legend. Values of p < 0.01 were considered significant.
RESULTS
Effect of 24S-OHC on Viability of Neuronal Cells
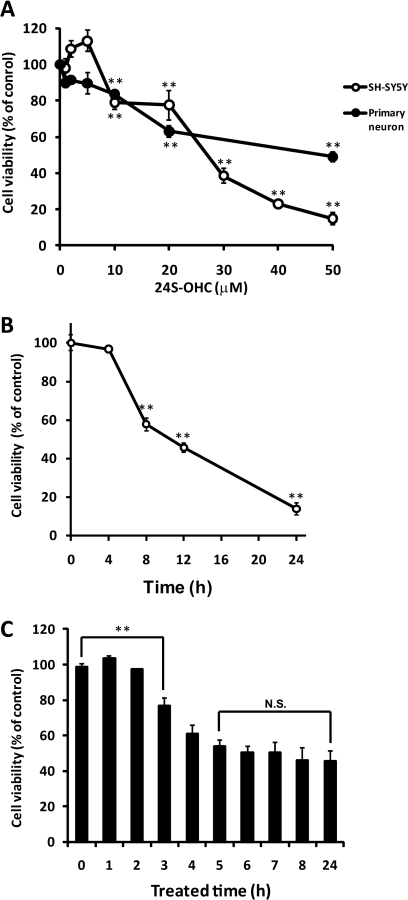
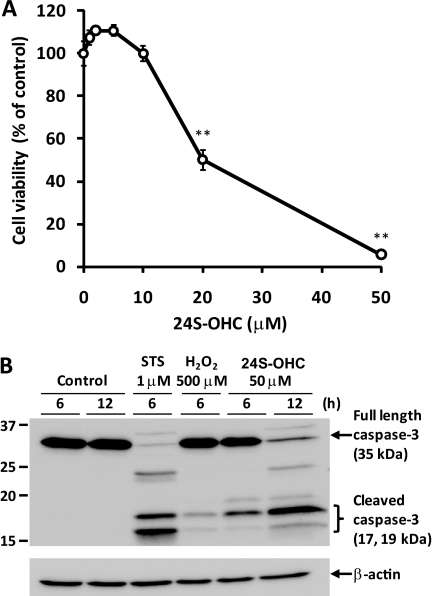
To determine the cytotoxicity of 24S-OHC, human neuroblastoma SH-SY5Y cells were treated with several concentrations of 24S-OHC for 24 h. The viability was measured by MTT assay, which is an indicator of mitochondrial function. 24S-OHC exhibited cytotoxicity in a concentration-dependent manner, and 24S-OHC at concentrations higher than 10 μm significantly decreased the cell viability (Fig. 1A). The cytotoxicity of 24S-OHC was also evaluated by measuring membrane integrity with LDH release assay. The membrane integrity decreased in a concentration-dependent manner (data not shown). 24S-OHC also showed cytotoxicity in the primary cortical neuronal cells at concentrations higher than 10 μm (Fig. 1A). Fig. 1B shows the time-dependent toxicity of 24S-OHC in SH-SY5Y cells, and the statistical decrease in cell viability was observed after 8 h of treatment with 24S-OHC. To estimate the incubation time for 24S-OHC-induced cell death, cells were treated with 24S-OHC for various durations and then cultured with fresh medium for a total of 24 h. Significant cell death was observed with incubation times longer than 3 h, and treatment of 24S-OHC for 5 h exhibited similar cytotoxicity as treatment for 24 h (Fig. 1C).
FIGURE 1.
Cell death induced by 24S-OHC in SH-SY5Y and cultured cortical neurons. A, SH-SY5Y cells and primary cortical neuronal cells were treated with variable concentrations of 24S-OHC for 24 h, and the viability was measured by MTT assay, as described under “Experimental Procedures.” **, p < 0.01, when compared with vehicle control (without 24S-OHC). B, SH-SY5Y cells were cultured with 50 μm 24S-OHC for the indicated incubation times, and the viability was measured by MTT assay. **, p < 0.01, when compared with time 0. C, SH-SY5Y cells were treated with 24S-OHC for the indicated times and then cultured in fresh medium for 24 h. Cell viability was measured by LDH assay. **, p < 0.01, when compared with treated time 0 h. N.S., not significant difference when compared with treated time 24 h.
Characterization of Cell Death Induced by 24S-OHC
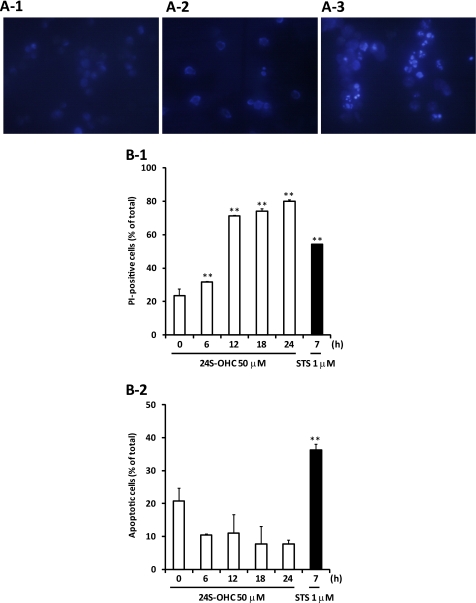
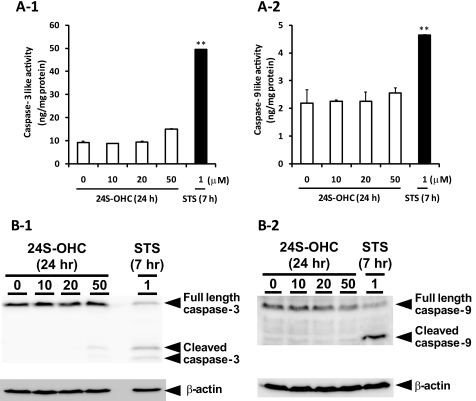
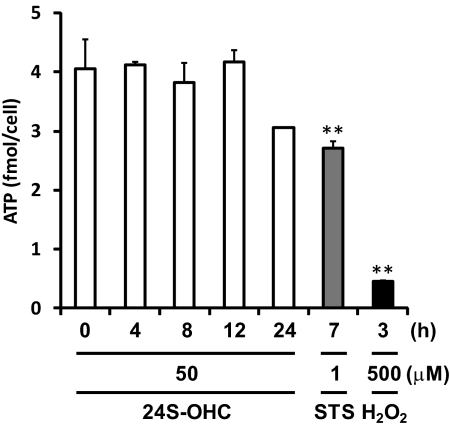
To identify the type of cell death induced by 24S-OHC, we analyzed morphological changes of chromatin and PS exposure. STS was used as an inducer of apoptosis. Apoptotic features such as condensed chromatin (Fig. 2A) and PS exposure (Fig. 2B-2) were observed in STS-treated cells. Morphological changes of chromatin were not observed in 24S-OHC-treated cells (Fig. 2A). 24S-OHC treatment significantly increased PI-positive cells, but it had no effect on apoptotic cells showing annexin V-FITC-positive and PI-negative cells at any incubation time tested (Fig. 2B). Furthermore, a significant increase in the activities of DEVDase (indicative of caspase-3; Fig. 3A-1) and LEHDase (indicative of caspase-9; Fig. 3A-2) was not observed in 24S-OHC-treated cells. In accordance with enzyme activities, cleaved and activated caspase-3 (Fig. 3B-1) and caspase-9 (Fig. 3B-2) were not observed in 24S-OHC-treated cells. These multiple lines of evidence indicate that 24S-OHC-induced neuronal cell death, in contrast to STS-induced cell death, was not induced by typical apoptotic mechanisms that depend on caspase activation. It has been known that some types of necrosis involve ATP depletion (29), but the significant decrease in ATP levels was not observed in 24S-OHC-treated cells (Fig. 4).
FIGURE 2.
Characterization of cell death induced by 24S-OHC in SH-SY5Y cells. A, SH-SY5Y cells treated with 50 μm 24S-OHC or 1 μm STS for 24 or 7 h were stained with Hoechst 33258 and viewed under a fluorescence microscope. B, cells were treated with 24S-OHC or STS for the indicated times, and the cell samples were then treated with annexin V-FITC and PI and subjected to flow cytometry. The percentage of cells exhibiting necrosis (PI-positive cells, panel 1) and apoptosis (annexin V-FITC-positive and PI-negative, panel 2) are shown. **, p < 0.01, when compared with time 0.
FIGURE 3.
Characterization of caspases in 24S-OHC-treated SH-SY5Y cells. The cells were treated with 24S-OHC or STS for the indicated times, and the cell samples were then subjected to the enzyme assay (A) using DEVD-AMC (panel 1) or LEHD-AMC (panel 2) as a substrate and Western blot analysis (B) using an antibody against caspase-3 (panel 1) or caspase-9 (panel 2). **, p < 0.01, when compared with vehicle control (without 24S-OHC).
FIGURE 4.
Analysis of the ATP levels in 24S-OHC-treated SH-SY5Y cells. ATP content in the cells treated with 24S-OHC or STS or H2O2 for the indicated times was determined. **, p < 0.01, when compared with vehicle control (without 24S-OHC).
24S-OHC-induced Cell Death Is Inhibited by Necrostatin-1 and RIPK1-siRNA Treatment
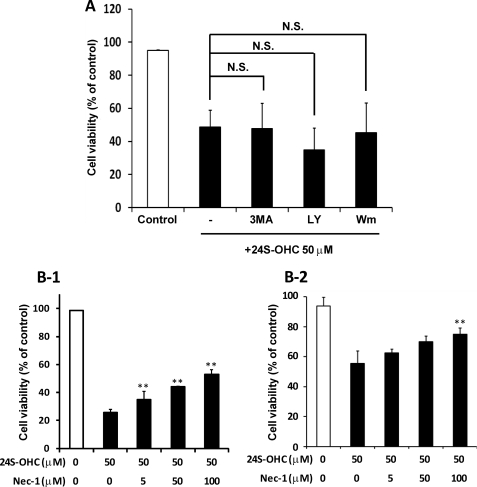
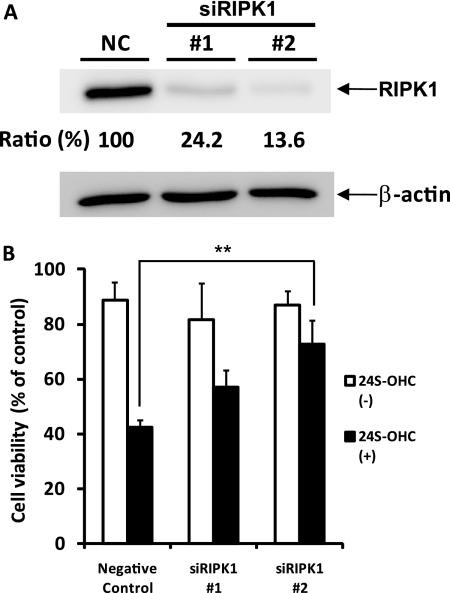
These findings suggest that the cell death induced by 24S-OHC is caspase-independent and does not have typical necrotic features. Since the implication of autophagic cell death or necroptosis has been reported in caspase-independent cell death (21, 31), we examined the effects of autophagic inhibitors on 24S-OHC-induced cell death. As shown in Fig. 5A, autophagic inhibitors such as 3-methyladenine, LY294002, and wortmannin did not prevent SH-SY5Y cell death induced by 24S-OHC. It has been shown that RIPK1, a target of Nec-1, plays a significant role in the caspase-independent cell death pathway of necroptosis (21–24). As shown in Fig. 5B, Nec-1, an inhibitor of necroptosis, inhibited the cell death induced by 24S-OHC in a concentration-dependent manner. The inhibitory effect of Nec-1 against 24S-OHC was also observed in primary cortical neuronal cells (Fig. 5B-2). To further confirm the involvement of RIPK1 in 24S-OHC-induced cell death, we investigated the effect of RIPK1-siRNA. SH-SY5Y cells treated with two independent RIPK1-siRNA for 3 days exhibited a marked decline in constitutive RIPK1 protein levels (Fig. 6A). In cells treated with RIPK1-siRNA, the cytotoxicity of 24S-OHC significantly decreased (Fig. 6B).
FIGURE 5.
Neuroprotective effect of Nec-1 against 24S-OHC-induced cell death. A, SH-SY5Y cells were exposed to 24S-OHC in the absence or presence of autophagy inhibitors (10 mm 3-methyladenine (3MA), 20 μm LY294002 (LY), and 100 nm wortmannin (Wm)) for 24 h, and the viability was measured by LDH assay. B, SH-SY5Y cells (panel 1) and cultured cortical neuronal cells (panel 2) were exposed to 24S-OHC in the absence or presence of Nec-1 for 24 h, and the viability was measured by LDH assay. **, p < 0.01, when compared with vehicle control (without Nec-1).
FIGURE 6.
Protective effect of RIPK1-siRNA treatment against 24S-OHC-induced cell death. A, SH-SY5Y cells were treated with RIPK1-siRNA (siRIPK1) or nonspecific RNA (negative control, NC) for 3 days, and the total protein lysate was subjected to Western blot analysis using an antibody against RIPK1. B, SH-SY5Y cells were pretreated with RIPK1-siRNA for 3 days and then treated with 24S-OHC for 24 h. Cell viability was determined by LDH assay. **, p < 0.01, when compared with negative control.
Expression Level of Caspase-8- and 24S-OHC-induced Caspase-3 Activation in Jurkat Cells
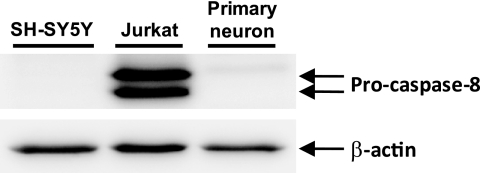
It has been suggested that the caspase-8-mediated degradation of RIPK1 may represent one of the key molecular switches between apoptosis and necroptosis (25). We therefore determined the expression levels of caspase-8 in both the SH-SY5Y cells and cortical neuronal cells. Western blot analysis using anti-caspase-8 antibody indicated the lower expression levels of caspase-8 in both the SH-SY5Y cells and cortical neuronal cells compared with human T lymphoma Jurkat cells that express obvious levels of caspase-8 protein (Fig. 7). Real time PCR analysis also showed the lower expression levels of caspase-8 mRNA in SH-SY5Y cells compared with Jurkat cells (data not shown). As observed in neuronal cells, 24S-OHC significantly decreased the cell viability in Jurkat cells (Fig. 8A). As shown in Fig. 8B, the activation of caspase-3 was observed in STS-treated Jurkat cells, but not in Jurkat cells treated with 500 μm hydrogen peroxide, which can induce necrosis in Jurkat cells (29). In contrast to the observation that 24S-OHC did not induce the caspase activation in SH-SY5Y cells (Fig. 3), 24S-OHC significantly induced the caspase-3 activation in Jurkat cells (Fig. 8B).
FIGURE 7.
Expression level of caspase-8 in SH-SY5Y cells, cortical neuronal cells, and Jurkat cells. Total protein lysates of SH-SY5Y cells, cortical neuronal cells, and Jurkat cells were subjected to Western blot analysis using an antibody against caspase-8.
FIGURE 8.
Cell death and activation of caspase-3 induced by 24S-OHC in Jurkat cells. A, cell death induced by 24S-OHC in Jurkat cells. The cells were treated with variable amounts of 24S-OHC for 24 h, and the viability was measured by MTT assay. **, p < 0.01, when compared with vehicle control (without 24S-OHC). B, Western blot analysis of caspase-3 in 24S-OHC-treated Jurkat cells. The cells were treated with 24S-OHC or STS or H2O2 for the indicated times, and then the cell samples were subjected to Western blot analysis using an antibody against caspase-3.
DISCUSSION
24S-OHC, an oxysterol related to cholesterol homeostasis in the brain, has been shown to possess potent cytotoxicity (16). This study clearly shows that neuronal cell death induced by 24S-OHC, at least partly, involves the necroptosis pathway. The absence of typical apoptotic features in 24S-OHC-treated cells also supports the involvement of programmed necrosis. It has been known that oxysterols such as 7-ketocholesterol can induce apoptotic cell death through association with membrane lipid raft domains (15, 32). We observed typical apoptotic features such as PS exposure and caspase activation in SH-SY5Y cells that were treated with 7-ketocholesterol (data not shown), suggesting that 7-ketocholesterol induces cell death via a different pathway with 24S-OHC. We further examined the effect of Nec-1 against SH-SY5Y cell death induced by other oxysterols such as 7α-OHC, 7β-OHC, 7-ketocholesterol, and 22R-OHC. These oxysterols decreased the viability of SH-SY5Y cells in a concentration-dependent manner (supplemental Fig. S-1). Nec-1 did not show a significant protective effect against SH-SY5Y cell death induced by the oxysterols described above (supplemental Fig. S-2), suggesting that, among the oxysterols tested in this study, only 24S-OHC induces necroptosis of SH-SY5Y cells. Although the details of the cell death mechanisms induced by oxysterols are still unclear, to our knowledge, this is the first report showing that an oxysterol can induce necroptosis.
Several studies have shown that Nec-1 can protect cells from cell death or tissue injury induced by the following stressors: mouse brain injury induced by ischemia (21), HT-22 cell death induced by glutamate (33), and macrophage cell death induced by sitosterol-containing lipoproteins (34). These reports suggest the involvement of the activation of RIPK1 in these types of cell injuries. It has been suggested that the caspase-8-mediated degradation of RIPK1 may represent one of the key molecular switches between apoptosis and necroptosis (25). We observed lower expression levels of caspase-8 in both the SH-SY5Y cells and cortical neuronal cells (Fig. 7). This observation prompted us to examine the type of 24S-OHC-induced cell death in human T lymphoma Jurkat cells, which express obvious levels of caspase-8 protein. Interestingly, significant cell death with typical apoptotic features such as caspase activation was observed in 24S-OHC-treated Jurkat cells (Fig. 8B). Although the physiological and pathological role of necroptosis has not been fully elucidated, our observations suggest that cell type or caspase-8 expression may be important factors that determine the fate of cell death even with the same stimulus.
It has been postulated that reactive oxygen species (ROS) play a role in the induction of necroptosis (35–37); however, the detailed molecular mechanisms are still unknown. It has been reported that ROS production is required for cell death induced by tumor necrosis factor (TNF) or TNF + benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone, and protective effects of antioxidants such as butylhydroxyanisol and N-acetylcysteine have been reported in mouse embryonic fibroblasts (36, 37). In contrast, N-acetylcysteine, superoxide dismutase, catalase, and vitamin E failed to suppress TNF + benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone-induced necrosis in U937 cells (21). In this study, we examined the protective effects of antioxidants with different actions, such as α-tocotrienol, deferoxamine, BO-653, curcumin, and N-acetylcysteine against 24S-OHC-induced cell death; however, significant protective effects were not observed (supplemental Figs. S-3 and S-4), suggesting that ROS might not play a critical role in 24S-OHC-induced necroptosis. The involvement of ROS in the induction of necroptosis might depend on the cell type or the type of stimulus, and further studies are necessary to elucidate the role of ROS in the induction or execution of necroptosis.
Several studies have shown that cholesterol metabolism is implicated in the pathogenesis of Alzheimer disease; hypercholesterolemia is unanimously recognized to be a risk factor for sporadic Alzheimer disease (38), and epidemiological studies have shown homozygosity for the APO-E4 allele is associated with an increased risk of Alzheimer disease (7). The increase of 24S-OHC in plasma and cerebrospinal fluid of Alzheimer disease patients has also been known (39, 40). The selective expression of cholesterol 24S-hydroxylase around the amyloid plaques has been suggested (41). Björkhem and co-workers (5) have reported that the concentration of 24S-OHC in the brain is elevated up to 30 μm. They also reported that 24S-OHC caused cell death when added at 50 μm to undifferentiated SH-SY5Y neuronal cells (16), which is the same concentration used in this study. Very recently, the action of 24S-OHC in amplifying neuronal damage of amyloid-β has been reported (42). Collectively, these studies suggest the implication of 24S-OHC in the pathogenesis of Alzheimer disease. Based on the our findings in this study, it should be significant to study the implication of necroptosis and RIPK1 in the amyloid plaques and the pathogenesis of Alzheimer disease.
In conclusion, we clearly show that 24S-OHC induced neuronal cell death through the necroptosis pathway involving RIPK1 in a cell type-dependent manner. These findings have important implications in the neuronal cell death induced by oxysterol. The pathological significance of neurotoxicity of 24S-OHC and the role of RIPK1 should be explored in future studies.
Supplementary Material
This work was supported in part by the Academic Frontier Research Project on “New Frontier of Biomedical Engineering Research” from the Ministry of Education, Culture, Sports, Science, and Technology (to N. N.), by the Cosmetology Research Foundation (to Y. S.), by a research grant from the Science and Engineering Research Institute (to Y. S.), and by Grant-in-aid for Research Activity Start-Up 21800075 (to Y. U.) from the Japan Society for the Promotion of Science.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- 24S-OHC
- 24(S)-hydroxycholesterol
- AMC
- aminomethylcoumarin
- LDH
- lactate dehydrogenase
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PI
- propidium iodide
- PS
- phosphatidylserine
- ROS
- reactive oxygen species
- STS
- staurosporine.
REFERENCES
- 1. Björkhem I., Meaney S. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 806–815 [DOI] [PubMed] [Google Scholar]
- 2. Jurevics H., Morell P. (1995) J. Neurochem. 64, 895–901 [DOI] [PubMed] [Google Scholar]
- 3. Björkhem I., Cedazo-Minguez A., Leoni V., Meaney S. (2009) Mol. Aspects Med. 30, 171–179 [DOI] [PubMed] [Google Scholar]
- 4. Björkhem I., Lütjohann D., Breuer O., Sakinis A., Wennmalm A. (1997) J. Biol. Chem. 272, 30178–30184 [DOI] [PubMed] [Google Scholar]
- 5. Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Sidén A., Diczfalusy U., Björkhem I. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 9799–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Björkhem I. (2007) Lipids 42, 5–14 [DOI] [PubMed] [Google Scholar]
- 7. Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kölsch H., Lütjohann D., Ludwig M., Schulte A., Ptok U., Jessen F., von Bergmann K., Rao M. L., Maier W., Heun R. (2002) Mol. Psychiatry 7, 899–902 [DOI] [PubMed] [Google Scholar]
- 9. Töröcsik D., Szanto A., Nagy L. (2009) Mol. Aspects Med. 30, 134–152 [DOI] [PubMed] [Google Scholar]
- 10. Saito Y., Yoshida Y., Niki E. (2007) FEBS Lett. 581, 4349–4354 [DOI] [PubMed] [Google Scholar]
- 11. Chen Z. H., Yoshida Y., Saito Y., Sekine A., Noguchi N., Niki E. (2006) J. Biol. Chem. 281, 14440–14445 [DOI] [PubMed] [Google Scholar]
- 12. Radhakrishnan A., Ikeda Y., Kwon H. J., Brown M. S., Goldstein J. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6511–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joseph S. B., McKilligin E., Pei L., Watson M. A., Collins A. R., Laffitte B. A., Chen M., Noh G., Goodman J., Hagger G. N., Tran J., Tippin T. K., Wang X., Lusis A. J., Hsueh W. A., Law R. E., Collins J. L., Willson T. M., Tontonoz P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7604–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vejux A., Guyot S., Montange T., Riedinger J. M., Kahn E., Lizard G. (2009) J. Nutr. Biochem. 20, 45–61 [DOI] [PubMed] [Google Scholar]
- 15. Royer M. C., Lemaire-Ewing S., Desrumaux C., Monier S., Pais de Barros J. P., Athias A., Néel D., Lagrost L. (2009) J. Biol. Chem. 284, 15826–15834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kölsch H., Lütjohann D., Tulke A., Björkhem I., Rao M. L. (1999) Brain Res. 818, 171–175 [DOI] [PubMed] [Google Scholar]
- 17. Wyllie A. H., Kerr J. F., Currie A. R. (1980) Int. Rev. Cytol. 68, 251–306 [DOI] [PubMed] [Google Scholar]
- 18. Schwartz L. M., Smith S. W., Jones M. E., Osborne B. A. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 980–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leist M., Single B., Castoldi A. F., Kühnle S., Nicotera P. (1997) J. Exp. Med. 185, 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicotera P., Melino G. (2004) Oncogene 23, 2757–2765 [DOI] [PubMed] [Google Scholar]
- 21. Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G. D., Mitchison T. J., Moskowitz M. A., Yuan J. (2005) Nat. Chem. Biol. 1, 112–119 [DOI] [PubMed] [Google Scholar]
- 22. Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J. L., Schneider P., Seed B., Tschopp J. (2000) Nat. Immunol. 1, 489–495 [DOI] [PubMed] [Google Scholar]
- 23. He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. (2009) Cell 137, 1100–1111 [DOI] [PubMed] [Google Scholar]
- 24. Hitomi J., Christofferson D. E., Ng A., Yao J., Degterev A., Xavier R. J., Yuan J. (2008) Cell 135, 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galluzzi L., Kroemer G. (2008) Cell 135, 1161–1163 [DOI] [PubMed] [Google Scholar]
- 26. Tsujimoto Y., Shimizu S. (2005) Cell Death Differ. 12, Suppl. 2, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 27. Saito Y., Nishio K., Ogawa Y., Kinumi T., Yoshida Y., Masuo Y., Niki E. (2007) Free Radic. Biol. Med. 42, 675–685 [DOI] [PubMed] [Google Scholar]
- 28. Saito Y., Nishio K., Numakawa Y., Ogawa Y., Yoshida Y., Noguchi N., Niki E. (2007) J. Neurochem. 102, 1625–1634 [DOI] [PubMed] [Google Scholar]
- 29. Saito Y., Nishio K., Ogawa Y., Kimata J., Kinumi T., Yoshida Y., Noguchi N., Niki E. (2006) Free Radic. Res. 40, 619–630 [DOI] [PubMed] [Google Scholar]
- 30. Ogawa Y., Saito Y., Nishio K., Yoshida Y., Ashida H., Niki E. (2008) Free Radic. Res. 42, 674–687 [DOI] [PubMed] [Google Scholar]
- 31. Gozuacik D., Kimchi A. (2004) Oncogene 23, 2891–2906 [DOI] [PubMed] [Google Scholar]
- 32. Berthier A., Lemaire-Ewing S., Prunet C., Monier S., Athias A., Bessède G., Pais de Barros J. P., Laubriet A., Gambert P., Lizard G., Néel D. (2004) Cell Death Differ. 11, 897–905 [DOI] [PubMed] [Google Scholar]
- 33. Xu X., Chua C. C., Kong J., Kostrzewa R. M., Kumaraguru U., Hamdy R. C., Chua B. H. (2007) J. Neurochem. 103, 2004–2014 [DOI] [PubMed] [Google Scholar]
- 34. Bao L., Li Y., Deng S. X., Landry D., Tabas I. (2006) J. Biol. Chem. 281, 33635–33649 [DOI] [PubMed] [Google Scholar]
- 35. Zhang D. W., Shao J., Lin J., Zhang N., Lu B. J., Lin S. C., Dong M. Q., Han J. (2009) Science 325, 332–336 [DOI] [PubMed] [Google Scholar]
- 36. Lin Y., Choksi S., Shen H. M., Yang Q. F., Hur G. M., Kim Y. S., Tran J. H., Nedospasov S. A., Liu Z. G. (2004) J. Biol. Chem. 279, 10822–10828 [DOI] [PubMed] [Google Scholar]
- 37. Chen T. Y., Chi K. H., Wang J. S., Chien C. L., Lin W. W. (2009) Free Radic. Biol. Med. 46, 643–655 [DOI] [PubMed] [Google Scholar]
- 38. Panza F., Capurso C., D'Introno A., Colacicco A. M., De Candia D., Capurso A., Solfrizzi V. (2007) J. Am. Geriatr. Soc. 55, 133–135 [DOI] [PubMed] [Google Scholar]
- 39. Lütjohann D., Papassotiropoulos A., Björkhem I., Locatelli S., Bagli M., Oehring R. D., Schlegel U., Jessen F., Rao M. L., von Bergmann K., Heun R. (2000) J. Lipid Res. 41, 195–198 [PubMed] [Google Scholar]
- 40. Shafaati M., Solomon A., Kivipelto M., Björkhem I., Leoni V. (2007) Neurosci. Lett. 425, 78–82 [DOI] [PubMed] [Google Scholar]
- 41. Brown J., 3rd, Theisler C., Silberman S., Magnuson D., Gottardi-Littell N., Lee J. M., Yager D., Crowley J., Sambamurti K., Rahman M. M., Reiss A. B., Eckman C. B., Wolozin B. (2004) J. Biol. Chem. 279, 34674–34681 [DOI] [PubMed] [Google Scholar]
- 42. Gamba P., Leonarduzzi G., Tamagno E., Guglielmotto M., Testa G., Sottero B., Gargiulo S., Biasi F., Mauro A., Viña J., Poli G. (2011) Aging Cell 10, 403–417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.