Abstract
Growth hormone (GH) stimulates growth plate chondrogenesis and longitudinal bone growth with its stimulatory effects primarily mediated by insulin-like growth factor-1 (IGF-1) both systemically and locally in the growth plate. It has been shown that the transcription factor Stat5b mediates the GH promoting effect on IGF-1 expression and on chondrogenesis, yet it is not known whether other signaling molecules are activated by GH in growth plate chondrocytes. We have previously demonstrated that nuclear factor-κB p65 is a transcription factor expressed in growth plate chondrocytes where it facilitates chondrogenesis. We have also shown that fibroblasts isolated from a patient with growth failure and a heterozygous mutation of inhibitor-κBα (IκB; component of the nuclear factor-κB (NF-κB) signaling pathway) exhibit GH insensitivity. In this study, we cultured rat metatarsal bones in the presence of GH and/or pyrrolidine dithiocarbamate (PDTC), a known NF-κB inhibitor. The GH-mediated stimulation of metatarsal longitudinal growth and growth plate chondrogenesis was neutralized by PDTC. In cultured chondrocytes isolated from rat metatarsal growth plates, GH induced NF-κB-DNA binding and chondrocyte proliferation and differentiation and prevented chondrocyte apoptosis. The inhibition of NF-κB p65 expression and activity (by NF-κB p65 siRNA and PDTC, respectively) in chondrocytes reversed the GH-mediated effects on chondrocyte proliferation, differentiation, and apoptosis. Lastly, the inhibition of Stat5b expression in chondrocytes prevented the GH promoting effects on NF-κB-DNA binding, whereas the inhibition of NF-κB p65 expression or activity prevented the GH-dependent activation of IGF-1 and bone morphogenetic protein-2 expression.
Keywords: Bone, Growth Factors, NF-κB Transcription Factor, Signal Transduction, STAT Transcription Factor, Growth Hormone, Chondrocyte, Growth Plate
Introduction
The NF-κB2 family is a group of transcription factors including seven members, p65 (RelA), c-Rel, RelB, p50/p105 (NF-κB1), and p52/p100 (NF-κB2), which can form various hetero- or homodimers (1). In an unstimulated cell, NF-κB homodimers or heterodimers are sequestered in the cytoplasm and bound to IκB proteins (2). Binding to IκB prevents the NF-κB·IκB complex from translocating to the nucleus, thereby maintaining NF-κB in an inactive state. Upon activation by a wide variety of stimuli (proinflammatory cytokines, growth factors, and viral proteins), the previously bound NF-κB is then released, and it is able to translocate to the nucleus where it modulates the expression of target genes involved in cell growth, survival, adhesion, and death (3, 4).
Previous evidence indicates that NF-κB exerts a regulatory role in bone growth and development. In chick embryo, overexpression of IκBα, which prevents NF-κB activation, results in abnormal limb development (5). It has also been shown that mice deficient in both the NF-κB subunits p50 and p52 have retarded growth and shortened long bones, suggesting that NF-κB may be involved in bone formation and growth (6).
We have recently demonstrated that NF-κB p65 is expressed in the murine growth plate, and it facilitates longitudinal bone growth and growth plate chondrogenesis in part by inducing bone morphogenetic protein-2 (BMP-2) expression (7). In addition, we have also shown that NF-κB p65 mediates the growth-promoting effects of insulin-like growth factor-1 (IGF-1) on chondrogenesis and longitudinal bone growth (8). Along with IGF-1, growth hormone (GH) is another critical regulator of longitudinal bone growth and growth plate chondrogenesis. Although it also elicits some IGF-independent effects, GH primarily stimulates chondrogenesis by inducing IGF-1 synthesis in chondrocytes via the activation of the transcription factor Stat5b. However, it is not clear whether other intracellular signaling pathways mediate the effects of GH on chondrocyte function. The aim of our study was to determine whether GH stimulates the activity of NF-κB in growth plate chondrocytes and whether such stimulatory effect is necessary for the GH promoting effect on chondrogenesis and IGF-1 expression.
EXPERIMENTAL PROCEDURES
Chondrocyte Culture
The cartilaginous portions of metatarsal bones isolated from Sprague-Dawley rat embryos (20 days postcoitum) were dissected, rinsed in PBS, and then incubated in 0.2% trypsin for 1 h and 0.2% collagenase for 3 h. The cell suspension was aspirated repeatedly and filtered through a 70-μm cell strainer, rinsed first in PBS and then in serum-free DMEM, and counted. Chondrocytes were seeded in 100-mm dishes at a density of 5 × 104/cm2 in DMEM with 100 units/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml ascorbic acid, and 10% FBS. The culture medium was changed at 72-h intervals. After reaching 70–80% confluence, cells were washed with serum-free medium and cultured without or with graded concentrations of recombinant mouse GH (1, 10, and 100 ng/ml) (Sigma) and/or 1 μm pyrrolidine dithiocarbamate (PDTC), a known NF-κB inhibitor. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (Department of Health, Education, and Welfare Publication (National Institutes of Health) 85-23, revised 1988).
siRNA Transfection
Chondrocytes were transfected with a pool of three target-specific, 20–25-nucleotide-long siRNAs designed to selectively inhibit NF-κB p65 or Stat5b mRNA expression (p65 siRNA: sense strand A, GAAGAAGAGUCCUUUCAAUtt (mRNA loc. 1000); sense strand B, CCAUCAACUUUGAUGAGUUtt (mRNA loc. 1131); sense strand C, GCAUUAACUUCCCUGAAGUtt (mRNA loc. 1893) (catalog number 61876, Santa Cruz Biotechnology, Inc., Santa Cruz, CA); Stat5b siRNA: sense strand A, CUGUAUCCGGCACAUUCUAtt (mRNA loc. 431); sense strand B, CGGAAGAGAAGUUCACAAUtt (mRNA loc. 1417); sense strand C, GUGUGAGUUUGCACGUUGUtt (mRNA loc. 2653) (catalog number 156026, Santa Cruz Biotechnology, Inc.)). A pool of siRNAs each comprising a scrambled sequence was similarly transfected as control siRNA (catalog number 44230, Santa Cruz Biotechnology, Inc.). siRNAs were introduced to cells using Lipofectamine 2000 (Invitrogen) according to the procedure recommended by the manufacturer. One day before transfection, cells were plated in 500 μl of growth medium without antibiotics such that they were 30–50% confluent at the time of transfection. The transfected cells were cultured in DMEM containing 10% FCS for 72 h after transfection.
To determine whether p65 siRNA and Stat5b siRNA silenced p65 and Sta5b expression, respectively, we analyzed the expression of these two target mRNAs by real time PCR. When compared with untransfected chondrocytes and control siRNA-transfected chondrocytes, cells transfected with p65 siRNA and Stat5b siRNA exhibited decreased p65 and Stat5b mRNA expression, respectively (supplemental Fig. 1).
NF-κB Transcription Factor Assay
NF-κB p65-DNA binding activity was determined by an enzyme-linked immunosorbent assay (Cayman Chemicals, Ann Arbor, MN) according to the manufacturer's instructions. A specific double-stranded DNA (dsDNA) sequence containing the NF-κB p65 response element was immobilized onto the bottom of wells of a 96-well plate. Chondrocytes were treated with GH and/or PDTC or p65 siRNA for 24 h, and then nuclear extracts were collected. Nuclear extracts containing NF-κB p65 were added to the plate and incubated overnight at 4 °C without agitation. NF-κB p65 was detected by addition of a specific primary antibody directed against NF-κB p65. A secondary antibody conjugated to HRP was added to provide a sensitive colorimetric readout at 450 nm. Data are expressed as A450/μg of nuclear extract and represent three separate experiments.
Whole Metatarsal Culture
The second, third, and fourth metatarsal bone rudiments were isolated from Sprague-Dawley rat embryos (20 days postcoitum) and cultured individually in 24-well plates (8). Each well contained 0.5 ml of minimum essential medium (Invitrogen) supplemented with 0.05 mg/ml ascorbic acid (Invitrogen), 1 mm sodium glycerophosphate (Sigma-Aldrich), 0.2% bovine serum albumin (Sigma), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) without or with 10 ng/ml recombinant mouse GH and/or 1 μm PDTC. The culture plates were incubated for 3 days in a humidified incubator with 5% CO2 in air at 37 °C, and the medium was changed every other day.
Measurement of Metatarsal Linear Growth
The length of each metatarsal bone was measured every day under a dissecting microscope using an eyepiece micrometer. The culture medium was briefly removed immediately before each measurement.
Quantitative Histology
At the end of the culture period (3 days), metatarsal bones were fixed with 4% paraformaldehyde overnight and embedded in paraffin. Three 5–7-μm-thick longitudinal sections from each bone were obtained and stained with hematoxylin. Metatarsal whole growth plate and growth plate epiphyseal, proliferative, and hypertrophic zone heights (μm) were measured. All measurements were performed by a single observer blinded to the treatment regimen.
[3H]Thymidine Incorporation
To assess cell proliferation in the metatarsal growth plate, we measured [3H]thymidine incorporation into newly synthesized DNA (9). After 3 days of culture, [3H]thymidine was added to the culture medium at a concentration of 5 μCi/ml (25 Ci/mmol; Amersham Biosciences). Bone rudiments were incubated for an additional 5 h. At the end of the incubation, all bones were fixed in 4% phosphate-buffered paraformaldehyde, embedded in paraffin, and cut in 5–7-μm-thick longitudinal sections. Autoradiography was performed by dipping the slides in Hypercoat emulsion, exposing them for 4 weeks, and then developing them with an Eastman Kodak Co. D-19 developer. Sections were counterstained with hematoxylin. The labeling index was calculated as the number of [3H]thymidine-labeled cells per grid divided by the total number of cells per grid. The grid circumscribed a portion of the growth plate zone as viewed through a 40× objective and generally contained an average of 50 cells. In each growth plate, the labeling index was calculated separately in three distinct grid locations of the epiphyseal and proliferative zones and then averaged. For each treatment group, we sampled eight bones and analyzed both growth plates of each of three longitudinal sections per bone. All determinations were made by the same observer blinded to the treatment category.
To assess proliferation in cultured chondrocytes, 2.5 μCi/well [3H]thymidine (Amersham Biosciences) was added to the culture medium for an additional 3 h at the end of the culture period. Cells were then released by trypsin and collected onto glass fiber filters. Incorporation of [3H]thymidine was measured by liquid scintillation counting.
In Situ Hybridization
At the end of the culture period, metatarsals were fixed overnight in 4% paraformaldehyde at 4 °C, then dehydrated in ethanol, and embedded in paraffin. Sections (5 μm thick) were hybridized to 35S-labeled Col10a1 antisense riboprobes. Slides were exposed to photographic emulsion at 4 °C for 4 days, then developed, fixed, and cleared. Sections were counterstained with hematoxylin and viewed using a light microscope. Sections hybridized with a labeled sense Col10a1 riboprobe were used as negative controls. The mouse Type X collagen (Col10a1) probe (a gift from Dr. Bjorn Olsen, Harvard Medical School, Boston, MA) was a 650-bp HindIII fragment containing 400 bp of non-collagenous (NC1) domain and 250 bp of 3′-untranslated sequence of the mouse Col10a1 gene in pBluescript (10).
Real Time PCR
At the end of the culture period, total RNA was extracted from chondrocytes using the Qiagen RNeasy Mini kit (Qiagen Inc., Valencia, CA). The recovered RNA was further processed using a First Strand cDNA Synthesis kit for RT-PCR (avian myeloblastosis virus) (Roche Diagnostics) to produce cDNA. 1 μg of total RNA and 1.6 μg of oligo(dT)15 primer were incubated for 10 min at 25 °C followed by incubation for 60 min at 42 °C in the presence of 20 units of avian myeloblastosis virus reverse transcriptase and 50 units of RNase inhibitor in a total reaction volume of 20 μl. The cDNA products were directly used for PCR or stored at −80 °C for later analysis. Real time quantitative PCR was carried out using the StepOne Real Time PCR System (Applied Biosystems, Foster City, CA) in a final volume of 25 μl containing 1 μl of cDNA, 12.5 μl of 2× SYBR Green Master Mix (Applied Biosystems), and 0.1 μm primers (Applied Biosystems) in DNase-free water. The primers used were as follows: rat collagen X (GenBankTM accession number AJ131848): forward, 5′-TCT GTA CAA CAG GCA GCA GCA CTA-3′; reverse, 5′-GTA CAT TGT GGG CGT GCC ATT CTT-3′; rat IGF-1 (M15481): forward, 5′-GGG CAT TGT GGA TGA GTG TTG CTT-3′; reverse, 5′-TGG AAC GAG CTG ACT TTG TAG GCT-3′; rat BMP-2 (NM_017178): forward, 5′-AAC CAT GGG TTT GTG GTG GAA GTG-3′; reverse, 5′-CCA GCT GTG TTC ATC TTG GTG CAA-3′; rat NF-κB p65 (NM_199267): forward, 5′-CAT GCG TTT CCG TTA CAA GTG CGA-3′; reverse, 5′-TGG TCC CGT GTA GCC ATT GAT CTT-3′; rat STAT5b (NM_022380): forward, 5′-GCG CTC AAC ATG AAG TTC AAG GCT-3′; reverse, 5′-AGG ACA CGG ACA TGC TGT TGT AGT-3′; and rat β-actin (NM_031144): forward, 5′-TGA GCG CAA GTA CTC TGT GTG GAT-3′; reverse, 5′-TAG AAG CAT TTG CGG TGC ACG ATG-3′. Product sizes were as follows: collagen X, 148 bp; IGF-1, 95 bp; NF-κB p65, 115 bp; STAT5b, 136 bp; and β-actin, 129 bp. The PCR conditions were 50 °C for 2 min followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Values were quantified using the comparative cycle threshold (CT) method, and samples were normalized to β-actin.
Western Blot
Whole cell lysates were solubilized with 1% SDS sample buffer and electrophoresed on a 4–15% SDS-polyacrylamide gel (Bio-Rad). Proteins were transferred onto a nitrocellulose membrane and probed with the following primary antibodies: goat polyclonal antibody against IGF-1 (Santa Cruz Biotechnology, Inc.), goal polyclonal antibody against BMP-2 (Santa Cruz Biotechnology, Inc.), rabbit polyclonal antibody against phospho-Stat5 (Cell Signaling Technology Inc., Danvers, MA), and rabbit polyclonal antibody against β-actin (Sigma-Aldrich). The blots were developed using a horseradish peroxidase-conjugated polyclonal donkey anti-goat/rabbit IgG antibody and the enhanced chemiluminescence system (GE Healthcare). The intensity of the bands on Western blots was analyzed by NIH ImageJ. The protein size was confirmed by molecular weight standards (Invitrogen).
In Situ Cell Death
At the end of the culture period, metatarsals were fixed in 4% phosphate-buffered paraformaldehyde, embedded in paraffin, and cut in 5–7-μm-thick longitudinal sections. From each bone, three sections parallel to the long axis of the bone were obtained. Apoptotic cells in the growth plate were identified by terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling according to the manufacturer's instructions (TdT-FragEL kit, Oncogene Research Products, Boston, MA) with slight modifications (deparaffinized and rehydrated sections were treated with proteinase K for 10 min instead of 20 min). A positive control was generated by covering the entire tissue section with 1 μg/liter DNase I in 1× Tris-buffered saline and 1 mm MgSO4 for 20 min following proteinase K treatment. A negative control was generated by substituting distilled H2O for the terminal deoxynucleotidyltransferase in the reaction mixture. Apoptosis was quantitated by determining the apoptotic index (calculated as the number of apoptotic cells per grid divided by the total number of cells per grid). The grid circumscribed a portion of the growth plate analyzed through a 40× objective and generally contained an average of 50 cells. In each growth plate, the apoptotic index was calculated separately in three distinct grid locations of the epiphyseal, proliferative, and hypertrophic zones and then averaged. For each treatment group, we sampled five bones and analyzed both growth plates of each of three longitudinal sections per bone. All determinations were made by the same observer blinded to the treatment category.
At the end of the culture period, cultured chondrocytes were briefly washed with PBS, fixed in −10 °C methanol for 5 min, and then air-dried. Apoptotic cells were identified by terminal deoxynucleotidyltransferase fragment end labeling (TdT-FragEL kit, Oncogene Research Products). Apoptosis was quantitated by determining the apoptotic index (calculated as the number of apoptotic cells per grid divided by the total number of cells per grid). The grid circumscribed a portion of chondrocytes analyzed through a 40× objective and generally contained an average of 30 cells. In each slide, the apoptotic index was calculated separately in four distinct grid locations and then averaged. For each group, we sampled three slides during each experiment. Results are expressed as the percentage of control and were obtained from three separate experiments. All determinations were made by the same observer blinded to the treatment category.
Caspase-3 Assay
Cytosolic caspase-3 activity was determined in a medium containing 50 mm Tris-HCl buffer (pH 7.0), 0.5 mm Na-EDTA, 20% glycerol, 500 μg of cytosolic protein, and a 75 μm concentration of a synthetic fluorogenic substrate containing the recognition sequence for caspase-3 (acetyl-DEVD-7-amido-4-methylcoumarin) (Upstate Biotechnology Inc., Lake Placid, NY). The caspase-3 activity was measured spectrofluorometrically at 460 nm using a 380-nm excitation wavelength at 37 °C for 150 s. The relative level of caspase-3 activity was expressed as nmol/mg of protein/h. Results are expressed as the percentage of control (mean ± S.E.) and were obtained from three separate experiments.
Statistics
All data are expressed as the mean ± S.E. Statistical significance was determined by t test or analysis of variance.
RESULTS
Effects of GH on NF-κB p65 DNA Binding in Growth Plate Chondrocytes
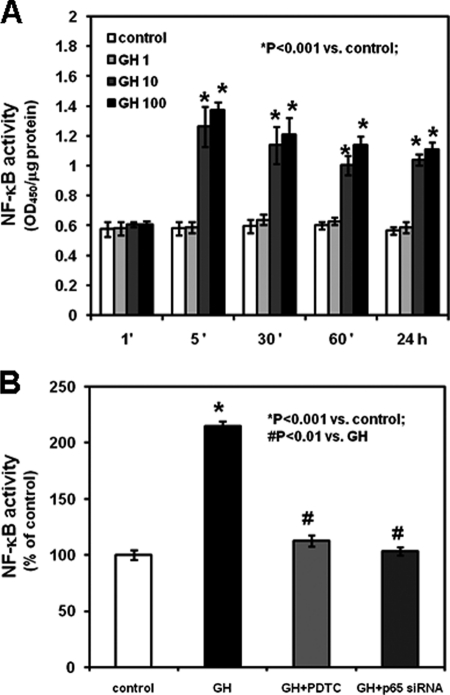
To determine whether GH specifically induces NF-κB activation in growth plate chondrocytes, we evaluated the binding of NF-κB p65 to nuclear DNA. Chondrocytes isolated from rat metatarsal growth plates were cultured in the absence or presence of graded concentrations of GH (1, 10, and 100 ng/ml). 10 and 100 ng/ml GH induced NF-κB-DNA binding in chondrocytes in a dose-dependent manner (Fig. 1A, p < 0.001 versus control). Such stimulatory effect was detected first after 5 min and up to 24 h of treatment. Co-treatment of chondrocytes with 10 ng/ml GH (the lowest concentration of GH inducing NF-κB-DNA binding) and PDTC prevented this stimulatory effect of GH, whereas the addition of 10 ng/ml GH to the culture medium of chondrocytes previously transfected with p65 siRNA did not modify NF-κB p65-DNA binding compared with untreated chondrocytes transfected with a control siRNA (Fig. 1B and supplemental Table 1).
FIGURE 1.
Effects of GH on NF-κB p65-DNA binding activity. NF-κB p65-DNA binding activity was determined by an enzyme-linked immunosorbent assay according to the manufacturer's instructions. A, chondrocytes were cultured in the absence or presence of 1, 10, or 100 ng/ml GH for the indicated time intervals (′, minutes). B, chondrocytes were transfected with a control siRNA or NF-κB p65 siRNA and cultured in the absence or presence of 10 ng/ml GH with or without 1 μm PDTC. Results are expressed as the percentage of control and represent means ± S.E. obtained from three independent experiments.
Effects of GH and NF-κB on Metatarsal Longitudinal Growth and Metatarsal Growth Plate Chondrogenesis
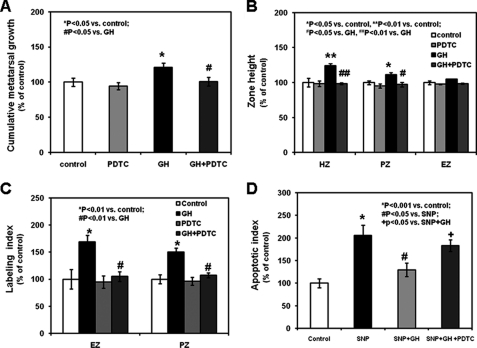
To determine whether the stimulatory effects of GH on longitudinal bone growth and growth plate chondrogenesis are mediated by NF-κB, we cultured rat metatarsal bones in serum-free medium in the absence or presence of the lowest effective concentration of GH (10 ng/ml). After 3 days of culture, GH significantly stimulated metatarsal longitudinal growth (Fig. 2A, p < 0.05, and supplemental Table 2). We then evaluated the effects of GH on the metatarsal growth plate morphology: 10 ng/ml GH increased the metatarsal growth plate proliferative and hypertrophic zone heights (Fig. 2B, p < 0.01, and supplemental Table 2). The addition of PDTC to the serum-free medium of cultured metatarsal bones neutralized all of the stimulatory effects of GH on metatarsal longitudinal growth and growth plate morphology (Fig. 2, A and B, p < 0.05 versus GH, and supplemental Table 2). To determine the effects of GH on growth plate chondrocyte proliferation, we examined the in situ [3H]thymidine incorporation into the metatarsal bones at the end of the culture period (3 days). GH significantly increased [3H]thymidine incorporation into the growth plate epiphyseal and proliferative zones, whereas co-treatment with PDTC abolished such effect (Fig. 2C, labeling index, and supplemental Fig. 2, representative sections of untreated and treated metatarsals). We then evaluated the effects of GH on chondrocyte differentiation by assessing mRNA expression of collagen X (a marker of chondrocyte differentiation) in the metatarsal growth plate. In situ hybridization revealed a more intense and more discrete expression in the hypertrophic zone of the metatarsals treated with GH when compared with untreated controls or PDTC-treated metatarsal bones (supplemental Fig. 3, in situ hybridization, representative sections of untreated and treated metatarsals); the addition of PDTC in the culture medium neutralized the stimulatory effect induced by GH on collagen X mRNA expression (supplemental Fig. 3).
FIGURE 2.
Effects of GH on metatarsal linear growth and metatarsal growth plate morphology. Fetal mouse metatarsals were cultured for 3 days in serum-free minimum essential medium in the absence or presence of GH (10 ng/ml, the lowest GH concentration inducing NF-κB p65-DNA binding activity) with or without 1 μm PDTC. A, bone length was measured daily using an eyepiece micrometer in a dissecting microscope. B, at the end of the experimental period, metatarsal bones were fixed and paraffin-embedded. Three 5–7-μm-thick longitudinal sections were obtained and stained with hematoxylin. The metatarsal growth plate zone heights were measured by one observer blinded to the treatment groups. EZ, epiphyseal zone; PZ, proliferative zone; HZ, hypertrophic zone. C, after 3 days in culture, [3H]thymidine was added to the culture medium at a final concentration of 5 μCi/ml. Bone rudiments were incubated for an additional 5 h. The labeling index was calculated as the number of [3H]thymidine-labeled cells per grid divided by the total number of cells per grid. For each treatment group, we sampled five bones and analyzed both growth plates of each of three longitudinal sections per bone (15 bone sections per group). All determinations were made by the same observer blinded to the treatment category. D, after 3 days in culture, metatarsal bones were fixed in 4% phosphate-buffered paraformaldehyde and embedded in paraffin. 5–7-μm-thick longitudinal sections were obtained and treated with terminal deoxynucleotidyltransferase-mediated deoxy-UTP nick end labeling (TUNEL) assay. The apoptotic index was calculated as described under “Experimental Procedures.” Results are expressed as the percentage of untreated control (mean ± S.E.).
In light of the regulatory role of NF-κB on chondrocyte apoptosis, we evaluated the effects of GH and PDTC on growth plate in situ cell death. Metatarsals cultured with 1 mm SNP (a known inducer of apoptosis) exhibited increased cell death (Fig. 2D, apoptotic index, p < 0.001 versus control, and supplemental Fig. 4, representative photographs) when compared with untreated metatarsals. The addition of 10 ng/ml GH to the culture medium of the SNP-treated metatarsals partially prevented the SNP-mediated increase of cell death, and such effect was partially neutralized by the addition of PDTC (Fig. 2D and supplemental Fig. 4).
Effects of GH, PDTC, and NF-κB p65 siRNA on Chondrocyte Proliferation, Differentiation, and Apoptosis
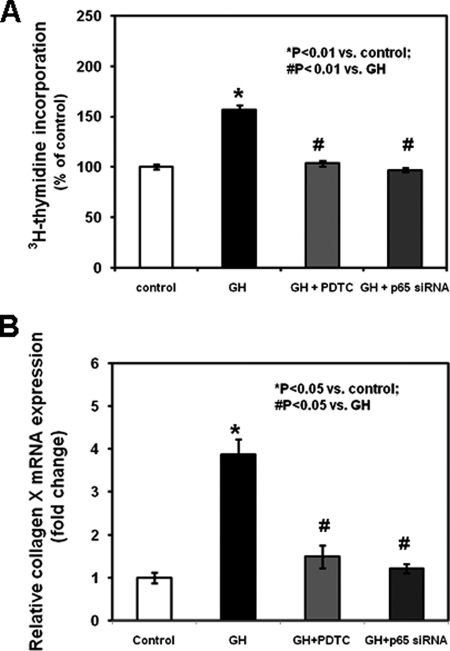
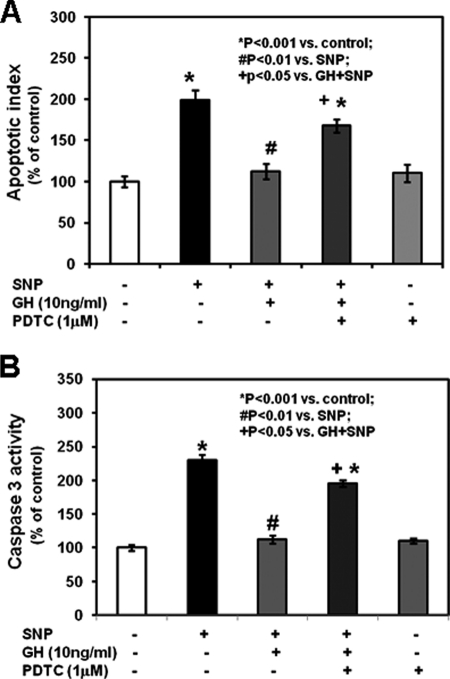
To evaluate the interaction between GH and NF-κB p65 in regulating growth plate chondrocyte function, we isolated chondrocytes from metatarsal growth plates and cultured them in serum-free medium in the absence or presence of the lowest GH concentration inducing NF-κB p65-DNA binding (10 ng/ml). GH increased chondrocyte proliferation (assessed by [3H]thymidine incorporation; Fig. 3A, p < 0.01 versus control, and supplemental Table 1) and differentiation (assessed by real time PCR analysis of collagen X mRNA expression; Fig. 3B, p < 0.05 versus control, and supplemental Table 1), whereas co-treatment with PDTC or chondrocyte transfection with p65 siRNA prevented such stimulatory effects (Fig. 3, A and B, and supplemental Table 1). To assess the interaction between GH and NF-κB p65 on chondrocyte apoptosis, we evaluated in situ cell death and caspase-3 activity. Chondrocytes cultured with 1 mm SNP exhibited increased cell death (Fig. 4A, apoptotic index, p < 0.001 versus control; supplemental Fig. 5, representative photographs; and supplemental Table 3) and caspase-3 activity (Fig. 4B, p < 0.001 versus control, and supplemental Table 3) when compared with untreated chondrocytes. The addition of 10 ng/ml GH to the culture medium of the SNP-treated chondrocytes partially prevented the SNP-mediated increase of cell death and caspase-3 activity, and both effects were partially neutralized by the addition of PDTC (Fig. 4, supplemental Fig. 5, and supplemental Table 3).
FIGURE 3.
Effects of GH, PDTC, and NF-κB p65 siRNA on chondrocyte proliferation and collagen X expression. Chondrocytes were washed with fresh serum-free DMEM, seeded in a 24-well plate, transfected with control siRNA or NF-κB p65 siRNA, and cultured in the absence or presence of 10 ng/ml GH with or without 1 μm PDTC. A, at the end of culture period, chondrocytes were added with 2.5 μCi/well [3H]thymidine (Amersham Biosciences) to the culture medium for an additional 3 h. Chondrocytes were released by trypsin and collected onto glass fiber filters. Incorporation of [3H]thymidine was measured by liquid scintillation counting. Results are expressed as the percentage of control and represent mean values obtained from three independent experiments. B, collagen X mRNA expression was determined by real time PCR. Total RNA was extracted from chondrocytes and then processed as described under “Experimental Procedures.” The relative expression levels of mRNA were normalized by β-actin in the same samples. Results are expressed as -fold change compared with control siRNA-transfected chondrocytes (mean ± S.E.).
FIGURE 4.
Effects of GH and PDTC on chondrocyte apoptotic index and caspase-3 activity. A, chondrocytes incubated with 1 mm SNP in the presence or absence of 10 ng/ml GH and/or 1 μm PDTC were washed with PBS three time, fixed in −10 °C methanol for 5 min, and then air-dried. Apoptotic cells were identified by TdT-FragEL assay according to the manufacturer's instructions. The apoptotic index was calculated as described under “Experimental Procedures.” Results are expressed as the percentage of untreated control (mean ± S.E.). B, cytosolic caspase-3 activity in chondrocytes was analyzed by a colorimetric assay. Results are expressed as the percentage of untreated control (mean ± S.E.).
Interaction between GH, Stat5b, and NF-κB p65
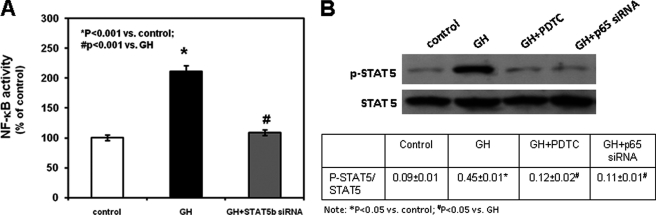
To determine whether GH activates NF-κB p65 via Stat5b, we cultured chondrocytes transfected with a control siRNA or Stat5b siRNA in the absence or presence of 10 ng/ml GH and then evaluated the binding of NF-κB p65 to DNA. The addition of GH in the medium of control siRNA-transfected chondrocytes induced NF-κB p65-DNA binding (Fig. 5A, p < 0.001 versus untreated control siRNA-transfected chondrocytes). However, no stimulatory effect was elicited by GH in Stat5b siRNA-transfected chondrocytes compared with untreated chondrocytes transfected with a control siRNA (Fig. 5A). We then studied Stat5b phosphorylation by Western blot in chondrocytes cultured in the absence or presence of 10 ng/ml GH. The addition of GH in the culture medium induced Stat5 phosphorylation as early as 5 min and up to 24 h of treatment (supplemental Fig. 6 and supplemental Table 4). Such GH stimulatory effect was prevented by the co-treatment with PDTC or by chondrocyte transfection with p65 siRNA (Fig. 5B). These findings suggest a reciprocal functional interaction between NF-κB p65 and Stat5b.
FIGURE 5.
Interaction between GH, NF-κB p65, and Stat5B. A, chondrocytes transfected with control siRNA or Stat5b siRNA were cultured in the absence or presence of 10 ng/ml GH. NF-κB p65-DNA binding was determined by an enzyme-linked immunosorbent assay according to the manufacturer's instructions. Results are expressed as the percentage of untreated control siRNA-transfected chondrocytes and represent mean values obtained from three independent experiments. B, chondrocytes transfected with control siRNA or NF-κB p65 siRNA were cultured in the absence or presence of 10 ng/ml GH with or without 1 μm PDTC. At the end of the culture period, chondrocytes were harvested, lysed, electrophoresed, and immunoblotted for phospho-STAT5 (p-STAT5) and the loading control β-actin. A representative blot from three independent experiments is presented. The intensity of the bands on Western blots was analyzed by NIH ImageJ. Results are expressed as the ratio of phospho-STAT5 to total STAT5 (mean ± S.E.).
Effects of GH, PDTC, and NF-κB p65 siRNA on IGF-1 and BMP-2 Expression
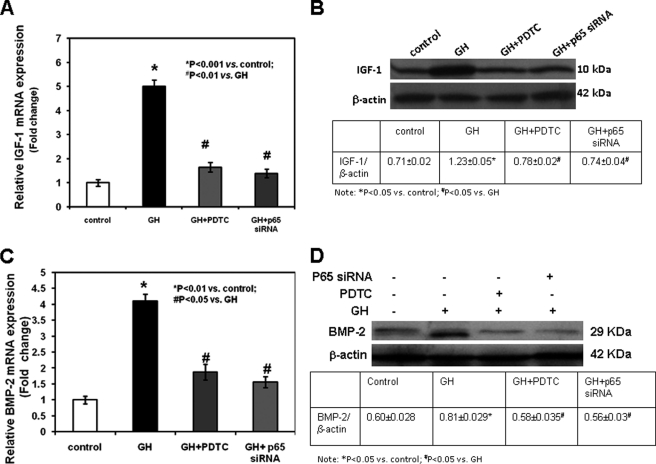
Because GH is known to modulate the expression of IGF-1, we evaluated whether this effect was mediated by NF-κB p65 activity. The addition of 10 ng/ml GH in the culture medium of control siRNA-transfected chondrocytes induced IGF-I mRNA expression (assessed by real time PCR; Fig. 6A, p < 0.001 versus control, and supplemental Table 1) and protein expression (assessed by Western blot; Fig. 6B) when compared with untreated control siRNA-transfected chondrocytes; in contrast, GH did not modify IGF-1 expression in chondrocytes transfected with p65 siRNA (Fig. 6, A and B, and supplemental Table 1). The stimulatory effects of GH on IGF-1 expression in control siRNA-transfected chondrocytes were neutralized by the addition of PDTC in the culture medium (Fig. 6, A and B, and supplemental Table 1).
FIGURE 6.
Effects of GH, PDTC, and NF-κB p65 siRNA on IGF-1 and BMP-2 expression. Chondrocytes transfected with control siRNA or NF-κB p65 siRNA were cultured in the absence or presence of 10 ng/ml GH with or without 1 μm PDTC. A and C, at the end of the culture period, IGF-1 (A) and BMP-2 (C) mRNA expression was determined by real time PCR. Total RNA was extracted from chondrocytes and then processed as described under “Experimental Procedures.” The relative expression levels of mRNA were normalized by β-actin in the same samples. Results are expressed as -fold change compared with untreated control siRNA-transfected chondrocytes (mean ± S.E.). B and D, cultured chondrocytes were harvested, lysed, electrophoresed, and immunoblotted for IGF-1 (B) or BMP-2 (D) and the loading control β-actin. A representative blot from three independent experiments is presented. The intensity of the bands on Western blots was analyzed by NIH ImageJ. Results are expressed as the ratio of IGF-1 or BMP-2 to β-actin (mean ± S.E.).
To determine whether GH regulates BMP-2 expression through NF-κB p65, we cultured growth plate chondrocytes in the presence or absence of GH, with or without PDTC, or after transfection of control siRNA or p65 siRNA. GH significantly stimulated BMP-2 mRNA (assessed by real time PCR; Fig. 6C and supplemental Table 1) and protein expression (assessed by Western blot; Fig. 6D) in control siRNA-transfected chondrocytes, whereas addition of PDTC neutralized such stimulatory effects (Fig. 6, C and D, and supplemental Table 1). GH did not modify BMP-2 expression in chondrocytes transfected with p65 siRNA (Fig. 6, C and D, and supplemental Table 1).
DISCUSSION
Our findings indicate that GH induces the activity of NF-κB p65 (an important member of the NF-κB family of transcription factors) in growth plate chondrocytes. In addition, we have demonstrated that NF-κB mediates the stimulatory effects of GH on metatarsal longitudinal growth and growth plate formation. Such effect on bone growth and growth plate chondrogenesis results from the permissive role of NF-κB p65 on the GH-mediated induction of chondrocyte proliferation and differentiation and prevention of chondrocyte apoptosis.
We have previously shown that NF-κB p65 is expressed in the murine growth plate, and it facilitates growth plate chondrogenesis (7). In addition, we have demonstrated that skin fibroblasts isolated from a child harboring a heterozygous mutation of IκBα (responsible for impaired NF-κB p65 activity) exhibited an absent proliferative response to GH in vitro (11). The addition of GH to the culture medium of mutated fibroblasts failed to induce IGF-1 mRNA and protein expression and Stat5b phosphorylation with all these findings reflecting GH insensitivity. Additional evidence supports a functional interaction between GH and NF-κB: it has been shown that GH exerts antiapoptotic and proliferative effects through NF-κB in murine pro-B lymphocytes (12, 13). In addition, GH has been found to increase NF-κB activity in neutrophils of rats treated with lipopolysaccharide (14).
GH is a potent stimulator of longitudinal bone growth: GH deficiency or insensitivity markedly impairs human postnatal growth (15–17). The role of the GH-IGF-1 axis in longitudinal bone growth has also been evaluated by genetic targeting of its components in mice. Mice lacking the GH gene exhibit a reduction in postnatal growth (18), whereas mice lacking the igf-1 gene show intrauterine as well as postnatal growth retardation. Although IGF-1 has long been known as the mediator of the systemic effects of GH on somatic growth, more recent evidence indicates that GH can also stimulate longitudinal bone growth by a local action on the growth plate. Injection of GH into the tibial growth plate accelerates longitudinal growth in the injected growth plate compared with the vehicle-injected contralateral growth plate (19). The addition of GH in the culture medium of whole rat metatarsal bones stimulates the metatarsal linear growth (20). In addition, in cultured growth plate chondrocytes, GH induces chondrocyte proliferation and IGF-I secretion (21). In support of a direct effect of GH on the growth plate, growth hormone receptor has been demonstrated in rabbit (22), rat (23), and human (24) growth plate chondrocytes.
GH action in mammalian cells is mediated by binding to the transmembrane growth hormone receptor, thereby triggering increased association with and activation of Janus kinases (JAKs) (25–27), which in turn leads to the activation of signal transducers and activators of transcription (STATs) (28–30), the phosphatidylinositol 3-kinase (PI3K)/Akt system (31, 32), and the extracellular signal-regulated kinases 1 and 2 (ERKs 1 and 2) (33–35). Recent studies have demonstrated that GH stimulates IGF-1 gene transcription through the activation of Stat5b (36), and findings in experimental animals and in humans have shown that the absence or mutations of the Stat5b gene are associated with diminished postnatal growth, GH resistance, and reduced IGF-1 synthesis (15, 37, 38).
In our study, the targeted silencing of Stat5b through Stat5b siRNA transfection prevented the GH-induced NF-κB p65-DNA binding, thus indicating that GH-mediated induction of NF-κB p65 activity in chondrocytes is Stat5b-dependent. In addition, the inhibition of NF-κB p65 via treatment with PDTC or p65 siRNA transfection prevented the GH-dependent Stat5b phosphorylation. Thus, these findings suggest a reciprocal functional interaction of these two GH-dependent signaling pathways. The timing of this functional interaction is not entirely clear because GH appears to induce both NF-κB nuclear translocation and Stat5b phosphorylation between 1 and 5 min of treatment. It is conceivable that NF-κB activation and Stat5b phosphorylation may be initiated independently and that they need to reciprocally interact to persist overtime. Recent evidence supports our findings relative to NF-κB p65 and Stat5b: depletion of Stat5 in lymphoid tumor cells leads to reduced NF-κB-DNA binding (39). On the other hand, increased activity of NF-κB resulting in up-regulated levels of STAT5 has been demonstrated in tumor cells (40) and transgenic mouse livers overexpressing IκB kinase (41). In contrast, thymocytes and T cells of transgenic mice with down-regulated NF-κB exhibit a dramatic reduction of STAT5 activation (42).
In light of the demonstrated interaction between NF-κB p65 and Stat5b in chondrocytes and of the known permissive role of Stat5b in the GH-dependent activation of IGF expression, we evaluated whether NF-κB p65 activity in chondrocytes mediates the GH effects on IGF-1. The targeted silencing of NF-κB p65 expression or activity prevented the GH stimulation of IGF-1 mRNA and protein expression, suggesting that both Stat5b and NF-κB p65 are necessary to mediate the GH stimulatory effects on IGF-1 in chondrocytes. Interestingly, we have previously shown that NF-κB p65 mediates the stimulatory effects of IGF-1 on chondrocyte proliferation and differentiation and demonstrated its preventive effects on chondrocyte apoptosis (8). Thus, our study supports a reciprocal functional interaction between NF-κB p65 and IGF-1 in which the transcription factor modulates both IGF-1 synthesis and action in growth plate chondrocytes.
BMPs are important modulators of skeletal growth and development. We have previously demonstrated that BMP-2 stimulates longitudinal bone growth and growth plate chondrogenesis (43), and BMP-2 expression and activity in growth plate chondrocyte function are regulated by NF-κB p65 (7). In this study, we show that the NF-κB p65 activation by GH also induces BMP-2 expression, which suggests that GH may promote growth plate chondrocyte function by activating both IGF-1 and BMP-2 activity.
In conclusion, our findings indicate that GH stimulates NF-κB p65 nuclear translocation and DNA binding in growth plate chondrocytes. Such stimulatory effect is necessary for the GH-dependent modulation of chondrocyte proliferation, differentiation, and apoptosis. The induction of IGF-1 and BMP-2 expression by GH in growth plate chondrocytes, via the activation of NF-κB p65, suggests that multiple signaling pathways mediate the promoting effects of GH on growth plate chondrogenesis and longitudinal bone growth.
Supplementary Material
This work was supported by a grant from Novo Nordisk.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6 and Tables 1–4.
- NF-κB
- nuclear factor-κB
- IGF
- insulin-like growth factor
- IκB
- inhibitor-κB
- PDTC
- pyrrolidine dithiocarbamate
- BMP
- bone morphogenetic protein
- GH
- growth hormone
- SNP
- sodium nitroprusside
- loc.
- location.
REFERENCES
- 1. Chen L. F., Greene W. C. (2004) Nat. Rev. Mol. Cell Biol. 5, 392–401 [DOI] [PubMed] [Google Scholar]
- 2. Baeuerle P. A., Baltimore D. (1996) Cell 87, 13–20 [DOI] [PubMed] [Google Scholar]
- 3. Karin M., Ben-Neriah Y. (2000) Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 4. Stancovski I., Baltimore D. (1997) Cell 91, 299–302 [DOI] [PubMed] [Google Scholar]
- 5. Kanegae Y., Tavares A. T., Izpisúa Belmonte J. C., Verma I. M. (1998) Nature 392, 611–614 [DOI] [PubMed] [Google Scholar]
- 6. Franzoso G., Carlson L., Xing L., Poljak L., Shores E. W., Brown K. D., Leonardi A., Tran T., Boyce B. F., Siebenlist U. (1997) Genes Dev. 11, 3482–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu S., Flint J. K., Rezvani G., De Luca F. (2007) J. Biol. Chem. 282, 33698–33706 [DOI] [PubMed] [Google Scholar]
- 8. Wu S., Fadoju D., Rezvani G., De Luca F. (2008) J. Biol. Chem. 283, 34037–34044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bagi C., Burger E. H. (1989) Calcif. Tissue Int. 45, 342–347 [DOI] [PubMed] [Google Scholar]
- 10. Apte S. S., Seldin M. F., Hayashi M., Olsen B. R. (1992) Eur. J. Biochem. 206, 217–224 [DOI] [PubMed] [Google Scholar]
- 11. Wu S., Walenkamp M. J., Lankester A., Bidlingmaier M., Wit J. M., De Luca F. (2010) J. Clin. Endocrinol. Metab. 95, 1220–1228 [DOI] [PubMed] [Google Scholar]
- 12. Jeay S., Sonenshein G. E., Postel-Vinay M. C., Baixeras E. (2000) Mol. Endocrinol. 14, 650–661 [DOI] [PubMed] [Google Scholar]
- 13. Jeay S., Sonenshein G. E., Kelly P. A., Postel-Vinay M. C., Baixeras E. (2001) Endocrinology 142, 147–156 [DOI] [PubMed] [Google Scholar]
- 14. Liu Z. H., Yu Y. Q., Li W. Q., Li J. S. (2002) Acta Pharmacol. Sin. 23, 887–892 [PubMed] [Google Scholar]
- 15. Kofoed E. M., Hwa V., Little B., Woods K. A., Buckway C. K., Tsubaki J., Pratt K. L., Bezrodnik L., Jasper H., Tepper A., Heinrich J. J., Rosenfeld R. G. (2003) N. Engl. J. Med. 349, 1139–1147 [DOI] [PubMed] [Google Scholar]
- 16. Rosenfeld R. G., Rosenbloom A. L., Guevara-Aguirre J. (1994) Endocr. Rev. 15, 369–390 [DOI] [PubMed] [Google Scholar]
- 17. Wit J. M., Kamp G. A., Rikken B. (1996) Pediatr. Res. 39, 295–302 [DOI] [PubMed] [Google Scholar]
- 18. Zhou Y., Xu B. C., Maheshwari H. G., He L., Reed M., Lozykowski M., Okada S., Cataldo L., Coschigamo K., Wagner T. E., Baumann G., Kopchick J. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Isaksson O. G., Jansson J. O., Gause I. A. (1982) Science 216, 1237–1239 [DOI] [PubMed] [Google Scholar]
- 20. Scheven B. A., Hamilton N. J. (1991) Acta Endocrinol. 124, 602–607 [DOI] [PubMed] [Google Scholar]
- 21. Isaksson O. G., Lindahl A., Nilsson A., Isgaard J. (1987) Endocr. Rev. 8, 426–438 [DOI] [PubMed] [Google Scholar]
- 22. Barnard R., Haynes K. M., Werther G. A., Waters M. J. (1988) Endocrinology 122, 2562–2569 [DOI] [PubMed] [Google Scholar]
- 23. Gevers E. F., van der Eerden B. C., Karperien M., Raap A. K., Robinson I. C., Wit J. M. (2002) J. Bone Miner. Res. 17, 1408–1419 [DOI] [PubMed] [Google Scholar]
- 24. Werther G. A., Haynes K., Edmonson S., Oakes S., Buchanan C. J., Herington A. C., Waters M. J. (1993) Acta Paediatr. Suppl. 82, Suppl. 391, 50–53 [DOI] [PubMed] [Google Scholar]
- 25. Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. (1993) Cell 74, 237–244 [DOI] [PubMed] [Google Scholar]
- 26. Foster C. M., Shafer J. A., Rozsa F. W., Wang X. Y., Lewis S. D., Renken D. A., Natale J. E., Schwartz J., Carter-Su C. (1988) Biochemistry 27, 326–334 [DOI] [PubMed] [Google Scholar]
- 27. Silva C. M., Day R. N., Weber M. J., Thorner M. O. (1993) Endocrinology 133, 2307–2312 [DOI] [PubMed] [Google Scholar]
- 28. Campbell G. S., Meyer D. J., Raz R., Levy D. E., Schwartz J., Carter-Su C. (1995) J. Biol. Chem. 270, 3974–3979 [DOI] [PubMed] [Google Scholar]
- 29. Choi H. K., Waxman D. J. (2000) Growth Horm. IGF Res. 10, Suppl. B, S1–S8 [DOI] [PubMed] [Google Scholar]
- 30. Lahuna O., Rastegar M., Maiter D., Thissen J. P., Lemaigre F. P., Rousseau G. G. (2000) Mol. Endocrinol. 14, 285–294 [DOI] [PubMed] [Google Scholar]
- 31. Costoya J. A., Finidori J., Moutoussamy S., Seãris R., Devesa J., Arce V. M. (1999) Endocrinology 140, 5937–5943 [DOI] [PubMed] [Google Scholar]
- 32. Liang L., Jiang J., Frank S. J. (2000) Endocrinology 141, 3328–3336 [DOI] [PubMed] [Google Scholar]
- 33. Campbell G. S., Pang L., Miyasaka T., Saltiel A. R., Carter-Su C. (1992) J. Biol. Chem. 267, 6074–6080 [PubMed] [Google Scholar]
- 34. Möller C., Hansson A., Enberg B., Lobie P. E., Norstedt G. (1992) J. Biol. Chem. 267, 23403–23408 [PubMed] [Google Scholar]
- 35. Winston L. A., Bertics P. J. (1992) J. Biol. Chem. 267, 4747–4751 [PubMed] [Google Scholar]
- 36. Woelfle J., Billiard J., Rotwein P. (2003) J. Biol. Chem. 278, 22696–22702 [DOI] [PubMed] [Google Scholar]
- 37. Udy G. B., Towers R. P., Snell R. G., Wilkins R. J., Park S. H., Ram P. A., Waxman D. J., Davey H. W. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teglund S., McKay C., Schuetz E., van Deursen J. M., Stravopodis D., Wang D., Brown M., Bodner S., Grosveld G., Ihle J. N. (1998) Cell 93, 841–850 [DOI] [PubMed] [Google Scholar]
- 39. Nagy Z. S., LeBaron M. J., Ross J. A., Mitra A., Rui H., Kirken R. A. (2009) Mol. Cancer 8, 67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hinz M., Lemke P., Anagnostopoulos I., Hacker C., Krappmann D., Mathas S., Dörken B., Zenke M., Stein H., Scheidereit C. (2002) J. Exp. Med. 196, 605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yau T. O., Chan C. F., Gee-San Lam S., Cheung O. F., Ching Y. P., Jin D. Y., Sham M. H., Ng I. O. (2009) J. Pathol. 217, 353–361 [DOI] [PubMed] [Google Scholar]
- 42. Mora A., Youn J., Keegan A., Boothby M. (2001) J. Immunol. 166, 2218–2227 [DOI] [PubMed] [Google Scholar]
- 43. De Luca F., Barnes K. M., Uyeda J. A., De-Levi S., Abad V., Palese T., Mericq V., Baron J. (2001) Endocrinology 142, 430–436 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.