Abstract
Mesenchymal stem cells (MSCs), which are modulated by cytokines present in the tumor microenvironment, play an important role in tumor progression. It is well documented that inflammation is an important part of the tumor microenvironment, so we investigated whether stimulation of MSCs by inflammatory cytokines would contribute to their ability to promote tumor growth. We first showed that MSCs could increase C26 colon cancer growth in mice. This growth-promoting effect was further accelerated when the MSCs were pre-stimulated by inflammatory factors IFN-γ and TNF-α. At the same time, we demonstrated that MSCs pre-stimulated by both inflammatory factors could promote tumor angiogenesis in vivo to a greater degree than untreated MSCs or MSCs pre-stimulated by either IFN-γ or TNF-α alone. A hen egg test-chorioallantoic membrane (HET-CAM) assay showed that treatment of MSC-conditioned medium can promote chorioallantoic membrane angiogenesis in vitro, especially treatment with conditioned medium of MSCs pretreated with IFN-γ and TNF-α together. This mechanism of promoting angiogenesis appears to take place via an increase in the expression of vascular endothelial growth factor (VEGF), which itself takes place through an increase in signaling in the hypoxia-inducible factor 1α (HIF-1α)-dependent pathway. Inhibition of HIF-1α in MSCs by siRNA was found to effectively reduce the ability of MSC to affect the growth of colon cancer in vivo in the inflammatory microenviroment. These results indicate that MSCs stimulated by inflammatory cytokines such as IFN-γ and TNF-α in the tumor microenvironment express higher levels of VEGF via the HIF-1α signaling pathway and that these MSCs then enhance tumor angiogenesis, finally leading to colon cancer growth in mice.
Keywords: Colon Cancer, Inflammation, MicroRNA, Stem Cell, Tumor
Introduction
Worldwide, more than 1 million new cases of colorectal cancer are diagnosed each year (1). Colorectal cancer is the third most common malignancy and the fourth most common cause of cancer mortality (1). Inflammatory bowel disease is an important risk factor for the development of colon cancer. Inflammation is also likely to be involved in other forms of sporadic and heritable colon cancer. The molecular mechanisms by which inflammation promotes cancer development, however, are still being uncovered and may differ between colitis-associated and other forms of colorectal cancer (2–4).
Mesenchymal stem cells (MSCs)3 are nonhematopoietic stem cells with the potential for both self-renewal and multiplex differentiation. They can differentiate into multiple lineages, such as osteoblasts, chondrocytes, and adipocytes (5, 6). Some studies have established that bone marrow-derived MSCs can engraft injured tissues, such as those of the lung, liver, heart, and brain, and recover their function. In the past 10 years, certain institutional studies have confirmed that the use of stem cell transplantation is an important tool in the treatment of several types of malignancies (7–9). For these reasons (10), such cells are currently being tested for the potential use in cell and gene therapy for tumors (11, 12). In contrast, newer studies have proposed that stem cells may be the direct cellular targets of the genetic alterations that lead to tumor formation and important contributors to the maintenance of human cancers (13, 14). Emerging evidence suggests that both bone marrow-derived MSCs and mature stromal cells can play an important role in the growth and development of human malignancies (15, 16).
The tumor microenvironment is very important with regard to the preservation and promotion of tumor growth and development. Indeed, changes occurring in the microenvironment of the progressing tumor resemble the process of chronic inflammation, which begins with ischemia followed by interstitial and cellular edema, the appearance of immune cells, and finally the growth of blood vessels (17). Chronic inflammation is clearly involved in shaping the tumor microenvironment and is an important constituent thereof (18). It has been well documented that tumors are generally infiltrated by inflammatory cells and inflammatory cytokines (19, 20). In response to these pro-inflammatory cytokine cascades, tumor and stromal cells produce a variety of soluble mediators with wide-ranging biologic effects. Thus, cell proliferation and differentiation, matrix remodeling, blood vessel growth, and cell migration and recruitment can all be re-programmed to benefit the tumor. IFN-γ and TNF-α are important inflammatory cytokines. The role of TNF-α in driving tumor progression has been emphasized by Balkwill and co-workers (21). Their work provides an example of how tumors usurp the normal process of inflammation to promote their own progression. It also suggests that blocking TNF-α, such as by anti-TNF-α antibodies, might be therapeutically useful (22). Some research institutions have found INF-γ to be an important inflammatory cytokine affecting tumor growth (9, 23, 24). Tumor angiogenesis is a major factor in the development of tumors. VEGF and HIF-1α are angiogenic cytokines that regulate angiogenesis in the tumor environment. The expression of these two pro-angiogenic cytokines can indicate the ability of the tumor to form new blood vessels in cancerous tissue.
In this study, we found that after being stimulated by inflammatory cytokines IFN-γ and TNF-α, MSCs showed a remarkable ability to accelerate tumor growth by up-regulating the expression of VEGF and significantly increasing the production of the VEGF protein, which promotes angiogenesis, providing nutrition for tumor cells. In addition, we observed that the mRNA and protein expression of HIF-1α became raised when MSCs were stimulated by the two inflammatory cytokines. The mechanism by which MSCs promote angiogenesis appears to be the increase in VEGF expression, which itself takes place through an increase in signaling in the HIF1α-dependent pathway within MSCs. Inhibition of HIF1α by siRNA in MSCs could effectively reduce the ability of MSC to affect the growth of colon cancer in vivo in the inflammatory microenviroment. Overall, this study supports the conclusion that MSCs promote tumor growth by contributing to angiogenesis in the colon cancer microenvironment. For this reason, medical practitioners should be cautious when using MSCs as part of cancer therapy.
EXPERIMENTAL PROCEDURES
Animals
Male BALB/c mice, 9–10 weeks old, were purchased from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences, Shanghai, China. All mice used in this study were housed in pathogen-free conditions, and all procedures were performed in accordance with guidelines established by the Chinese Academy of Sciences' Committee on Animals.
Cell Culture
Mouse colon carcinoma cell line C26 was obtained from the Chinese Academy of Sciences Cell Bank and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (all from Invitrogen). Cultures were maintained in a humidified atmosphere of 5% CO2 at 37 °C.
Preparation of Mouse Bone Marrow Mesenchymal Stem Cells
MSCs were generated from bone marrow flushed out of the tibias and femurs of 4–6-week-old BALB/c mice. Cells were cultured in α-minimal essential medium supplemented with 10% FBS, 2 mm glutamine, 100 units/ml of penicillin, and 100 mg/ml of streptomycin (all from Invitrogen). Non-adherent cells were removed after 72 h, and adherent cells were maintained in media replenished every 3 days. Cells were used at the 5th to 20th passage (47). The MSCs were divided into groups and exposed to the following: 1) control MSCs: pretreated with no inflammatory cytokines; 2) IFN-γ group: pretreated with IFN-γ (20 ng/ml, PeproTech) alone; 3) TNF-α group: pretreated with TNF-α (20 ng/ml; PeproTech) alone; 4) I+T group: pretreated with TNF-α and IFN-γ together (20 ng/ml each). After incubation for 12 h, we collected MSCs for in vitro and in vivo experiments.
C26 Murine Colon Cancer Model
C26 colon cancer cells and MSCs were prepared either as single-cell type suspensions (1 × 106 cells in 100 μl of PBS) or as mixtures of cells (1 × 106 C26 cells and 1 × 105 MSCs in 100 μl of PBS). Subcutaneous administration of C26 cells (alone or mixed with MSCs) was performed in the armpit areas of Balb/c mice. Mice were examined three times per week and tumor growth was evaluated by measuring the length and width of the tumor mass. The animals were euthanized and the tumors were removed at the end of the experiment. Tumor masses were weighed and analyzed by histology.
Conditioned Medium
MSCs were stimulated with IFN-γ (20 ng/ml) and TNF-α (20 ng/ml) for 12 h. Then the culture medium was replaced with fresh DMEM F-12. After being cultured for a further 24 h, the conditioned medium was obtained by collection and 0.22 μm filtration of the supernatant media from MSCs.
Immunohistochemistry
Tumor tissue sections (4 μm thick) were cut and mounted on 3-aminopropyltriethoxysilane-coated slides. Incubation with monoclonal anti-mouse CD34 antibody (Abbiotec, La Jolla, CA) was performed at room temperature for 1 h, after blocking endogenous peroxidase activity. Detection of the primary antibody was performed using rabbit anti-mouse antibody (DAKO A/S, Denmark) and streptavidin-biotin-horseradish peroxidase complex (SABC/HRP, DAKO A/S, Denmark). The peroxidase reaction was visualized using diaminobenzidine/H2O2 (0.05% (w/v)/0.03% (v/v)).
Chorioallantoic Membrane Angiogenic Assay
Chicken embryos hatched for 8 days were randomly divided into five groups (10 chick embryos per group). The air chambers were opened under sterile conditions, and the crust endomembrane was exposed. The normal medium (control group) and MSC-conditioned medium were daubed onto the chicken embryonic allantois. The holes were then sealed with adhesive tape sterilized by Co-60 irradiation. The air chambers were reopened when these chicken embryos had hatched until the 11th day. The allantoides were then fixed on cover slides (48). Blood vessels (BVs) were divided into two classes: bole vessels and branches on the bole vessels with vessel diameters not less than 1 mm were defined as class I BVs; branches on the bole vessels with vessel diameters less than 1 mm were defined as class II BVs (49, 50). The number of each order of BVs was double blindly determined by three investigators.
Real-time PCR
Cells were collected to extract the total cellular mRNA using TRIzol reagent (Invitrogen). cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Promega) and 2 μg of total RNA and oligo(dT)18 primers. Two-microliter aliquots of cDNA were used for PCR amplification. Real-time PCR was performed in triplicate using the SYBR PrimeScript RT-PCR Kit (Takara, Dalian, China) and the sequence of primers listed in Table 1. Total sample RNA was normalized to endogenous β-actin mRNA. Thermocycler conditions included an initial hold at 50 °C for 2 min and then 95 °C for 10 min; this was followed by a two-step PCR program of 95 °C for 15 s and 60 °C for 60 s repeated for 40 cycles on an Mx4000 system (Stratagene, La Jolla, CA), on which data were collected and quantitatively analyzed. The expression level of mRNA was presented as fold-change relative to an untreated control.
TABLE 1.
Oligonucleotide sequences used in real-time PCR and siRNA assays
| Assay | Gene | Sequence (5′ → 3′) |
|---|---|---|
| Real-time PCR | VEGF | |
| F | GGAGATCCTTCGAGGAGCACTT | |
| R | GGCGATTTAGCAGCAGATATAAGAA | |
| HIF-1α | ||
| F | CGGCGAAGCAAAGAGTCTGAAGT | |
| R | TCGCCGTCATATGTTAGCACCAT | |
| β-Actin | ||
| F | CTCCATCCTGGCCTCGCTGT | |
| R | GCTGTCACCTTCACCGTTCC | |
| VEGF siRNA | Sequence 1 | |
| Sense | CGAGATAGAGTACATCTTCAA | |
| Antisense | TTGAAGATGTACTCTATCTCG | |
| Sequence 2 | ||
| Sense | TGCGGATCAAACCTCACCAAA | |
| Antisense | TTTGGTGAGGTTTGATCCGCA | |
| Sequence 3 | ||
| Sense | GATCAAGTTCATGGATGTCTA | |
| Antisense | TAGACATCCATGAACTTGATC | |
| Control | ||
| Sense | UUCUCCGAACGUGUCACGUTT | |
| Antisense | ACGUGACACGUUCGGAGAATT | |
| HIF-1α siRNA | Sequence 1 | |
| Sense | CCCATTCCTCATCCGTCAAAT | |
| Antisense | ATTTGACGGATGAGGAATGGG | |
| Sequence 2 | ||
| Sense | AGTCGACACAGCCTCGATATG | |
| Antisense | CATATCGAGGCTGTGTCGACT | |
| Sequence 3 | ||
| Sense | TGGATAGCGATATGGTCAATG | |
| Antisense | CATTGACCATATCGCTATCCA | |
| Control | ||
| Sense | UUCUCCGAACGUGUCACGUTT | |
| Antisense | ACGUGACACGUUCGGAGAATT | |
Enzyme-linked Immunosorbent Assay
ELISA assays were performed using a commercial VEGF ELISA kit (R&D Systems). MSC- conditioned medium was collected and stored at −80 °C for further experiments. Assays were performed in duplicate, and readings were compared with standard curves obtained with standard proteins provided with the kits. Mean ± S.D. of concentrations in triplicate samples were compared by t test.
Western Blotting
Cells were washed in PBS solution, and protein was extracted according to an established protocol. Nuclear extract proteins were quantified using the Bio-Rad protein assay. Proteins were then mixed with Laemmli sample buffer, heated at 65 °C for 10 min, loaded (20 μg/sample), separated by SDS-polyacrylamide gel (7.5%) electrophoresis under denaturing conditions, and electroblotted on nitrocellulose membranes. The nitrocellulose membranes were blocked by incubation in blocking buffer (1% BSA in Tris-buffered saline, 0.1% Tween 20), incubated with anti-HIF-1α antibody (1:500 polyclonal; Bethyl), washed, and incubated with anti-rabbit peroxidase-conjugated secondary antibody (1:10,000; Sigma). Signals were visualized by chemiluminescent detection. Blots were quantified using Quantity One software from Bio-Rad, and HIF-1α expression (peak intensity) was normalized to values in the control group. Equal loading of samples was verified by Coomassie Blue staining of simultaneously run gels. Gels were run four times, and the images shown are representative.
Statistical Analysis
Statistical analysis of the data were performed using GraphPad Prism 4 software. Student's t test was used to compare the mean values of the two groups. Data from three or more groups were compared using the one-way analysis of variance, followed by the Dunnett's post hoc test. Final values are expressed as mean ± S.E. A difference of at least p < 0.05 was considered statistically significant.
RESULTS
Acceleration of the Growth of Colon Cancer in Mice by MSCs Pre-stimulated with Inflammatory Cytokines
MSCs were isolated from mouse bone marrow and expanded. The resulting cells exhibited spindle-shaped morphology and continuous proliferation (supplemental Fig. S1A). The MSCs generated were capable of differentiating into adipocytes and osteoblast-like cells (supplemental Fig. S1, B and C). We used MSCs from the 8th passage (propagated for 6 weeks) to analyze the expression of cell surface molecules by flow cytometry and found these cells to be positive for CD90, CD105, CD166, CD44, and CD29 and negative for CD34, CD14, and CD45 (supplemental Fig. S1D).
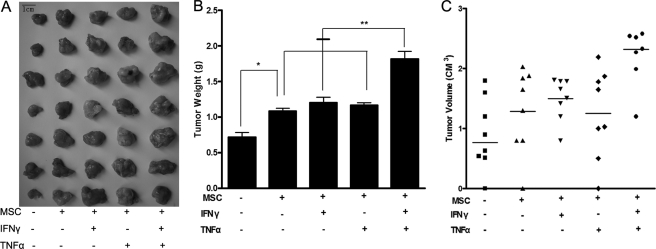
The pro-tumorigenic effects of MSCs have been demonstrated in multiple systems. Recent studies have shown that MSCs contribute to the growth of breast and ovarian cancer (25, 26). The specific mechanisms by which this occurs have not yet been elucidated. To determine the effects of MSCs stimulated by inflammatory cytokines on tumor growth in mice, C26 colon cancer cells mixed with MSCs were injected subcutaneously into BALB/c mice, which served as the allograft tumor model. Subcutaneous C26 tumor-bearing mice that had not been exposed to MSCs were used as controls. The other groups were treatment groups. Tumor growth was measured after the mice were euthanized on day 18. Relative to controls, C26 cells administrated with MSCs were found to increase colon cancer growth in vivo, with MSCs pretreated by both IFN-γ and TNF-α showing the greatest tumor-promoting effects (Fig. 1A). Tumor weight was higher in all four treatment groups than in the control group (p < 0.05). No statistical difference in tumor weight was observed between the groups exposed to MSCs pretreated with IFN-γ or TNF-α and the MSC control group (p > 0.05). However, the tumors of the IFN-γ + TNF-α group were significantly more massive than those of other groups (p < 0.01) (Fig. 1B). The same results were seen for tumor volumes (Fig. 1C).
FIGURE 1.
Acceleration of the growth of colon cancer in mice by MSCs pre-stimulated by inflammatory cytokines. Mice were randomized into five groups, with one control group (n = 7) only receiving 1 × 106 C26 cells and the other groups receiving 1 × 106 C26 cells and 1 × 105 MSCs together. MSCs were pre-stimulated in advance by inflammatory cytokines for 12 h. A, photographic illustration of tumors excised from all experimental mice on the day of euthanasia (day 18). B, tumor weights of mice treated with MSCs, particularly the IFN-γ + TNF-α group, were significantly increased (*, p < 0.05) relative to those of the control group (**, p < 0.01). C, similar results occurred for tumor volumes.
Promotion of in Vivo Tumor Angiogenesis by MSCs Pretreated with Inflammatory Cytokines
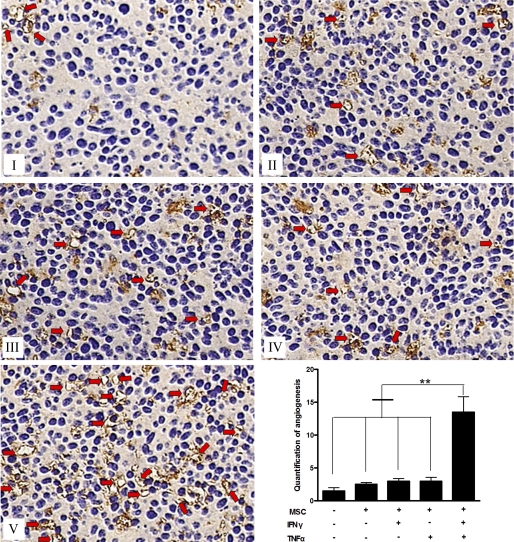
Tumor progression is closely linked with microvessel formation, which is usually associated with the inflammatory microenvironment. Inflammation is an essential element in tumor microenvironment, but the role of inflammation in angiogenesis and the underlying mechanism by which it influences the process have yet to be established. Thus, we assessed the density of newly formed microvessels in tumor tissue sections via immunohistochemistry for CD34, a marker for vascular endothelial cells. Our study demonstrated that MSCs could enhance microvessel formation in tumor tissues, with MSCs pretreated with two inflammatory cytokines (TNF-α and IFN-γ) showing the most marked effects. As shown in Fig. 2, whereas there is no significant difference in microvessel-stimulating effect among non-treated MSCs and MSCs pretreated with single inflammatory cytokine, TNF-α, or IFN-γ (p > 0.05), the three groups showed stronger CD34 staining than the control group (p < 0.05). Furthermore, MSCs pre-stimulated by both inflammatory cytokines, TNF-α and IFN-γ, showed a striking ability to promote angiogenesis, showing three times as many new vessels than any other treatment group (p < 0.01). All the above results demonstrate that MSCs stimulated by exogenous inflammatory cytokines and MSCs in the inflammatory microenvironment have remarkable stimulating effects on tumor angiogenesis.
FIGURE 2.
In vivo promotion of cancer angiogenesis by MSCs pretreated with inflammatory cytokines. I–V, CD34 immunostaining showing the numbers of newly formed vessels in colon cancer tissues: (I) control, (II) MSCs, (III) MSCs (IFN-γ), (IV) MSCs (TNF-α), and (V) MSCs (IFN-γ + TNF-α). Bottom right, quantification of the vessels was estimated by counting five randomly chosen high-power fields. MSCs were stimulated by two cytokines showing the most marked effect in enhancing microvessel formation in tumor tissues compared with the others groups (**, p < 0.01).
Promotion of Angiogenesis in Chicken Embryonic Allantois in Vitro by MSCs Pretreated with Inflammatory Cytokines
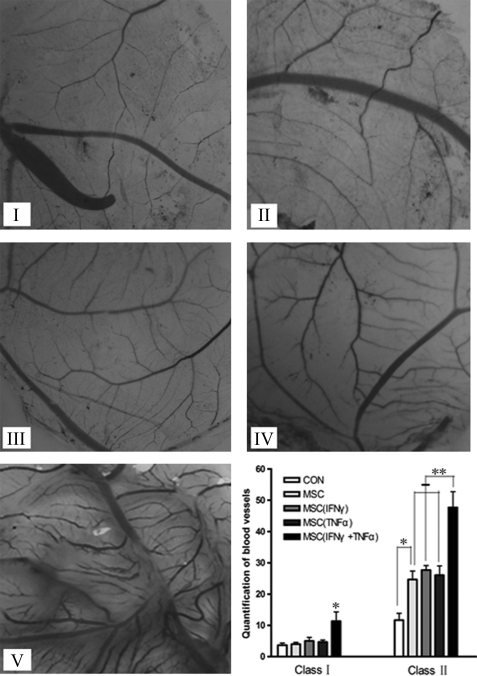
Three days after hatching, the allantoides were fixed on cover slides, and the BVs on the slides were counted. As shown in Fig. 3, relative to the control group, the conditioned medium of MSCs pretreated with no or single inflammatory cytokines had no stimulating effects in the formation of class I BVs, but conditioned medium of MSCs pretreated with both inflammatory cytokines could significantly enhance class I BV formation (p < 0.05). All treatment groups had higher density of class II BVs than the control group (p < 0.05), with the group with both inflammatory cytokines showing the most striking effects (p < 0.01). These results indicate that, after stimulation with both TNF-α and IFN-γ, MSCs promoted angiogenesis in chicken embryonic allantois in vitro by secreting certain angiogenesis-related cytokines.
FIGURE 3.
In vitro promotion of angiogenesis in chicken embryonic allantoides by MSCs pretreated with inflammatory cytokines. MSCs were stimulated in advance by inflammatory cytokines for 12 h. BVs were broken down into two classes: bole vessels and branches on the bole vessels with vessel diameters not less than 1 mm defined as class I BVs; branches on the bole vessels with vessel diameters less than 1 mm were defined as class II BVs. I–V, chicken embryonic allantois microvessels: (I) control, (II) MSCs, (III) MSCs (IFN-γ), (IV) MSCs (TNF-α), and (V) MSCs (IFN-γ + TNF-α). Bottom right, chicken embryonic allantois microvessel formation in all groups (*, p < 0.05; **, p < 0.01).
Promotion of Angiogenesis via the Production and Secretion of VEGF by MSCs Pretreated with Inflammatory Cytokines
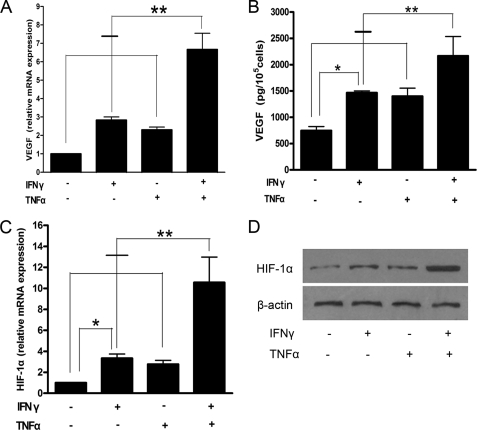
As shown above, angiogenesis can be enhanced by MSCs that have been pretreated with inflammatory cytokines IFN-γ and TNF-α. VEGF and HIF-1α were chosen and investigated because of their known pro-angiogenic functions. The levels of mRNA expression of VEGF and HIF-1α were higher in MSCs pretreated with IFN-γ and TNF-α, either alone (p < 0.05) or in combination (p < 0.01) than in the MSC control group. mRNA levels of the double pretreatment group were almost three times higher than those of either single pretreatment group (Fig. 4, A and C). VEGF protein expression in conditioned medium of MSCs pretreated with both inflammatory cytokines was significantly elevated over that of other groups (p < 0.01) (Fig. 4B). Western blot results showed that pre-stimulation with inflammatory cytokines promoted the expression of HIF-1α in MSCs, especially in MSCs pre-stimulated with both IFN-γ and TNF-α (Fig. 4D). These findings demonstrate that, in the tumor inflammation microenvironment, MSCs might secrete VEGF and so enhance angiogenesis and accelerated tumor growth.
FIGURE 4.
Production/secretion of HIF-1α and VEGF by MSCs pretreated with inflammatory factors. A, real-time PCR analysis of the expression of VEGF mRNA in MSCs. B, enzyme-linked immunosorbent assay analysis of the protein expression of VEGF in the supernatant of MSCs. C, real-time PCR analysis of the expression of HIF-1α mRNA in MSCs. D, Western blot detection of the expression of HIF-1α protein in tumor tissue. Data are representative of three independent experiments and shown as mean ± S.E. *, p < 0.05; **, p < 0.01 versus control.
Regulation of VEGF Expression by the HIF-1α-dependent Pathway Signaling
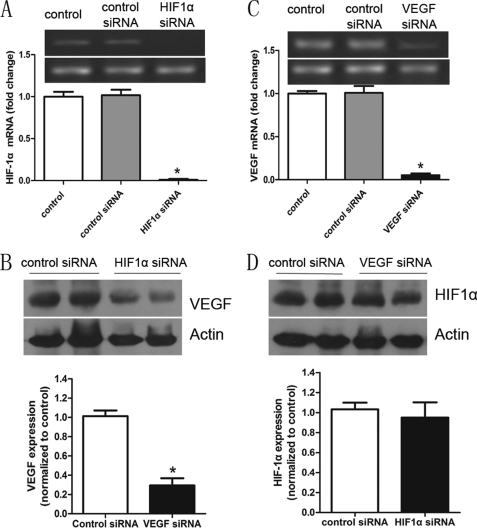
The mechanism by which VEGF levels increase in response to inflammatory cytokines is not clear. Because HIF-1α activity and levels are influenced by cellular glucose availability and metabolism, we hypothesized that the increase in VEGF in response to IFN-γ and TNF-α might take place through a HIF-1α-dependent pathway. The above results show that IFN-γ and TNF-α can increase levels of HIF-1α. To further test this hypothesis, we measured VEGF protein levels in control or HIF-1α siRNA-transfected MSCs, which were in advance pretreated with both inflammatory cytokines. The levels of VEGF were significantly lower (Fig. 5B) when the expression of HIF-1α was inhibited by siRNA (Fig. 5A). However, HIF-1α expression did not change significantly when VEGF expression was inhibited by siRNA (Fig. 5, C and D). Overall, these results suggest that IFN-γ and TNF-α increase VEGF levels through a HIF-1α-dependent pathway.
FIGURE 5.
Regulation of the expression of VEGF in MSCs by the HIF-1α signaling pathway. A, real-time PCR analysis of the expression of HIF-1α in MSCs that received no pretreatment and in MSCs treated with control solution or HIF-1α siRNA under inflammatory conditions (all groups were pretreated with two inflammatory cytokines). B, Western blot of VEGF in MSCs treated with control or HIF-1α siRNA under inflammatory conditions (pretreated with two inflammatory cytokines). C, real-time PCR analysis of the expression of VEGF (see A). D, Western blot of HIF-1α in MSCs treated with control solution or VEGF siRNA under inflammatory conditions. The bar graph below the blot represents a summary of the results. Data are representative of three independent experiments and shown as mean ± S.E. *, p < 0.05 versus control.
Inhibition of the Growth of C26 Colon Cancer Cells in Vivo by VEGF siRNA and HIF1α siRNA
As has been shown above, the growth of C26 cells can be enhanced by MSCs pretreated with inflammatory cytokines IFN-γ and TNF-α. This enhancement might be correlated with angiogenesis associated with VEGF and HIF1α expressed by MSCs. The pro-angiogenic capabilities of MSC are dependent upon the expression of VEGF, which can be induced by inflammatory cytokines via a HIF-1α-dependent pathway. Therefore, we demonstrated that the stimulating effect of MSCs induced by inflammatory cytokines on C26 cancer cell growth could be inhibited by VEGF siRNA and HIF-1α siRNA (Fig. 6A). Similar results were found for tumor volume (Fig. 6, B and C). We also assessed the density of newly formed microvessels in tumor tissue sections via immunohistochemistry for CD34. Our data demonstrate that suppression of VEGF and HIF-1α expression in MSCs can reduce microvessel formation in tumor tissues (Fig. 6, D and E). These data support the idea that angiogenesis mediated by MSCs via the HIF1α-VEGF pathway play an important role in the growth of C26 colon cancer cells in vivo.
FIGURE 6.
Inhibition of colon cancer growth favored by MSCs in vivo by HIF1α siRNA and VEGF siRNA. Transfections of VEGF siRNA and HIF1α siRNA into MSCs were performed using Lipofectamine. The MSCs siRNA-VEGF (1 × 105) and MSCs siRNA-HIF1α (1 × 105) were stimulated by both IFN-γ and TNF-α for 12 h and then mixed with C26 colon cancer cells (1 × 106) for subcutaneous administration to BALB/c mice via the armpit area. Eighteen days after implantation, the animals were euthanized and their tumors were dissected. A, photographic illustration of tumors. B and C, the weight and volume of C26 tumors. D, CD34 immunostaining showing the numbers of newly formed vessels in colon cancer tissues: (I) control siRNA, (II) VEGF siRNA, (III) HIF1α siRNA. Bottom right, quantification of the vessels was estimated by counting five randomly chosen high power fields. *, p < 0.05.
DISCUSSION
It has recently been recognized that MSCs contribute to the regeneration of a wide variety of organs and to the healing of some diseases (27–29). To clarify the utility of MSCs and avoid inappropriate use of MSCs in clinical therapy, we must fully understand the role of MSCs in the tumor microenviroment. In this study, we observed that MSCs produced and secreted pro-angiogenic cytokines such as VEGF, promoting angiogenesis and thereby contributing to colon cancer growth. In the meantime, our studies showed that the pro-angiogenic effects of MSCs were not innate but rather induced by inflammatory cytokines INF-γ and TNF-α. MSCs may be the mediators that form a link between inflammatory cytokines and cancer.
INF-γ has been reported to be an important agonist to MSCs (30). However, we found that INF-γ alone was insufficient to increase the cancer-promoting effects of MSC. TNF-α or perhaps other cytokines are required to cause MSCs to produce and secrete cytokines. It has been reported previously that IFN-γ and TNF-α synergize in the induction of gene expression in various cells (31–33), including MSCs (34), but little is known about the expression of VEGF and other pro-angiogenic factors. The mechanism of this synergy between these two cytokines is not fully understood. Only Xu (35) reported that C/EBPβ is likely to be a major transcription factor for regulating this synergy. As shown under supplemental Fig. S2, the synergy of IFN-γ and TNF-α can lead to up-regulation of C/EBPβ in MSCs. But inhibition of C/EBPβ in MSCs by siRNA does not effectively down-regulate the expression of HIF-1α relative to control groups. Certainly MSCs are also activated by other pro-inflammatory cytokines. Wang et al. (36) showed TGF-α to cause human MSCs to secrete VEGF by MEK- and PI3K- but not JNK- or ERK-dependent mechanisms. Tang et al. (37) discovered that MSCs overexpressing SDF-1α could promote angiogenesis.
Tumor angiogenesis is a key step in tumor growth and cancer development. VEGF is one of the important angiogenic cytokines, which have not only angiogenic properties but also an autocrine ability to regulate the synthesis of multiple angiogenic proteins, including angiogenin, IL-6, IL-8, TGF-β1, and MCP-1 in multiple cells (38–41). Tumors always exist in ischemic and hypoxic conditions. In the tumor microenvironment, hypoxia can cause some effector cells to produce HIF-1α, which is the upstream regulatory gene of VEGF (42). VEGF also has been demonstrated to interact with the HIF-1α via an autocrine loop (43, 44). The level of VEGF and HIF-1α could directly and indirectly indicate the ability of a tumor to cause angiogenesis.
In conclusion, in contrast with recent studies demonstrating that certain stem cell populations can promote or give rise to neoplastic growth (45, 46), our findings highlight the pro-angiogenic effects of MSCs in tumor tissues. Furthermore, our studies suggest that MSCs represent a unique stromal cell population that can directly contribute to tumorigenesis, in contrast to the reported indirect effects of normal or carcinoma-associated fibroblasts. Some reports have shown that MSCs can also suppress the immune reaction and tumor cells the chance to evade immune monitoring, resulting in proliferation of tumor cells and leading to tumor metastasis and recurrence. Therefore, the pro-angiogenic effects of MSCs may not be the only but are undoubtedly one of the important ways in which cancer growth is regulated in the inflammatory environment. Overall, MSCs may exert adverse effects in certain microenvironments that involve inflammation, such as the tumor microenvironment. Thus, MSCs should be incorporated into clinical applications only with great caution.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grants 30870974, 30801347, 30901722, 81000970, and 81030041, Shanghai Science and Technology Committee Grants 11ZR1449500, 10411963100, 10ZR1439900, and 10ZR1439600, Key Basic Research Project of China Grants 2010CB945600 and 2011CB966200, Science Fund for Creative Research Groups, NSFC, China Grant 30921006, the Stem Cell and Medicine Research Center's Innovation Research Program, and Second Military Medical University Grants SCOP101913, SCOP102004, and SCOP101812.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- MSC
- mesenchymal stem cells
- HIF-1α
- hypoxia-inducible factor 1α
- BV
- blood vessel.
REFERENCES
- 1. Tenesa A., Dunlop M. G. (2009) Nat. Rev. Genet. 10, 353–358 [DOI] [PubMed] [Google Scholar]
- 2. Atreya I., Neurath M. F. (2008) Exp. Rev. Anticancer Ther. 8, 561–572 [DOI] [PubMed] [Google Scholar]
- 3. Atreya I., Atreya R., Neurath M. F. (2008) J. Intern Med. 263, 591–596 [DOI] [PubMed] [Google Scholar]
- 4. Clevers H. (2004) Cell 118, 671–674 [DOI] [PubMed] [Google Scholar]
- 5. Prockop D. J. (1997) Science 276, 71–74 [DOI] [PubMed] [Google Scholar]
- 6. Bruder S. P., Jaiswal N., Haynesworth S. E. (1997) J. Cell. Biochem. 64, 278–294 [DOI] [PubMed] [Google Scholar]
- 7. Tang S. J., Ho M. Y., Cho H. C., Lin Y. C., Sun G. H., Chi K. H., Wang Y. S., Jhou R. S., Yang W., Sun K. H. (2008) Int. J. Cancer 123, 2840–2848 [DOI] [PubMed] [Google Scholar]
- 8. Moreno M., Molling J. W., von Mensdorff-Pouilly S., Verheijen R. H., Hooijberg E., Kramer D., Reurs A. W., van den Eertwegh A. J., von Blomberg B. M., Scheper R. J., Bontkes H. J. (2008) J. Immunol. 181, 2446–2454 [DOI] [PubMed] [Google Scholar]
- 9. Müller-Hermelink N., Braumüller H., Pichler B., Wieder T., Mailhammer R., Schaak K., Ghoreschi K., Yazdi A., Haubner R., Sander C. A., Mocikat R., Schwaiger M., Förster I., Huss R., Weber W. A., Kneilling M., Röcken M. (2008) Cancer Cell 13, 507–518 [DOI] [PubMed] [Google Scholar]
- 10. Eriksson F., Culp W. D., Massey R., Egevad L., Garland D., Persson M. A., Pisa P. (2007) Cancer Immunol. Immunother. 56, 677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horwitz E. M., Prockop D. J., Fitzpatrick L. A., Koo W. W., Gordon P. L., Neel M., Sussman M., Orchard P., Marx J. C., Pyeritz R. E., Brenner M. K. (1999) Nat. Med. 5, 309–313 [DOI] [PubMed] [Google Scholar]
- 12. Caplan A. I. (2000) Clin. Orthop. Relat. Res. 397, (suppl.) S67–S70 [DOI] [PubMed] [Google Scholar]
- 13. Reya T., Morrison S. J., Clarke M. F., Weissman I. L. (2001) Nature 414, 105–111 [DOI] [PubMed] [Google Scholar]
- 14. Sell S. (2004) Crit. Rev. Oncol. Hematol. 51, 1–28 [DOI] [PubMed] [Google Scholar]
- 15. Hamada H., Kobune M., Nakamura K., Kawano Y., Kato K., Honmou O., Houkin K., Matsunaga T., Niitsu Y. (2005) Cancer Sci. 96, 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khakoo A. Y., Pati S., Anderson S. A., Reid W., Elshal M. F., Rovira I. I., Nguyen A. T., Malide D., Combs C. A., Hall G., Zhang J., Raffeld M., Rogers T. B., Stetler-Stevenson W., Frank J. A., Reitz M., Finkel T. (2006) J. Exp. Med. 203, 1235–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balkwill F., Mantovani A. (2001) Lancet 357, 539–545 [DOI] [PubMed] [Google Scholar]
- 18. Aller M. A., Arias J. L., Nava M. P., Arias J. (2004) Exp. Biol. Med. 229, 170–181 [DOI] [PubMed] [Google Scholar]
- 19. Chiou S. H., Sheu B. C., Chang W. C., Huang S. C., Hong-Nerng H. (2005) J. Reprod. Immunol. 67, 35–50 [DOI] [PubMed] [Google Scholar]
- 20. Taylor R. C., Patel A., Panageas K. S., Busam K. J., Brady M. S. (2007) J. Clin. Oncol. 25, 869–875 [DOI] [PubMed] [Google Scholar]
- 21. Balkwill F. R., Ward B. G., Moodie E., Fiers W. (1987) Cancer Res. 47, 4755–4758 [PubMed] [Google Scholar]
- 22. Harrison M. L., Obermueller E., Maisey N. R., Hoare S., Edmonds K., Li N. F., Chao D., Hall K., Lee C., Timotheadou E., Charles K., Ahern R., King D. M., Eisen T., Corringham R., DeWitte M., Balkwill F., Gore M. (2007) J. Clin. Oncol. 25, 4542–4549 [DOI] [PubMed] [Google Scholar]
- 23. Yang C. H., Murti A., Pfeffer L. M. (2005) J. Biol. Chem. 280, 31530–31536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hiroishi K., Tüting T., Lotze M. T. (2000) J. Immunol. 164, 567–572 [DOI] [PubMed] [Google Scholar]
- 25. Sun B., Roh K. H., Park J. R., Lee S. R., Park S. B., Jung J. W., Kang S. K., Lee Y. S., Kang K. S. (2009) Cytotherapy 11, 289–298 [DOI] [PubMed] [Google Scholar]
- 26. Coffelt S. B., Marini F. C., Watson K., Zwezdaryk K. J., Dembinski J. L., LaMarca H. L., Tomchuck S. L., Honer zu Bentrup K., Danka E. S., Henkle S. L., Scandurro A. B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3806–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D'Agostino B., Sullo N., Siniscalco D., De Angelis A., Rossi F. (2010) Expert Opin. Biol. Ther. 10, 681–687 [DOI] [PubMed] [Google Scholar]
- 28. Mazzini L., Ferrero I., Luparello V., Rustichelli D., Gunetti M., Mareschi K., Testa L., Stecco A., Tarletti R., Miglioretti M., Fava E., Nasuelli N., Cisari C., Massara M., Vercelli R., Oggioni G. D., Carriero A., Cantello R., Monaco F., Fagioli F. (2010) Exp. Neurol. 223, 229–237 [DOI] [PubMed] [Google Scholar]
- 29. Loebinger M. R., Eddaoudi A., Davies D., Janes S. M. (2009) Cancer Res. 69, 4134–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., Santarlasci V., Mazzinghi B., Pizzolo G., Vinante F., Romagnani P., Maggi E., Romagnani S., Annunziato F. (2006) Stem Cells 24, 386–398 [DOI] [PubMed] [Google Scholar]
- 31. Hattori A., Iwasaki S., Murase K., Tsujimoto M., Sato M., Hayashi K., Kohno M. (1994) FEBS Lett. 340, 177–180 [DOI] [PubMed] [Google Scholar]
- 32. Sekine N., Ishikawa T., Okazaki T., Hayashi M., Wollheim C. B., Fujita T. (2000) J. Cell. Physiol. 184, 46–57 [DOI] [PubMed] [Google Scholar]
- 33. Lee A. H., Hong J. H., Seo Y. S. (2000) Biochem. J. 350, 131–138 [PMC free article] [PubMed] [Google Scholar]
- 34. English K., Barry F. P., Field-Corbett C. P., Mahon B. P. (2007) Immunol. Lett. 110, 91–100 [DOI] [PubMed] [Google Scholar]
- 35. Xu G., Zhang Y., Zhang L., Roberts A. I., Shi Y. (2009) Stem Cells 27, 942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y., Crisostomo P. R., Wang M., Markel T. A., Novotny N. M., Meldrum D. R. (2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1115–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang J., Wang J., Yang J., Kong X., Zheng F., Guo L., Zhang L., Huang Y. (2009) Eur. J. Cardiothorac. Surg. 36, 644–650 [DOI] [PubMed] [Google Scholar]
- 38. McClintock J. Y., Wagner E. M. (2005) J. Appl. Physiol. 99, 861–866 [DOI] [PubMed] [Google Scholar]
- 39. Gessi S., Fogli E., Sacchetto V., Merighi S., Varani K., Preti D., Leung E., Maclennan S., Borea P. A. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 90–97 [DOI] [PubMed] [Google Scholar]
- 40. Zheng W., Seftor E. A., Meininger C. J., Hendrix M. J., Tomanek R. J. (2001) Am. J. Physiol. Heart Circ. Physiol. 280, H909–917 [DOI] [PubMed] [Google Scholar]
- 41. Niu J., Azfer A., Zhelyabovska O., Fatma S., Kolattukudy P. E. (2008) J. Biol. Chem. 283, 14542–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forooghian F., Das B. (2007) Am. J. Ophthalmol. 144, 761–768 [DOI] [PubMed] [Google Scholar]
- 43. Takata K., Morishige K., Takahashi T., Hashimoto K., Tsutsumi S., Yin L., Ohta T., Kawagoe J., Takahashi K., Kurachi H. (2008) Mol. Cancer Ther. 7, 1551–1561 [DOI] [PubMed] [Google Scholar]
- 44. Das B., Yeger H., Tsuchida R., Torkin R., Gee M. F., Thorner P. S., Shibuya M., Malkin D., Baruchel S. (2005) Cancer Res. 65, 7267–7275 [DOI] [PubMed] [Google Scholar]
- 45. Bhowmick N. A., Chytil A., Plieth D., Gorska A. E., Dumont N., Shappell S., Washington M. K., Neilson E. G., Moses H. L. (2004) Science 303, 848–851 [DOI] [PubMed] [Google Scholar]
- 46. Singh S. K., Clarke I. D., Terasaki M., Bonn V. E., Hawkins C., Squire J., Dirks P. B. (2003) Cancer Res. 63, 5821–5828 [PubMed] [Google Scholar]
- 47. Alhadlaq A., Mao J. J. (2004) Stem Cells Dev. 13, 436–448 [DOI] [PubMed] [Google Scholar]
- 48. Auerbach R., Kubai L., Knighton D., Folkman J. (1974) Dev. Biol. 41, 391–394 [DOI] [PubMed] [Google Scholar]
- 49. DeFouw D. O., Rizzo V. J., Steinfeld R., Feinberg R. N. (1989) Microvasc. Res. 38, 136–147 [DOI] [PubMed] [Google Scholar]
- 50. Rizzo V., DeFouw D. O. (1996) Microvasc. Res. 52, 245–257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.