Abstract
A host of diverse stress techniques was applied to a monoclonal antibody (IgG2) to yield protein particles with varying attributes and morphologies. Aggregated solutions were evaluated for percent aggregation, particle counts, size distribution, morphology, changes in secondary and tertiary structure, surface hydrophobicity, metal content, and reversibility. Chemical modifications were also identified in a separate report (Luo, Q., Joubert, M. K., Stevenson, R., Narhi, L. O., and Wypych, J. (2011) J. Biol. Chem. 286, 25134–25144). Aggregates were categorized into seven discrete classes, based on the traits described. Several additional molecules (from the IgG1 and IgG2 subtypes as well as intravenous IgG) were stressed and found to be defined with the same classification system. The mechanism of protein aggregation and the type of aggregate formed depends on the nature of the stress applied. Different IgG molecules appear to aggregate by a similar mechanism under the same applied stress. Aggregates created by harsh mechanical stress showed the largest number of subvisible particles, and the class generated by thermal stress displayed the largest number of visible particles. Most classes showed a disruption of the higher order structure, with the degree of disorder depending on the stress process. Particles in all classes (except thermal stress) were at least partially reversible upon dilution in pH 5 buffer. High copper content was detected in isolated metal-catalyzed aggregates, a stress previously shown to produce immunogenic aggregates. In conclusion, protein aggregates can be a very heterogeneous population, whose qualities are the result of the type of stress that was experienced.
Keywords: Antibodies, Fluorescence, Fourier Transform IR (FTIR), Oxidative Stress, Protein Metal Ion Interaction, Immunogenicity, Aggregation, Mechanism, Particles, Reversibility
Introduction
Aggregation of biotherapeutics is a key issue for the advancement of marketed products (1–3). The characterization of protein aggregates as caused by assorted kinds of stress is an essential step to understanding the features, underlying causes, and potential biological effects of these particles (4). The control of particulation is necessary for ensuring the safety and efficacy of protein drug products (5).
Much of our current understanding of the immunogenicity of aggregates comes from studies on vaccine development and the immunogenicity of protein assemblies. Regulatory agencies are concerned about the safety impact of submicron and micron protein particles (2, 6), as rigidly organized protein arrays in the micron range have been shown to be immunogenic (7–10). Protein aggregates may present self-antigens that break B-cell tolerance (stimulating B-cells without T-cell help) to produce neutralizing antibodies (11, 12). Unlike rigid arrays, protein aggregates, however, appear to be irregular and amorphous and thus unlikely to display repeating antigenic motifs (13). Also, subvisible particles, most of them likely to be aggregates of the biotherapeutic, are found in marketed products with no evidence of safety risks (14). However, the exact role of aggregates in causing an immune response is not known.
Immunogenicity assays have shown that the nature of the aggregate and the type of protein are critical for eliciting an immune response as seen in several cases, IFN-α (9, 15–17), FVIII (18, 19), and recombinant human growth hormone (20). Metal-catalyzed aggregates of IFN-α showed the highest level of response and were able to break the tolerance of transgenic mice (9, 15–17), whereas heat-aggregated FVIII was found to be less immunogenic than the monomeric protein (18, 19). Studies that assess aggregated immunoglobulins (IgG) (4, 5), a prominent category of biopharmaceutical medication, would help shed light on the potential health issues surrounding this type of therapeutic. Although the true propensity of aggregates to trigger immune responses will only be revealed upon clinical analysis, detailed studies describing these distinct sets of IgG aggregates will provide needed information for understanding the effect of these specific subsets of antibody aggregates on the immune system.
Biotherapeutics for clinical trials must be fully investigated and accurately quantitated for particulates. Acceptance criteria have been defined for visible and subvisible particles in the United States Pharmacopeia (USP),3 which specifies that particulates ≥10 μm in size are limited to 6000 particles/container and particles ≥25 μm are to be less than 600 particles/container (21–24). However, USP testing was not designed to control the risk of potentially immunogenic aggregates. USP limits are numerical, do not reflect the quality of the product, and show no differentiation between different types of aggregates. Analytical methods that tie aggregate characteristics to immunogenicity are critical for developing risk mitigation strategies.
The propensity of proteins to aggregate is an inherent and problematic property of all proteins (15, 25). Aggregation of protein particles can occur under a wide range of conditions (26, 27). The control of protein stability during biotherapeutic drug production is a challenging task for many pharmaceutical companies. Biopharmaceutical products can aggregate during any stage of the manufacturing process, including fermentation, purification, formulation, filling, storage, shipping, handling, and administration. Protein aggregation can be accelerated by various external factors such as heating, freezing-thawing, mechanical stress, chemical treatment, cross-linking, formulation changes, storage, and exposure to extractables/leachables (28–30). Different stress treatments can generate aggregates with widely varying properties (31). Stressed samples can potentially have different particle size distributions, particle morphology, chemical modifications, reversibility, percent aggregation, conformations (native-like versus unfolded), and particle surface hydrophobicity. Full characterization of different aggregate types is essential to understanding the origin of aggregate formation and the dependence of potential biological impact on specific traits.
No single analytical technique is sufficient for assessing and monitoring aggregates (30). In this work we used one IgG1 protein, two IgG2 proteins, and intravenous IgG (containing all IgG subtypes) as representative antibody biotherapeutics. A collection of protein aggregates was prepared by using a wide range of stress approaches. A host of analytical techniques was used to differentiate and compare aggregates, including size-exclusion chromatography, light obscuration, nanoparticle tracking analysis, micro-flow imaging, FTIR, and UV-visible spectroscopies, fluorescence, hydrophobic dye binding, and ICP-MS. These techniques were selected as they are effective at probing the desired biophysical attributes; however, other convenient methods that are equally informative could be substituted to obtain the desired information. Aggregate groups were then classified based on the properties identified by these analyses. Future studies that test a wider array of molecules will be essential for establishing the extent of suitability of the classification scheme.
EXPERIMENTAL PROCEDURES
Aggregate Preparation
Purified human IgG2 monoclonal antibodies (mAb1 and mAb2) and a mouse IgG1 monoclonal antibody (mAb4) were supplied by an Amgen process development group as high concentration solutions. Human IgG1 monoclonal antibody (mAb3) and intravenous human IgG (IVIG, containing a mixture of IgG antibodies from all subtypes), are commercially available as highly purified solutions used therapeutically. The protein solutions were diluted to both 1 and 10 mg/ml in 10 mm acetate, pH 5.0 (except for mAb3 where, according to the manufacturer's instructions, water was used for all subsequent steps), and then stressed to make aggregated solutions at concentrations needed by the various assays. The diluted samples before stress treatment were used as negative controls (untreated).
Aggregates were synthesized to resemble those that can occur during the storage, manufacture, shipping, and administration of biotherapeutics. 11 different stress conditions were used as described below. To imitate aggregation during storage below freezing temperatures, the protein solution was subjected to 10 cycles of either placement in a freezer at −80 °C followed by thawing in a 37 °C bath (ft-slow) or flash freezing in liquid nitrogen followed by thawing in a 37 °C bath (ft-fast). To accelerate aggregation occurring during long term storage at 4–8 °C, the protein solution was incubated in a 37 °C bath for 19 months (store).
For aggregates that were made upon the change in pH, the high concentration protein solution was diluted to 1 mg/ml in 10 mm acetate at varying pH values (pH 3.5, 4.3, and 8.5) and 10 mm Tris, pH 11, and it was incubated overnight at 37 °C.
Aggregates were created by simulated mechanical stress to reproduce those caused during the manufacturing and shipping of therapeutic antibodies. One condition used was pipetting; the protein solution was pumped through a disposable pipette tip (Fisher) 100 times (pipette). To apply agitation stress, 0.5 ml of sample was subjected to shaking at 500 rounds/min in a 3-ml glass vial capped and placed vertically in a VWR Scientific (West Chester, PA) analog orbital shaker (model OS-500) as described previously (27), at either 4 °C for 7 days in the absence (agitate-4C-so−) or presence of 1.5% (w/v) silicone oil (agitate-4C-so+) or 22 °C for 3 days in the absence (agitate-so−) or presence of silicone oil (agitate-so+). A 3% (w/v) stock solution of silicone oil in 10 mm acetate, pH 5.0, was used that had been previously pumped 10 times through an 18-gauge × 1.5-inch needle (VWR Scientific) attached to a 3-ml disposable syringe (Fisher) to make an emulsion. For stirring stress, 2 ml of the protein sample was stirred with a 6 × 6-mm Teflon stirrer bar at ∼700 rpm, creating a vortex, in a 5-ml glass vial capped and placed vertically on a magnetic stir plate over 20 h (stir-20h) or 3 days (stir-3d).
To replicate aggregates that can be formed during the administration of biotherapeutics via syringes, two different approaches were taken. The protein samples were pumped 50 times through a disposable 18-gauge × 1.5-inch needle (VWR Scientific) attached to either a Daikyo Crystal Zenith cyclic olefin copolymer syringe that was silicone oil-free (West Pharmaceutical Services) (syringe-so−) or through a 3-ml disposable polyethylene syringe containing silicone oil (Fisher) (syringe-so+). The plastic cyclic olefin copolymer syringe was made of material commonly found in commercially available plastic pre-filled syringes (32, 33), and the disposable polyethylene syringe was the device commonly used for administration during clinical drug development. These two syringes differ in several aspects, including material composition and the presence of silicone oil.
To model aggregates that become chemically modified during manufacturing, the protein solution was exposed to various chemical treatments. Oxidation of proteins during manufacturing was imitated by adding 0.1% hydrogen peroxide (H2O2) to the protein, incubating for 20 h at 37 °C, and quenching with 12 mg/ml methionine (H2O2), as similarly described by other groups (16, 34). Metal-catalyzed oxidation was also employed. This condition was chosen because much of the literature on the immunogenicity of aggregates was obtained using aggregates generated by this technique. Protein solutions were oxidized with 5 mm CuSO4 and 4 mm ascorbic acid overnight at 37 °C and quenched with 5 mm EDTA (metal), similar to that described previously (16, 17). Lower copper concentrations were also tested, and similar results were obtained. Chemically cross-linked aggregates were generated as well. Although these conditions do not emulate any conditions encountered during manufacturing, these aggregates are good controls as they are irreversible once generated. Samples were dialyzed into 20 mm HEPES, pH 5.5, then incubated for 1 h with 0.8 mg/ml 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride and 1.2 mg/ml N-hydroxysuccinimide (Pierce), and quenched with 10 mm ethanolamine (xlink). After quenching, the H2O2, metal, and xlink samples were dialyzed at a volume ratio of 1:100 into 10 mm acetate, pH 5.0, in two steps for 2 h and then overnight.
Thermal stress was used to study aggregate that can be generated due to unfolding of the protein, reflecting the conformational stability of the mAb. As a condition of extreme stress, the protein solution was incubated in a 90 °C bath overnight (90C), well above the thermal transition of the Fc and Fab domains. As a less extreme stress condition, the protein solution was diluted to 1 mg/ml in 10 mm acetate, pH 8.5, followed by incubation in a 65 °C bath for 1 h (65C/pH 8.5). 65 °C was chosen as this is below the melting temperature (Tm) of the protein (the Tm of mAb1 in 10 mm acetate, pH 5, was determined by differential scanning calorimetry to be 74.2 °C). Exposing the protein to 65 °C or pH 8.5 separately produced a minimal amount of aggregation. However, in combination, the protein was destabilized enough to aggregate substantially.
Aggregate Concentration
To estimate the percent of original protein present as aggregates, stressed samples were centrifuged at 12,000 rpm to pellet the insoluble material, and the protein concentration before and after centrifugation was determined by measuring the A280 on a Cary 300 Bio UV-visible spectrophotometer (Varian). For samples that contained insoluble aggregates (aggregates that could be pelleted by centrifugation at 12,000 rpm), an aliquot of each solution was first dissolved in 6 m guanidine hydrochloride before measuring the A280. A denaturing agent was used to dissolve protein aggregates as they can interfere with the absorbance reading by quenching the signal or scattering light. The percentage of aggregation was calculated from the difference in protein concentration before and after centrifugation, taken as a percentage of the protein originally present. The average of 3–4 different sample preparations is reported, where the error is the standard deviation. Resolubilization was confirmed by comparing the absorbance of the unfractionated sample before and after stress treatment to ensure that the readings were equal.
For all experiments where the supernatant and pellet components were separated after stress treatment, the following protocol was used. Total stressed samples were centrifuged at 12,000 rpm, and the supernatant fraction was removed for analysis. The tube containing pelleted aggregates was inverted and lightly tapped to remove residual supernatant, followed by gentle resuspending of the pellet in 100 μl of 10 mm acetate, pH 5.0 (pellet fraction). The protein concentration of the total sample, supernatant, and pellet fractions was then determined as above.
Size-exclusion-High Performance Liquid Chromatography
SE-HPLC was carried out on an Agilent 1100 HPLC system with a binary pump and equipped with a UV detector. 100 μl of the supernatant of each stressed sample prepared at 1 mg/ml was injected onto a Tosoh Bioscience TSK-Gel G3000SWxl (7.8 × 300 mm, 5-μm particle size) column operated at 25 °C, and the absorbance at 280 nm was recorded. The flow rate was 0.5 ml/min with a total elution time of 35 min. The mobile phase contained 100 mm sodium phosphate, pH 6.8, and 250 mm sodium chloride. Data acquisition and peak area integration were achieved by the Agilent ChemStation software version B.04.02. The main peak corresponded to the monomeric peak of the untreated sample. High molecular weight (HMW) peaks included everything in the range between the excluded volume and the start of the main peak, and low molecular weight (LMW) peaks included all peaks up to ∼6 min after the main peak (buffer peak excluded). The integrated area of the Main, HMW, and LMW peaks were taken as a percentage of the total integrated peak area and called % Main, % HMW, and % LMW, respectively.
Light Obscuration
Particle counting and size distribution were achieved via the light obscuration method described in USP and adapted for a smaller sample volume. An HIAC/Royco liquid particle counter model 9703 with an HRLD-150 sensor and the software PharmSpec (HACH Ultra Analytics, Grants Pass, OR) were used. Particle-free water was washed through the system before each sample analysis to provide for a clean base line. Total stressed samples made at 1 mg/ml were diluted (20–440×) in 10 mm acetate, pH 5.0, to keep the particle counts within the detection limits of the instrument. Samples were degassed in a vacuum chamber at 75 torr for at least 1 h, gently swirled, and then measured four times at a volume of 0.5 ml per injection. The first run was discarded, and the last three runs were averaged. Particles were counted as differential counts per 0.5 ml in size ranges of 2–5, 5–10, 10–15, 15–20, 20–25, and 25–150 μm. Final particle counts were adjusted based on the initial dilution factor.
Nanoparticle Tracking Analysis
Nanoparticle detection was performed on a NanoSight LM10-HSB (NanoSight, Amesbury, UK), containing a high sensitivity EMCCD camera and 405 nm blue laser. Total stressed samples made at 1 mg/ml were diluted 10-fold in 10 mm acetate, pH 5.0, and then centrifuged at 12,000 rpm for 2 min to reduce interference from very large aggregates. Samples were injected in the sample chamber using silicone oil-free syringes, and measurements were taken at room temperature with an assumed viscosity of 0.95 centipoise. Videos were recorded for 10 s with manual shutter and gain adjustments. The NanoSight NTA version 2.0 Build 0252 software was used for data analysis. Final counts were adjusted based on the dilution factor.
Micro-flow Imaging (MFI)
Particle images were captured on a liquid-borne particle MFI System DPA4100 (Brightwell Technologies, Inc.). MFI captures images as the sample passes through a flow cell and then analyzes each particle's image to gain specific information about the properties of the particle (13). Particle-free water was flushed through the system before each sample analysis to provide for a clean base line. Total stressed samples made at 1 mg/ml were diluted (20–440×) in 10 mm acetate, pH 5.0 (in the same manner as described under “Light Obscuration”). Samples were degassed in a vacuum chamber at 75 torr for at least 1 h, gently swirled, and then drawn from a 1-ml pipette tip into the flow cell using a peristaltic pump. 0.8 ml was loaded at a time; the first 0.3 ml was discarded and only the following 0.5 ml was analyzed. 500 images were collected per sample and processed by the system software to extract each particle and its characteristics, including size, shape, transparency, and an individual image. An array of typical particle images from each sample and the longest dimension (feret diameter) were used for further comparison.
Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectra of the untreated and stressed protein were obtained with the Vertex 70 FT-IR spectrophotometer with the attenuated total reflectance device and OPUS spectroscopy software version 6.5 (Bruker Optics). Stressed samples prepared at 10 mg/ml were used for analysis. 20 μl of the total sample, supernatant, and pellet fractions (which created a slurry upon resolubilization because of the higher protein concentration used) was placed directly in the attenuated total reflectance cell for 270 scans at a resolution of 2 cm−1. To improve contact between the aggregate in the pellet fraction and the cell surface, standing liquid was removed from the cell, and the remaining thin layer of particles was air-dried for 20 s before taking the spectrum. A protein-free blank control was prepared for each stressed sample, where buffer was stress-treated in a manner identical to the protein. The spectrum of the relevant control was subtracted from the stressed protein spectrum for each sample, to minimize differences in solutions. Vector normalization of the second derivative spectra was used to minimize the effects of concentration differences. The correlation coefficient (CC) was calculated using the Thermal Electron OMNIC software version 5.1b (Nicolet Instrument Corp.) (35), where sample spectra were compared against the untreated sample in the second derivative amide I region, 1600–1700 wavenumber cm−1. This method correlates the spectral information across the specified region of the sample and the reference spectra to determine the similarity between the two. The result of the analysis is reported as a value between 0 and 100%. The value indicates how well the sample spectrum matches the reference spectrum. A correlation value of ≥93% indicates an identical match (36).
Fluorescence Measurements
All measurements were recorded using a Cary-Eclipse fluorescence spectrophotometer and accompanying software (Varian). The total sample, supernatant, and pellet fractions were diluted to 0.1 mg/ml with 10 mm acetate, pH 5.0, based on concentration estimates described under “Aggregate Preparation.” Protein-free buffer controls containing components used to cause stress were also tested.
For intrinsic tryptophan fluorescence, an excitation wavelength of 280 nm and a photo multiplier tube voltage of 600 V were used. The emission was monitored from 285 to 450 nm, with a bandwidth of 5 nm, where 10 scans were taken and averaged. 600 μl of each sample was measured one at a time in a 1 × 1-cm path length cuvette. Raman peaks were removed by subtracting the buffer emission spectra from the protein emission spectra using the Cary-Eclipse scan application software version 1.1.
Hydrophobic Dye Binding
For 1-anilinonaphthalene-8-sulfonate (ANS) dye binding, an excitation wavelength of 380 nm and a photo multiplier tube voltage of 650 V were used. The emission was monitored from 400 to 600 nm, where five scans were recorded and averaged, and the fluorescence intensity at 480 nm was reported. ANS was titrated into 400 μl of each protein sample at 0.1 mg/ml to final concentrations of 2.5, 6.3, 12.5, 25, and 50 μm ANS, lightly tapped, and tested for fluorescence. An initial measurement of each sample was also taken in the absence of ANS. For SYPRO Orange dye binding, the experiment was performed according to He et al. (37).
Reversibility
Stressed samples were tested for reversibility by diluting each stressed sample prepared at 1 or 10 mg/ml (pH 11 only) in a total volume of 2 ml of either a pH 5 buffer (10 mm acetate, pH 5.0) or PBS. Samples were diluted either 100× (untreated, syringe-so−, stir-20h, stir-3d, metal, 90C) or 17× (pH 11, H2O2) and placed in a 3-ml vial. Vials were capped, covered with parafilm, and gently rotated for 0, 1, 3, 6, 17, 24, or 48 h at room temperature. Samples were then uncapped, degassed for 1 h, and tested by light obscuration as described above. The 0-h time point for the pH 11 sample was tested without degassing as particle disappearance was faster than the degassing period. Final counts are reported directly with no adjustment for dilution.
ICP-MS
The mAb1 and mAb4 untreated and metal samples at 1 mg/ml and a negative control buffer (treated in the same fashion as the metal samples) were analyzed for copper content. A PerkinElmer Life Sciences/SCIEX Elan DRC II ICP/MS equipped with an autosampler (AS-93Plus) was used in this work. The instrument was optimized following the standard procedures recommended by the vendor. Sampling was performed at sample flush 45 s/24 rpm, read delay 25 s/20 rpm, analysis/20 rpm, and wash 210 s/35 rpm with 0.5% (v/v) nitric acid solution. For each sample, raw data of six replicates of 20 sweeps each were acquired. Copper standard solution (Ricca Chemicals, MSCU1KN-100) and cobalt standard solution (BDH, 82026-152) were purchased. Nitric acid (catalog no. A467-500) was purchased from Fisher. Water from a laboratory water purification system (Millipore, Milli-Q, Billerica, MA, ≥18.0 megohms cm) was used. Standard calibration solutions of copper were prepared at concentrations 0.5, 5, 25, 100, and 200 ppb in 0.5% (v/v) nitric acid solution. Cobalt was used as an internal standard at 50 ppb. Analysis monitored mass at 65 m/z for tungsten and 59 m/z for cobalt. Calibration curves had correlations (R2) 0.999 for the copper/cobalt curve. Samples were diluted to 3 ml with 0.5% nitric acid solution and contained 50 ppb cobalt. A blank 0.5% nitric acid solution was measured prior to each sample analysis to subtract background noise. The accuracy of the method was tested against three quality control (QC) samples at concentrations of 2.5, 25.0, and 180.0 ppb copper. The method was accurate to ±5% of the theoretical concentrations of copper for all the QC levels. Spike recovery of copper at 5 and 50 ppb to mAb1 diluted 1:30 was better than 95%.
RESULTS
Production of Aggregates under Accelerated Stress
To compare and classify aggregates created via different mechanisms that might be encountered during the manufacturing, storage, and delivery of biotherapeutics, an IgG2 monoclonal antibody (mAb1) was subjected to a mixture of varied stress techniques. These stress techniques are meant to act as accelerated conditions for stresses that might be commonly encountered during manufacturing to amplify the effect for further analysis and increase the detectable differences between samples. As described under “Experimental Procedures,” different types of stress were utilized including the following: freeze-thawing (ft-slow and ft-fast), storing under accelerated circumstances (store), change in pH from 5.0 (to pH 3.5, 4.3, 8.5, and 11), chemical cross-linking (xlink), pipetting (pipette), stirring (stir-20h and stir-3d), oxidation by hydrogen peroxide (H2O2) or metal-catalyzed aggregation (metal), heating and raising the pH (65C/pH 8.5), and heating to just below boiling (90C). Mechanical stress methods were also tested in the absence and presence of silicone oil as follows: agitating both at 4 °C (agitate-4C-so− and agitate-4C-so+) and at room temperature (agitate-so− and agitate-so+) and pumping through a syringe (syringe-so− and syringe-so+).
Percentage Aggregation
To estimate the percentage of mAb1 aggregation as a function of the total protein, stressed samples were centrifuged, and the concentrations of protein before and after spinning were compared. As shown in Table 1, the total protein that formed insoluble aggregates was below detection in the untreated, ft-slow, ft-fast, store, pH 3.5, and pipette stressed samples. A slightly higher percentage (≤10%) was seen in the pH 11, xlink, syringe-so−, syringe-so+, H2O2, and metal samples. The highest percentage was found in the agitate-so+ (73 ± 5%), stir-20h (28 ± 6%), stir-3d (97 ± 3%), 65C/pH 8.5 (86 ± 5%), and 90C (92 ± 4%) samples. These results reveal that different stress conditions caused varying amounts of protein to form insoluble aggregates, with most being produced under the harshest conditions (stirring and heat), and are consistent with previous reports for some of these treatments (30).
TABLE 1.
Percent aggregation of mAb1 stress-treated samples
| Stress treatment | % Insol. agg.a | SE-HPLCb |
||
|---|---|---|---|---|
| % HMW | % Main | % LMW | ||
| untreated | NDc | 0.4 ± 0.0 | 99.6 ± 0.0 | ND |
| ft-slow | ND | 0.4 ± 0.0 | 99.6 ± 0.0 | ND |
| ft-fast | ND | 0.4 ± 0.0 | 99.6 ± 0.0 | ND |
| store | ND | 6.9 ± 0.1 | 93.1 ± 0.1 | ND |
| pH 3.5 | ND | 0.3 ± 0.0 | 99.7 ± 0.0 | ND |
| pH 11 | 4 ± 1 | 1.0 ± 0.0 | 99.0 ± 0.0 | ND |
| xlink | 3 ± 2 | 2.2 ± 0.1 | 97.8 ± 0.1 | ND |
| pipette | ND | 0.4 ± 0.0 | 99.6 ± 0.0 | ND |
| agitate-so+ | 73 ± 5 | 6.9 ± 0.1 | 93.1 ± 0.1 | ND |
| syringe-so− | 7 ± 5 | 0.3 ± 0.0 | 99.7 ± 0.0 | ND |
| syringe-so+ | 7 ± 5 | 0.4 ± 0.0 | 99.6 ± 0.0 | ND |
| stir-20h | 28 ± 6 | 11.1 ± 0.7 | 88.9 ± 0.7 | ND |
| stir-3d | 97 ± 3 | ND | ND | ND |
| H2O2 | 3 ± 1 | 0.4 ± 0.0 | 99.6 ± 0.0 | ND |
| metal | 3 ± 2 | –c | – | – |
| 65C/pH 8.5 | 86 ± 5 | 3.3 ± 0.4 | 95.6 ± 0.4 | 1.1 ± 0.3 |
| 90C | 92 ± 4 | ND | ND | 100.0 ± 0.0 |
a Percent insoluble aggregation (% Insol. agg.) was determined by comparing the protein concentration before and after centrifugation. The average of 3–4 different sample preparations is shown, where the error is the standard deviation.
b SE-HPLC was used to assess the % HMW, % Main, and % LMW present in the supernatant fraction. The average of three replicate runs is shown where the error is the standard deviation.
c ND indicates not detected (values were below the limit of detection, <1% for % aggregation or not present on the SE-HPLC);–indicates sample could not be assessed due to interference.
Detection of oligomers and clipped fragments in stressed samples after centrifugation was performed by SE-HPLC. As described under “Experimental Procedures,” the molecular weight distribution of the protein that eluted from the column was calculated as the percent high molecular weight (% HMW), percent main peak (% Main), and percent low molecular weight (% LMW) to determine the percentage of protein present in the supernatant as oligomers, monomers, and clipped fragments, respectively (supplemental Fig. S1). An increase in HMW species above that detected in the untreated sample (0.4 ± 0.0%) was seen for the store (6.9 ± 0.1%), pH 11 (1.0 ± 0.0%), xlink (2.2 ± 0.1%), agitate-so+ (6.9 ± 0.1%), stir-20h (11.1 ± 0.7%), and 65C/pH 8.5 (3.3 ± 0.4%) samples but not for the ft-slow, ft-fast, pH 3.5, pipette, syringe-so−, syringe-so+, H2O2, and 90C samples (Table 1). LMW species were found only in the 65C/pH 8.5 (1.1 ± 0.3%) and 90C samples (100.0 ± 0.0%), indicating that for the high heat stressed sample the small amount of protein that remained in solution had all been fragmented. The stir-3d sample did not contain sufficient protein in the supernatant to be detected by SE-HPLC (due to the aggregation of all of the protein into larger species), and the metal-treated sample could not be assessed due to interference with the signal.
Particle Count and Size Distribution
Particle count and size distribution in the micron-sized range (≥2 μm) for each mAb1 stressed sample were determined through analysis by light obscuration. Few particles (<30,000 particles/ml) were created by stresses such as freezing-thawing, storing, pH change, chemical cross-linking, pipetting, and hydrogen peroxide oxidation (Fig. 1). However, a large number of particles (>30,000 particles/ml) resulted from most types of mechanical stress (agitate-4C-so+, agitate-so+, syringe-so−, syringe-so+, stir-20h, and stir-3d), metal-catalyzed oxidation, and heat (65C/pH 8.5 and 90C). The largest sized particles (>20 μm) were found in the greatest abundance in the 65C/pH 8.5 and 90C samples.
FIGURE 1.
Micron-sized particle counts and size distribution for stress-treated samples. Stress-treated mAb1 samples were examined by HIAC to determine the number and size range of particles present. The differential particle counts per ml of samples tested are shown for each discrete size range listed. Vertical bars group similar types of stress treatment and show that mechanical stress creates the greatest number of 2–20 μm particles, whereas heat creates the most particles sized at 20 μm and larger.
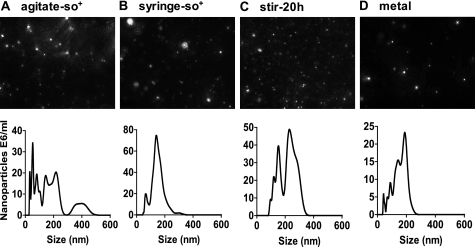
Detection of particles in the nanometer range (20–1000 nm) for each stressed sample was achieved through nanoparticle tracking analysis (NTA). The small size of nano-sized protein aggregates has made them particularly challenging to monitor by conventional techniques (38); thus, an innovative system was employed using NTA, which had recently been shown to be suitable for analyzing heat-induced IgG protein aggregates (39). NTA appears to be an effective comparative tool for assessing nanoparticle concentration in different stressed samples. Although nanoparticles were detected in all samples, nanoparticle concentration was the same or less than the untreated sample for weak stresses such as freeze-thaw, pH change, and hydrogen peroxide oxidation, as well as the most severe stresses, stir-3d and 90C (Table 2). The greatest number of nanoparticles were created by mechanical stress (agitate-so+, syringe-so+, and stir-20h), followed by the metal and 65C/pH 8.5 samples. Size distribution profiles varied based on the stress treatment (Fig. 2) and were the widest for agitate-so+ and stir-20h.
TABLE 2.
Nanometer-sized particle concentration and mean size for mAb1 stress-treated samples by nanoparticle tracking analysis
| Stress treatment | Particle conc.a | Meanb | S.D.b |
|---|---|---|---|
| E7/ml | nm | nm | |
| bufferc | 4 | 106 | 21 |
| buffer-so+c | 6 | 115 | 35 |
| untreated | 59 | 145 | 40 |
| ft-slow | 19 | 181 | 12 |
| pH 11 | 38 | 120 | 35 |
| agitate-so+ | 407 | 180 | 112 |
| syringe-so+ | 597 | 149 | 44 |
| stir-20h | 642 | 217 | 60 |
| stir-3d | 38 | 118 | 49 |
| H2O2 | 24 | 149 | 34 |
| metal | 216 | 156 | 46 |
| 65C/pH 8.5 | 109 | 97 | 24 |
| 90C | 29 | 124 | 35 |
a Concentration of particles in E7/ml was measured by nanoparticle tracking analysis.
b Mean ± S.D. was calculated by the NanoSight NTA 2.0 software. See under “Experimental Procedures” for details.
c A pH 5 buffer with silicone oil (buffer-so+) and without (buffer) was tested for nanoparticles.
FIGURE 2.
Nanometer-sized particle images and size distribution for stress-treated samples. Stress-treated mAb1 samples were tested by nanoparticle tracking analysis to determine the number and size range of particles present. NanoSight video frames (top panel) are shown with their corresponding size distribution profiles of 1-nm size bins (bottom panel). The four samples with the highest particle counts are depicted as follows: A, agitate-so+; B, syringe-so+; C, stir-20h; D, metal.
Particle Morphology
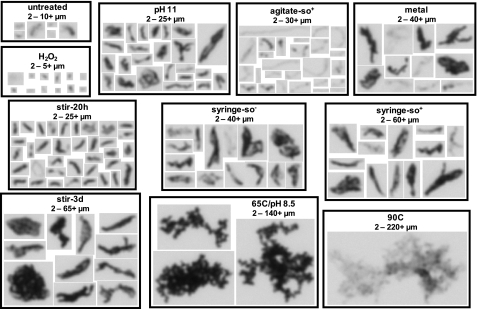
To decipher differences in morphology between protein aggregates, MFI technology was used to visualize and quantitate particles in solution. MFI images of mAb1 protein particles were obtained that varied widely in size, shape, and transparency. Fig. 3 shows typical images taken of the largest particles detected per sample, because larger particles exhibit more detailed resolution. Overall, samples contain a heterogeneous mixture of protein particles where particles appear disordered, amorphous, and irregular in shape. The longest particle dimension (based on the size threshold of the bin with the largest 20 particles) was up to 30+ μm for the untreated, pH 11, agitate-so+, stir-20h, and H2O2 samples, up to 65+ μm for the syringe-so−, syringe-so+, stir-3d, and metal samples, and up to 220+ μm for the 65C/pH 8.5 and 90C samples (which appeared particularly translucent and fragile). Overall, these results indicate that changes in pH, harsh mechanical stress (agitation and stirring for 1 day), and hydrogen peroxide oxidation produce the smallest sized particles. Medium sized particles are caused by more severe mechanical stress (syringe and stirring for 3 days) and metal-catalyzed oxidation. Finally, the largest, most amorphous visible particles (≥125 μm) were found only in the aggregates created with high heat (65C/pH 8.5 and 90C). Visible particles are defined as ≥125 μm (set above the threshold of 100 μm shown previously to be detected by visual inspection 70% of the time (40)) and were visualized and quantitated by MFI.
FIGURE 3.
Panel of particle images for aggregates created under different stress conditions. Aggregates of mAb1 were examined by MFI, and representative images of the largest particles detected are shown. Size ranges represent the span of particle sizes detected, where the lower limit of quantitation of the instrument is 2 μm and the upper limit is the size threshold of the bin with the largest 20 particles.
Changes in Secondary Structure
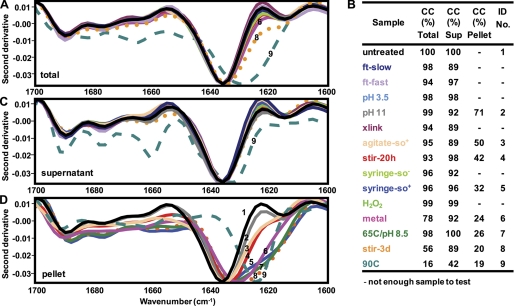
To assess secondary structural changes upon stress treatment, mAb1 samples were examined by FTIR. Fig. 4, A and B, shows an overlay of the second derivative FTIR spectra of the untreated and 14 stress-treated samples after treatment (the total sample with no separation of the particles from the bulk protein in the samples). To compare the spectral similarity between the untreated and each stressed sample, the OMNIC QC Compare software (35) was used to calculate a CC between the spectra in the amide I region. This method compares the information in a defined region of the spectra of different samples, to determine the similarity between them, and is a way of quantitatively comparing results from the FTIR analysis of multiple samples. In a separate study, the precision and sensitivity of the FTIR method using the OMNIC QC Compare tool was investigated and found to be reproducible with suitable method precision (spectral similarity of replicate measurements gives a correlation coefficient that is ≥93%) and is sensitive enough to detect differences caused by common manufacturing stresses (36). Furthermore, CCs of ≥93% are considered identical, within experimental error. The spectrum of the total untreated sample displays a predominantly β-sheet structure with a typical minimum at 1636 cm−1 (Fig. 4, 1st spectra). 11 of the total stress-treated samples display a spectrum similar to untreated protein and have a CC of at least 93% between these spectra (Fig. 4B). The remaining three are the metal sample (Fig. 4A, spectra 6), which has a CC of 78% but shows no shift of the minima, the stir-3d sample (Fig. 4A, spectra 8), which has a CC of 56% and shows a shift of the minima to 1634 cm−1, and the sample treated at 90C (Fig. 4A, spectra 9), which has a CC of 16% and shows a more pronounced shift to 1630 cm−1, demonstrating that these are the most extreme cases of disruption of secondary structure.
FIGURE 4.
Second derivative FTIR spectra show varying degrees of mAb1 protein structural changes upon stress treatment. A, overlaid spectra of the total untreated (solid black line) and stressed samples (colored lines) show a high degree of similarity except for metal (solid pink line), stir-3d (dotted orange line) and treatment at 90C (dashed teal line). B, colors and numbers indicate the identity of the spectra. The CC (%) between the spectra of the untreated protein and the total, supernatant (sup), and pellet of each stressed sample was calculated. C, spectra of the supernatant of each stressed sample (colored lines) also shows a high degree of similarity to untreated (solid black line); except for the 90 stress treatment (dashed teal line). D, spectra of the pellet of each stressed sample (colored lines) shows the greatest shift from the untreated sample (solid black line) for metal (solid pink line), 65C/pH 8.5 (solid green line), stir-3d (dotted orange line), and at 90C (dashed teal line).
To investigate differences in secondary structure of the pellets versus the protein remaining in solution, each sample was centrifuged and divided into a supernatant and pellet fraction for further analysis. The supernatant of 13 of the stressed samples had a CC to the untreated sample of at least 89% (Fig. 4, B and C), demonstrating that the portion of each stressed sample that remained in solution has a mostly unaltered folded β-sheet structure. The 90C sample differs from the others (Fig. 4, B and C, spectra 9), displaying a CC of 42% and a shift in the minima to 1632 cm−1, showing that even the secondary structure of the protein that remained in solution has been disrupted for this extreme case.
The secondary structure of the pellets, however, showed some degree of perturbation for all samples (Fig. 4, B and D). The CC of the pellets revealed that the pH 11 sample (Fig. 4D, spectra 2) had the greatest similarity to the folded structure (71%); the agitate-so+ (Fig. 4D, spectra 3), stir-20h (Fig. 4D, spectra 4), and syringe-so+ (Fig. 4D, spectra 5) samples had intermediate similarity (30–50%), and the metal (Fig. 4D, spectra 6), 65C/pH 8.5 (Fig. 4D, spectra 7), stir-3d (Fig. 4D, spectra 8), and 90C (Fig. 4D, spectra 9) samples had the lowest similarity to the folded conformation (less than 30%). In addition, the shift in the minima of the pellets showed a similar trend to the CC. The minima were as follows: agitate-so+ (1636 cm−1), pH 11 (1634 cm−1), stir-20h (1634 cm−1), syringe-so+ (1634 cm−1), 65C/pH 8.5 (1632 cm−1), metal (1630 cm−1), stir-3d (1630 cm−1), and 90C (1624 cm−1), revealing a progressive increase in perturbations of the folded β-sheet secondary structure (Fig. 4D).
Changes in Tertiary Structure
Alterations in the tertiary structures were monitored by assessing untreated and stressed samples for changes in intrinsic fluorescence. Fig. 5 shows the shift in the wavelength of maximum fluorescence (λmax) of the mAb1 total sample and the supernatant and pellet fractions. The untreated sample has a λmax of 337 nm, which does not shift significantly for the total sample or the supernatant of stressed samples (ft-slow, pH 11, xlink, agitate-so+, syringe-so−, syringe-so+, stir-20h, and H2O2), showing that the Trp environment of the major species in these samples has not changed. In contrast, the total sample and supernatant of the samples where more particles were generated have a λmax that has red-shifted (moved to a longer wavelength) by greater than 2 nm (Fig. 5, A and B), showing that Trp has become more solvent-exposed in these samples (pH 3.5, stir-3d, metal, 65C/pH 8.5, and 90C). For the stir-3d sample, the protein concentration of the supernatant was too dilute to assess, and so for this aggregate only the total sample was analyzed. The protein that stayed in the supernatant of the 90C sample had the most Trp exposure (red-shifted by 13 nm) of any fraction tested, indicating complete unfolding of the protein. This is consistent with the morphological assessment, with this treatment being classified as the most extreme condition. Of the seven pellets that were at a high enough concentration for testing, six were red-shifted (agitate-so+, syringe-so+, stir-20h, stir-3d, 65C/pH 8.5, and 90C) suggesting Trp exposure to solvent had increased, and one (pH 11) was not shifted, indicating that the Trp environment in these particles had not changed (Fig. 5C).
FIGURE 5.
Wavelength of maximum fluorescence (λmax) shifts depending on the aggregate type. Graphs represent the mAb1 λmax of the total untreated and stress treated samples (A), the supernatant of the untreated and stress-treated samples (B), and the total untreated sample in comparison with the pellet of the stressed samples (C). Diagonal stripes indicate when a shift greater than or equal to 2 nm has occurred.
Increase in Surface Hydrophobicity
Changes in surface aspects of aggregates created by the assorted stress methods were evaluated using the fluorescent dye ANS. ANS was titrated into equivalent concentrations of the mAb1 total samples and the supernatant and pellet fractions, and the fluorescence intensity was monitored (Fig. 6). To allow comparative analysis, the fluorescence intensity of each stressed sample was calculated as a percentage of the ANS fluorescence of the total 90C sample, which had the highest fluorescence intensity. Notable ANS binding was observed for some of the stressed samples as follows: agitate-so+ (52%), syringe-so− (31%), stir-3d (49%), metal (27%), 65C/pH 8.5 (95%), and 90C (100%). The remaining untreated and stressed samples showed less than 17% ANS binding as follows: untreated (11%), ft-slow (12%), pH 3.5 (16%), pH 11 (9%), xlink (8%), syringe-so+ (16%), stir-20h (14%), and H2O2 (11%).
FIGURE 6.
Hydrophobic dye ANS binds preferentially to certain aggregate types. ANS was titrated into equivalent concentrations of the mAb1-untreated (black line) and stress-treated samples (colored lines), and the fluorescence intensity was measured at 480 nm. ANS titration curves show the untreated and stress-treated total samples (A), supernatants (B), and pellets (C) in comparison with the total untreated sample. AU, arbitrary units.
To determine which sample components have exposed hydrophobic patches, the supernatant and pellet fractions were analyzed. The supernatant of all samples showed less ANS binding than their initial unfractionated sample counterparts (Fig. 6, A and B). All supernatants displayed less than 17% ANS binding, except for agitate-so+ (23%), 65C/pH 8.5 (63%), and 90C (35%).
In contrast, the pellets, from samples where they were isolated in high enough concentration for testing, were found to exhibit the largest amount of ANS binding (Fig. 6C). Substantial ANS binding was detected for the samples stir-20h (76%), stir-3d (126%), 65C/pH 8.5 (153%), and 90C (36%), and minimal ANS binding for the sample at pH 11 (18%). The 90C pellet displayed lower binding than expected as a result of the difficulties that are associated in studying this extreme case. The 90C supernatant continually aggregates, even during fractionation. The pellet contains very large sized aggregates, which may result from association of the hydrophobic patches of individual molecules or smaller aggregates, resulting in protection of these regions within the pellet and thus blocking dye binding, a process that may become accelerated upon centrifugation. All samples were also tested for their ability to bind a second hydrophobic dye, SYPRO Orange (37). Trends in SYPRO Orange binding were similar to ANS binding with the highest binding seen for the total metal, stir-20h, stir-3d, agitate-so+, 65C/pH 8.5, and 90C samples, in increasing order (data not shown). The remaining untreated and stressed samples, untreated, ft-slow, pH 3.5, pH 11, xlink, syringe-so−, syringe-so+, and H2O2, all showed minimal SYPRO Orange binding.
Reversibility
To gain insight into protein particle reversibility, defined here as the disintegration of the particles into smaller pieces upon dilution as indicated by a reduction in particle counts over time, mAb1 stressed samples were diluted in a pH 5 buffer (similar to the storage buffer), gently rotated, and tested for a decrease in particle counts over 48 h. In other words, the samples were returned to the original solution conditions, and the amount of aggregate that persisted was determined. Most stressed samples (pH 11, syringe-so−, syringe-so+, stir-20h, stir-3d, H2O2, metal, and 65C/pH 8.5) showed a decrease in the number of particles, in all size ranges detected, over time (Fig. 7) (represented data shown). By 48 h, these samples showed a >70% reduction of particles in the 2–5 μm size range. The pH 11 sample showed the most dramatic decline, where the opacity of the sample disappeared immediately upon dilution, and the particle counts had decreased by 98% within 1 min of dilution (Fig. 7F). The 90C sample showed a decrease in only the number of particles between 2 and 20 μm, and little change in the number of particles at ≥20 μm. The untreated sample showed little change over time. Although every sample did show a reduction in particle counts for some sizes, it is unclear whether particles are reversing to the monomeric state or breaking apart into smaller sized particles that are below the detection limit of the instrument (about 2 μm).
FIGURE 7.
Time course showing a decrease in particle counts upon dilution in a pH 5 buffer. Stressed samples of mAb1 were diluted in a pH 5 buffer and then measured for particle counts and size distribution by HIAC at different time points. Particle count profiles vary depending on the stress treatment. A, untreated and size range key; B and C, mechanical; D and E, chemical; F, pH change; and G, heat.
To explore the behavior of particles at a more physiologically relevant pH, stressed samples were next tested for the reversibility of aggregate upon dilution in a neutral pH buffer. All stressed samples showed a much slower decrease in the number of particles at neutral pH as compared with the pH 5 buffer (supplemental Fig. 2). Syringe-so− and metal samples showed a large initial increase (>2.5-fold), followed by a rapid decrease, indicating that solution conditions (including neutral pH and higher salt concentration) may temporarily induce aggregation (27, 28). The pH 11, stir-20h, and H2O2 samples showed a steady decrease, although at a slower rate than was seen upon dilution into the pH 5 buffer. 90C showed a decrease in subvisible particles in only the 2–5 μm range and either no change or a slight increase in particles ≥10 μm.
Copper Content of Metal-catalyzed Samples
Metal-catalyzed aggregation is the stress most frequently used to generate protein aggregates for testing immunogenicity in vivo (16, 17). These aggregates are generated by exposure of the protein to a large excess of copper beyond what is encountered during manufacturing. To determine whether the EDTA treatment and extensive dialysis had successfully removed the copper following protein treatment, ICP-MS was used to analyze both the mAb1 metal sample and a mouse IgG1 monoclonal antibody (mAb4) that had been subjected to the metal-catalyzed oxidation via copper. Untreated mAb1 and mAb4 (purified under normal manufacturing conditions) and a negative control buffer (treated in the same manner as the metal samples) were also tested. Very high levels of copper were detected in both metal-treated samples (>5000 ppb), corresponding to a molecular ratio of protein to copper of 1:20 for mAb1 and 1:12 for mAb4 (Table 3). The untreated mAb1, untreated mAb4, and negative control buffer were found to be free of any residual copper (<15 ppb). These results indicate that even after overnight dialysis, high copper levels were retained in the aggregate generated by treatment with copper, whereas the amount of this metal found in the protein purified with the normal manufacturing process is undetectable.
TABLE 3.
Elemental copper was detected in metal-catalyzed aggregates by ICP-MS
| Sample | Coppera | Molecule of mAb/molecule of copperb |
|---|---|---|
| ppb | ||
| mAb1 untreated | <15c | |
| mAb1 metal | 11,335 | 1:20 |
| mAb4 untreated | <15c | – |
| mAb4 metal | 5,611 | 1:12 |
a Elemental copper in ppb as detected by ICP-MS.
b Calculated ratio of molecules of antibody (mAb) to molecules of copper is shown. See under “Experimental Procedures” for details.
c Lower quantitation level (0.5 ppb) was multiplied by dilution (30).
Aggregate Classification
Aggregate populations were divided into seven classes based on the biophysical qualities identified. Table 4 shows a summary of the mAb1 aggregate members, classes, and trends in biophysical characteristics. Qualities used to classify aggregates include the following: particle concentrations ≤1 and ≥2 μm, dynamic size ranges, folded secondary structure, folded tertiary structure, surface hydrophobicity, reversibility (defined as reversible (R) = 90–100% reduction in 2–5 μm particles; partially reversible (PR) = 70–89% reduction in 2–5 μm particles; or irreversible (I) = ≤50% reduction in 20+ μm particles), and percent aggregation. Chemical modifications as identified by Luo et al. (41) were also included. Aggregates were partitioned into classes as follows: class 1 contains samples with a low number of particles (untreated, ft-slow, ft-fast, store, pH 3.5, pH 4.3, pH 8.5, pipette, agitate-4C-so− and agitate-so−); class 2 contains those that are metal-containing (metal); class 3 contains those that are small sized, folded, and mostly reversible (H2O2, pH 11, syringe-so−, and syringe-so+); class 4 contains those that are irreversibly cross-linked; class 5 contains those that are small sized, partially folded, and partially reversible (stir-20h and agitate-so+); class 6 contains those that are medium sized, mostly unfolded, and partially reversible (stir-3d and 65C/pH 8.5); and class 7 contains those that are large sized, unfolded, and irreversible (90C).
TABLE 4.
Classification scheme for mAb1 aggregates based on biophysical characterization
a Class descriptions refer to metal content or aggregate properties (size, degree of native-like conformation, and reversibility) if present.
b Values for the total stressed sample are displayed except for the FTIR data where both the supernatant and pellet are shown.
c Oligomer (oligo.) concentration as determined by SE-HPLC (see Table 1), where the + symbol indicates the relative % HMW. Nanoparticle (nano.) concentration as determined by NTA (Table 2 and Fig. 2), where the + symbol indicates the relative fold increase. Total micron (micro.)-sized particle counts as determined by HIAC (Fig. 1), where approximate particles/ml = 10n × 102 (n equals the number of + symbols). Relative visible (vis.) particles as determined by MFI.
d Size threshold of the bin with the largest 20 particles as measured by MFI (Fig. 3).
e Correlation coefficient (%) between the FTIR spectra of the untreated and the supernatant (sup) or pellet fractions (Fig. 4).
f Red shift of the wavelength (nm) of maximum fluorescence from untreated sample (Fig. 5).
g Fluorescence intensity upon binding ANS as a percentage of the total 90 °C sample (Fig. 6).
h Reversibility (Fig. 7) was listed as reversible (R) 90–100% reduction in 2–5 μm, partially reversible (PR) 70–89% reduction in 2–5 μm, or irreversible (I) ≤50% reduction in 20 + μm.
i For determination of chemical modifications (Chem. Mod.) see Ref. 41. + represents the relative extent of chemical modification. For class 1, only untreated was tested.
j Percent insoluble aggregation (% Insol. Agg.) after stress treatment (Table 1).
k Values are for members of class 1, except for pH 3.5 and store which varied slightly in some cases.
l Explanation of symbols: −, not tested; ND, not detected.
To test if the classification system has broader applicability, several additional IgG molecules, an IgG2 monoclonal antibody (mAb2), an IgG1 monoclonal antibody (mAb3), and intravenous IgG containing a mixture of IgG subtypes (IVIG), were stressed by one technique from each class, and the resulting aggregated solutions were biophysically assessed (similar to mAb1). mAb2, mAb3, and IVIG aggregate groups showed similar trends to mAb1 in micron-sized particle counts and size distribution (Fig. 8), nanoparticle concentration (supplemental Fig. 3), gross morphology (Fig. 9), percent aggregation, changes in secondary and tertiary structure, surface hydrophobicity, and reversibility (data not shown). mAb1, mAb2, mAb3, and IVIG aggregate groups created by the same stress are therefore placed into the same classes, demonstrating that the classification system can apply to more than one molecule.
FIGURE 8.
Aggregates of different IgG molecules show similar trends in micron-sized particle counts and size distribution. Particle number was quantitated by HIAC for mAb1 (IgG2) (A), mAb2 (IgG2) (B), mAb3 (IgG1) (C), and IVIG (containing a mixture of all IgG subtypes) (D). Bar height represents the differential particle counts per ml of sample tested (average of three runs) for each specific particle size range.
FIGURE 9.
Morphology of aggregates from distinct IgG molecules is comparable. Particle images of stress-treated mAb1 (IgG2), mAb2 (IgG2), mAb3 (IgG1), and IVIG (containing a mixture of IgG from all subtypes) were captured on a Micro-flow Imaging system. Representative images of the largest particles detected are shown. The size threshold indicates the lower size limit of the particles that were used for comparison. The black arrow emphasizes the increase in particle size seen over prolonged stirring.
DISCUSSION
In this study, aggregates induced under a wide variety of stress conditions were extensively characterized and then sorted into seven classes based on the identified traits (Table 4). To accomplish this, an array of techniques was used including the following: size-exclusion chromatography, light obscuration, nanoparticle tracking analysis, micro-flow imaging, FTIR and UV-visible spectroscopies, fluorescence, hydrophobic dye binding, and ICP-MS. The methods used here are merely a selection of potential bioanalytical procedures. Alternative techniques can be chosen to ascertain the defined aggregate properties followed by subsequent mapping to the classification system. Results were used to differentiate aggregated solutions by percent aggregation, particle counts, size distribution, gross morphology, differences in secondary and tertiary structure, surface hydrophobicity, reversibility, and metal content. An in-depth investigation of chemical modifications introduced by the various stress conditions was also conducted in a separate study (41). The fact that aggregates from multiple IgG antibodies, mAb1, mAb2, and mAb3 (from both the IgG1 and IgG2 subclasses), and IVIG (containing all IgG subtypes) show the same attributes when subjected to the same treatment demonstrates that a similar pathway of aggregation may be used by distinct IgG molecules under the same applied stress. Although these results suggest that the classification scheme could have broader applicability, its potential widespread utility will only be revealed upon the testing of a wider range of molecules (beyond IgGs) and by additional techniques that probe new aggregate properties. An aggregate classification scheme is a useful tool for protein formulation development that highlights similarities between aggregates created under different conditions, as well as identifying features that have the potential for immunogenicity. Conversely, isolation and characterization of antibody aggregates isolated from actual therapeutic protein solutions could help identify what stress resulted in the aggregation. This information could then be used to help identify root cause and remediation/amelioration steps.
Our results suggest that the mechanism that drives aggregate formation may be dependent on the stress to which the protein was exposed (25, 30, 42). Examining the size spectrum across oligomers, nanoparticles, micron-sized particles, and visible particles revealed that each stress has a preference for populating certain sizes (Table 4). Furthermore, the populated size range correlated with the degree of unfolding of the molecule. For example, the mildest stress condition, accelerated storage, produced aggregates only in the oligomer size range, indicating that this may have induced subtle conformational changes that shifted the equilibrium from monomer to oligomers but not to micron and larger particles. At high concentrations, some oligomers are present in equilibrium with the monomer; during storage it appears that the equilibrium may have shifted slightly toward the oligomers. For classes with intermediate levels of stress, most of the members of class 3–5 (high pH, cross-linking, and harsh mechanical stress) produced aggregates in the oligomer, nanometer, and micron sized ranges, which maintained some of the original secondary and tertiary structure of the molecule. This was demonstrated by the mAb1 FTIR CC (42–71%) to the untreated sample with no significant shift in the mAb1 λmax from untreated and an intermediate mAb1 surface hydrophobicity (9–52% ANS binding). The remaining members of class 2 and 3 (metal-catalyzed oxidation and syringe stress) produced aggregates in the nanometer and micron-sized ranges but not oligomers, and displayed slightly more unfolding of the molecule. This was shown by a further decrease in the mAb1 FTIR CC (24–32%) to the untreated sample, and a shift in the mAb1 λmax (≤2 nm) from the untreated sample. For the harshest levels of stress, class 6 and 7 (prolonged harsh mechanical stress and thermal stress) produced aggregates only in the micron and visible size ranges, and these were mostly unfolded as shown by the lowest mAb1 FTIR CC (19–26%) to the untreated sample, a larger shift in the mAb1 λmax (2–4 nm) from the untreated sample, and the highest mAb1 surface hydrophobicity (49–100% ANS binding). In contrast, the mAb1 65C/pH 8.5 sample displayed a unique profile that populated all sizes from oligomers to visible particles to some extent. This may be a result of a combination of stresses, where both oligomers (perhaps because of high pH) and visible particles (perhaps because of thermal stress) can be generated. It appears that only mild to intermediate conditions (accelerated storage, high pH, cross-linking, harsh mechanical stress, and heat/pH) are able to limit the unfolding of the molecule and thus exposure of surface hydrophobic patches that increase the aggregation propensity of the particle. As the severity of the stress is increased, the molecule further unfolds, exposing hydrophobic areas that can now aggregate into larger particles. Overall, these results suggest that each stress technique is associated with a specific mechanism of protein aggregation, where the end point is dependent on the degree of conformational unfolding as well as the type and severity of the stress that was applied. However, it is important to note that these results reflect the end points of each pathway at the time tested. This includes the results of both equilibrium and irreversible reactions, and the exact size distribution obtained could depend on the time points chosen for analysis.
Unlike the other stress conditions tested, high pH (pH 11) generated aggregates that retained a higher order structure of the molecule that most resembled the folded molecule. This was seen in that the mAb1 pH 11 sample had the highest FTIR CC (71%) to the untreated sample, no significant shift in λmax from the untreated sample, and the same percent ANS binding as the untreated sample (9%). This was also confirmed by the complete reversibility of the particles within minutes of dilution. Consistent with the folded structure being conserved, the size distribution profile of the particles populated the oligomer, nanometer, and micron size ranges. This suggests that this stress condition follows a different pathway than the other conditions examined and affects the colloidal stability to a greater degree than the conformational stability of the protein (43) or sensitivity to interfaces.
Stress-induced oligomers may be potential intermediates on the pathway to particle formation under certain mechanisms of aggregation (30, 44, 45). Oligomers that were generated by moderate stress conditions were no longer detected when the severity of the stress was increased. This was coupled with the simultaneous shift of the particle distribution profile to larger sizes. For example, the mAb1 stir-20h sample had the highest percentage of oligomers (11.1%) of all samples; however, when stirring was prolonged over 3 days, oligomers, monomers, and fragments could not be detected, and the number of small sized micron aggregates (2–10 μm) decreased, although the number of particles greater than or equal to 10 μm increased. The accelerated storage of mAb1 at 37 °C produced oligomers (6%) but few larger particles, whereas incubation of mAb1 at 90 °C resulted in primarily subvisible and visible particle formation but not detectable aggregated oligomers. It appears that the degree of stress, as well as the type of stress, is important in determining the extent of unfolding and aggregation. Oligomers of other proteins have been shown to have locally unfolded hydrophobic patches and have been suggested as intermediates on certain pathways to aggregation (46–48). Conversion of oligomers to larger particles may require a minimum input of energy in the form of stress to overcome the energy barrier to further unfolding and aggregation. Some oligomers may therefore be intermediates on one of the pathways to particle formation under certain types of stress, although others could be natively folded oligomers that are in equilibrium with the monomer and are not able to grow into larger aggregates.
Two different types of syringes (which differed in various properties, including the presence of silicone oil (32, 33)) produced mAb1 aggregates similar in morphology, size, degree of folded structure, reversibility, and percent aggregation, indicating that silicone oil may not have an effect on the type of aggregate formed. In addition, oxidation of methionine in mAb1 was found to be the major chemical modification caused by syringe stress and was comparable between the two types of syringes (41). Interactions at the air-liquid interface due to syringe stress could be one of the dominant contributors to aggregation from this physical manipulation. Syringe stress may therefore follow the same pathway of aggregation irrespective of the presence of silicone oil or perhaps material composition.
Uncertainty surrounding subvisible particles in the 0.1–10 μm range has heightened concerns about their potential risk for immunogenicity (14). The greatest number of mAb1 subvisible particles in the 0.2–10 μm range was found in class 3 and 5 (harsh mechanical stress), followed by class 2 and 6 (metal-catalyzed oxidation, prolonged harsh mechanical stress, and heat/pH). Although the submicron and micron aggregates in the mechanically stressed samples in the presence of silicone oil are artificially high due to the presence of silicone oil droplets themselves, an abundance of protein particles was confirmed by MFI analysis. Limited stirring produced by far the greatest number of subvisible particles, which is in agreement with previous studies that identified stirring as a particularly harsh stress condition (4). Stirring may induce higher particle counts than other mechanically stressed samples tested here due to a unique combination of stresses in this method, including shear (49), interfacial effects with the stirrer bar and glass vial (50, 51), cavitations because of voids/trapped air bubbles (52), continual renewal and interactions of the protein at the air-liquid interface (41), local heating, and rapid movement of aggregated or unfolded species from the surface into the bulk solution because of turbulent flow, to name a few.
Engineered particle arrays not traditionally found in biotherapeutic products have been shown to be immunogenic (7–10, 53). Protein aggregates are suspected of eliciting an immune response due to the clustering of repeating motifs (6, 11, 54); however, the higher order structure of these particles is not known. Our results imply that the internal organization of the protein aggregates generated in this study is somewhat disordered as reflected in visual images, changes in the secondary and tertiary structure, and an increase in the surface hydrophobicity of the molecule; however, the degree of unfolding depends on the type of stress to which the molecule was subjected. The MFI images of protein particles reveal a heterogeneous mixture of particles that appear amorphous in shape. The morphology of individual particles varies depending on the stress condition. For example, stirring creates particles that appear more compact and dense than heating, which produced particles that appear far more complex, branched, and translucent. FTIR analysis revealed that a CC of less than 50% was seen between the mAb1 untreated protein and all stressed samples (except pH 11), indicating a general change in conformation from a folded β-sheet to an unfolded or amorphous β-sheet structure within the particles. Tertiary structural changes in all of the mAb1 aggregate pellets (except pH 11) were demonstrated by intrinsic fluorescence as a shift in the λmax (≥2 nm). It is important to note that additional components may exist with a different Trp exposure but are too dilute to be reflected in the overall λmax. Light scattering due to particles may also have an effect on the λmax (55); however, we have minimized these effects as other groups have done previously (56), and we believe that the overall trends are accurate. An increase in surface hydrophobicity of the stressed samples was demonstrated by ANS and SYPRO Orange binding. ANS and SYPRO Orange act as general probes of the hydrophobic surface of the protein that might be exposed to changes in conformation and unfolding (37, 57), by fluorescing when bound to hydrophobic surfaces. An increase in surface hydrophobicity was found in all of the mAb1 particle pellets; however, the protein that remained in solution also displayed hydrophobic exposure in extreme cases, such as heat treatment. Dramatic changes in the protein structure reported here would be unlikely to support the sort of rigid multimeric epitope presentation needed to break B-cell tolerance (53), such as regular spacing of antigens at 5–10 nm (58, 59); however, the ability of ordered or semi-ordered aggregates to induce an immune response is not known.
Reversibility of protein aggregate formation has been described as the dissociation of protein particles upon reversal of aggregate-generating conditions (45). Upon dilution of aggregates into a pH 5 buffer (the original storage solution conditions for these proteins prior to stress), some members of class 3, 5, and 6 (pH 11 and stirring) showed substantial reversibility, followed by other members of class 2 and 3 (metal-catalyzed oxidation and H2O2 treatment), suggesting that a particulate population exists in each of these groups that is at an early aggregation stage and can disaggregate. Although the particle count decreases over time, it is unclear if the molecules are reverting to the monomeric state or to smaller aggregates below the range that can be detected by the instrument used. Class 7 particles (≥20 μm) appeared irreversible, as is often the case for aggregates created under thermal stress, and may indicate that particles are at a later aggregation stage. Aggregation was also shown to initially increase in some cases upon dilution in a neutral pH buffer, suggesting that aggregation could temporarily increase when introduced in vivo. Overall, the reduction in the number of particles over time may ease concerns about their effect on the immune system.
Hydrostatic pressure is a useful tool for studying the mechanisms of protein misfolding and aggregate formation (60–63). High pressure may cause aggregates to form reversibly, permitting the kinetic process of protein aggregation to be assessed (61). This technique can also be used to stabilize folding intermediates allowing for the characterization of their structure and dynamics (62, 63). Characterization of pressure-induced antibody aggregates will be necessary to confirm their placement in the classification scheme; however, their partially folded conformation (62) and their partial reversibility (61) suggest that they will most likely fall into class 3 or class 5. Further experiments that use hydrostatic pressure could prove beneficial for probing the reversibility and mechanism of aggregation of the different IgG molecules described here.
Previous reports on the immunogenicity of aggregates used metal-aggregated IFN-α to break the tolerance of transgenic mice (9, 15–17). These artificially induced aggregates were generated with excess copper well beyond what would be expected during manufacturing. Here, metal-catalyzed aggregates of mAb1 and mAb4, prepared in a similar manner, were shown to contain high levels of copper in at least a 12-fold excess of molecules of metal to protein. Metals are a well known adjuvant that in the presence of protein antigens can enhance the response by the immune system (64). Adjuvants can attract antigen-presenting cells and enhance their activation, which can lead to an increase in the helper T-cell response and result in B-cell activation and antibody production. The presence of both protein aggregates and an excess of copper would naturally confound any result showing a breakage of tolerance in a mouse model system. These ICP-MS results indicate that even after overnight dialysis in the presence of a chelating agent, high copper levels were retained, and this could contribute to the enhanced immunogenicity previously seen with metal-catalyzed aggregates.
In conclusion, classification of aggregate groups could help to develop protein formulation strategies as well as to identify the aggregate classes and attributes that pose problems in terms of quality and safety. Some aggregate components may raise concern because of their small size, high subvisible particle concentration, semi-ordered conformation, and production techniques that most resemble the manufacturing process. Their tendency toward reversibility, however, may make their presence less of a concern in a physiological environment. Classification of the particles into these seven categories can be used to design further experiments to test for biological effects of these aggregates, such as safety due to immunogenicity and/or decreased or enhanced activity. The classification scheme could also be used as a forensic aid to identify aggregates when only a few aggregate properties are known as well as to decipher the mechanism of aggregate formation. Future experiments that test the immunogenicity of these protein aggregate classes in vitro and in vivo will be essential for ascertaining the risk of particles in a therapeutic setting.
Supplementary Material
Acknowledgments
We thank Steve Brych, Nancy Jiao, Cynthia Li, Ranjini Ramachander, and Riki Stevenson for scientific assistance and David Brems and Izydor Apostol for their support on this project.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- USP
- United States Pharmacopeia
- H2O2
- hydrogen peroxide
- ICP-MS
- inductively coupled plasma-mass spectrometry
- MFI
- micro-flow imaging
- NTA
- nanoparticle tracking analysis
- so
- silicone oil; cross-linking
- HMW
- high molecular weight
- LMW
- low molecular weight
- Main
- main peak
- ANS
- 1-anilinonaphthalene-8-sulfonate
- CC
- correlation coefficient
- IVIG
- intravenous human IgG
- SE-HPLC
- size-exclusion-HPLC
- QC
- quality control.
REFERENCES
- 1. Kessler M., Goldsmith D., Schellekens H. (2006) Nephrol. Dial. Transplant. 21, Suppl. 5, 9–12 [DOI] [PubMed] [Google Scholar]
- 2. Seaver S. (2008) Bioquality 13, 3–4 [Google Scholar]
- 3. Arvinte T. (2007) BioWorld Europe 1, 6–9 [Google Scholar]
- 4. Kiese S., Papppenberger A., Friess W., Mahler H. C. (2008) J. Pharm. Sci. 97, 4347–4366 [DOI] [PubMed] [Google Scholar]
- 5. Demeule B., Gurny R., Arvinte T. (2006) Eur. J. Pharm. Biopharm. 62, 121–130 [DOI] [PubMed] [Google Scholar]
- 6. Rosenberg A. (2009) 13th Symposium of the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products, January 12–14, 2009, p. 23, California Separation Science Society and Food and Drug Administration, San Francisco [Google Scholar]
- 7. Denis J., Majeau N., Acosta-Ramirez E., Savard C., Bedard M. C., Simard S., Lecours K., Bolduc M., Pare C., Willems B., Shoukry N., Tessier P., Lacasse P., Lamarre A., Lapointe R., Lopez Macias C., Leclerc D. (2007) Virology 363, 59–68 [DOI] [PubMed] [Google Scholar]
- 8. Morefield G. L., Sokolovska A., Jiang D., HogenEsch H., Robinson J. P., Hem S. L. (2005) Vaccine 23, 1588–1595 [DOI] [PubMed] [Google Scholar]
- 9. Braun A., Kwee L., Labow M. A., Alsenz J. (1997) Pharm. Res. 14, 1472–1478 [DOI] [PubMed] [Google Scholar]
- 10. Fifis T., Gamvrellis A., Crimeen-Irwin B., Pietersz G. A., Li J., Mottram P. L., McKenzie I. F., Plebanski M. (2004) J. Immunol. 173, 3148–3154 [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg A. S. (2006) AAPS J. 8, E501–E507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carpenter J. F., Randolph T. W., Jiskoot W., Crommelin D. J., Middaugh C. R., Winter G., Fan Y. X., Kirshner S., Verthelyi D., Kozlowski S., Clouse K. A., Swann P. G., Rosenberg A., Cherney B. (2009) J. Pharm. Sci. 98, 1201–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C. T., Sharma D., Oma P., Krishnamurthy R. (2009) J. Pharm. Sci. 98, 3058–3071 [DOI] [PubMed] [Google Scholar]
- 14. Singh S. K., Afonina N., Awwad M., Bechtold-Peters K., Blue J. T., Chou D., Cromwell M., Krause H. J., Mahler H. C., Meyer B. K., Narhi L., Nesta D. P., Spitznagel T. (2010) J. Pharm. Sci. 99, 3302–3321 [DOI] [PubMed] [Google Scholar]
- 15. Maas C., Hermeling S., Bouma B., Jiskoot W., Gebbink M. F. (2007) J. Biol. Chem. 282, 2229–2236 [DOI] [PubMed] [Google Scholar]
- 16. Hermeling S., Aranha L., Damen J. M., Slijper M., Schellekens H., Crommelin D. J., Jiskoot W. (2005) Pharm. Res. 22, 1997–2006 [DOI] [PubMed] [Google Scholar]
- 17. Hermeling S., Schellekens H., Maas C., Gebbink M. F., Crommelin D. J., Jiskoot W. (2006) J. Pharm. Sci. 95, 1084–1096 [DOI] [PubMed] [Google Scholar]
- 18. Reipert B. M., van Helden P. M., van den, Helden P. M., Schwarz H. P., Hausl C. (2007) Br. J. Haematol. 136, 12–25 [DOI] [PubMed] [Google Scholar]
- 19. Purohit V. S., Middaugh C. R., Balasubramanian S. V. (2006) J. Pharm. Sci. 95, 358–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fradkin A. H., Carpenter J. F., Randolph T. W. (2009) J. Pharm. Sci. 98, 3247–3264 [DOI] [PubMed] [Google Scholar]
- 21. United States Pharmacopeia (2010) Particulate Matter in Injections, United States Pharmacopeia-National Formulary, USP 34-NF, 29 ed, United States Pharmacopeia, Rockville, MD [Google Scholar]
- 22. European Directorate for the Quality of Medicines and Healthcare (2010) Particulate Contamination, Sub-visible Particles, 7.1 ed, European Pharmacopeia Commission, Council of Europe [Google Scholar]
- 23. United States Pharmacopeia (2010) Visual Inspection, United States Pharmacopeia-National Formulary, USP 34-NF, 29 ed, United States Pharmacopeia, Rockville, MD [Google Scholar]
- 24. European Directorate for the Quality of Medicines and Healthcare (2010) Particulate Contamination, Visible Particles, 7.1 ed, European Pharmacopeia Commission, Council of Europe [Google Scholar]
- 25. Krishnan S., Raibekas A. A. (2009) Biophys. J. 96, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen S., Lau H., Brodsky Y., Kleemann G. R., Latypov R. F. (2010) Protein Sci. 19, 1191–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fesinmeyer R. M., Hogan S., Saluja A., Brych S. R., Kras E., Narhi L. O., Brems D. N., Gokarn Y. R. (2009) Pharm. Res. 26, 903–913 [DOI] [PubMed] [Google Scholar]
- 28. Hari S. B., Lau H., Razinkov V. I., Chen S., Latypov R. F. (2010) Biochemistry 49, 9328–9338 [DOI] [PubMed] [Google Scholar]
- 29. Jiang Y., Nashed-Samuel Y., Li C., Liu W., Pollastrini J., Mallard D., Wen Z. Q., Fujimori K., Pallitto M., Donahue L., Chu G., Torraca G., Vance A., Mire-Sluis T., Freund E., Davis J., Narhi L. (2009) J. Pharm. Sci. 98, 4695–4710 [DOI] [PubMed] [Google Scholar]
- 30. Mahler H. C., Friess W., Grauschopf U., Kiese S. (2009) J. Pharm. Sci. 98, 2909–2934 [DOI] [PubMed] [Google Scholar]
- 31. Hawe A., Kasper J. C., Friess W., Jiskoot W. (2009) Eur. J. Pharm. Sci. 38, 79–87 [DOI] [PubMed] [Google Scholar]
- 32. Eakins M. N. (2010) Pharmaceutical Outsourcing 11, 10–14 [Google Scholar]
- 33. Lahendro B. (2008) OnDrugDelivery, 24–26 [Google Scholar]
- 34. European Pharmacopeia 5.0 (2004) Interferon alpha 2b, Vol. 2, 1812–1815, European Pharmacopeia [Google Scholar]
- 35. Cover T. M., Hart P. E. (1967) IEEE Transactions on Information Theory, 13, 21–27 [Google Scholar]
- 36. Jiang Y., Li C., Nguyen X., Muzammil S., Towers E., Gabrielson J., Narhi L. (2011) J. Pharm. Sci., in press [DOI] [PubMed] [Google Scholar]
- 37. He F., Phan D. H., Hogan S., Bailey R., Becker G. W., Narhi L. O., Razinkov V. I. (2010) J. Pharm. Sci. 99, 2598–2608 [DOI] [PubMed] [Google Scholar]
- 38. Narhi L. O., Jiang Y., Cao S., Benedek K., Shnek D. (2009) Curr. Pharm. Biotechnol. 10, 373–381 [DOI] [PubMed] [Google Scholar]
- 39. Filipe V., Hawe A., Jiskoot W. (2010) Pharm. Res. 27, 796–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knapp J. Z. (2000) PDA J. Pharm. Sci. Technol. 54, 218–232 [PubMed] [Google Scholar]
- 41. Luo Q., Joubert M. K., Stevenson R., Narhi L. O., Wypych J. (2011) J. Biol. Chem. 286, 25134–25144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krishnan S., Chi E. Y., Wood S. J., Kendrick B. S., Li C., Garzon-Rodriguez W., Wypych J., Randolph T. W., Narhi L. O., Biere A. L., Citron M., Carpenter J. F. (2003) Biochemistry 42, 829–837 [DOI] [PubMed] [Google Scholar]
- 43. Valente J. J., Payne R. W., Manning M. C., Wilson W. W., Henry C. S. (2005) Curr. Pharm. Biotechnol. 6, 427–436 [DOI] [PubMed] [Google Scholar]
- 44. Morris A. M., Watzky M. A., Finke R. G. (2009) Biochim. Biophys. Acta 1794, 375–397 [DOI] [PubMed] [Google Scholar]
- 45. Wang W., Nema S., Teagarden D. (2010) Int. J. Pharm. 390, 89–99 [DOI] [PubMed] [Google Scholar]
- 46. Frare E., Mossuto M. F., de Laureto P. P., Tolin S., Menzer L., Dumoulin M., Dobson C. M., Fontana A. (2009) J. Mol. Biol. 387, 17–27 [DOI] [PubMed] [Google Scholar]
- 47. Juárez J., Taboada P., Mosquera V. (2009) Biophys. J. 96, 2353–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindgren M., Sörgjerd K., Hammarström P. (2005) Biophys. J. 88, 4200–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li C. H., Li T. (2009) Curr. Pharm. Biotechnol. 10, 391–399 [DOI] [PubMed] [Google Scholar]
- 50. Chang S. H., Chen L. Y., Chen W. Y. (2005) Colloids Surf. 41, 1–6 [DOI] [PubMed] [Google Scholar]
- 51. Baszkin A., Boissonnade M. M., Kamyshny A., Magdassi S. (2001) J. Colloid Interface Sci. 239, 1–9 [DOI] [PubMed] [Google Scholar]
- 52. Gülseren I., Güzey D., Bruce B. D., Weiss J. (2007) Ultrason. Sonochem. 14, 173–183 [DOI] [PubMed] [Google Scholar]
- 53. Bachmann M. F., Zinkernagel R. M. (1997) Annu. Rev. Immunol. 15, 235–270 [DOI] [PubMed] [Google Scholar]
- 54. De Groot A. S., Scott D. W. (2007) Trends Immunol. 28, 482–490 [DOI] [PubMed] [Google Scholar]
- 55. Carpenter M. L., Oliver A. W., Kneale G. G. (2001) Methods Mol. Biol. 148, 491–502 [DOI] [PubMed] [Google Scholar]
- 56. Souillac P. O. (2005) J. Pharm. Sci. 94, 2069–2083 [DOI] [PubMed] [Google Scholar]
- 57. Hawe A., Sutter M., Jiskoot W. (2008) Pharm. Res. 25, 1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dintzis H. M., Dintzis R. Z., Vogelstein B. (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 3671–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chackerian B., Lenz P., Lowy D. R., Schiller J. T. (2002) J. Immunol. 169, 6120–6126 [DOI] [PubMed] [Google Scholar]
- 60. Foguel D., Silva J. L. (2004) Biochemistry 43, 11361–11370 [DOI] [PubMed] [Google Scholar]
- 61. Randolph T. W., Seefeldt M., Carpenter J. F. (2002) Biochim. Biophys. Acta 1595, 224–234 [DOI] [PubMed] [Google Scholar]
- 62. Silva J. L., Cordeiro Y., Foguel D. (2006) Biochim. Biophys. Acta 1764, 443–451 [DOI] [PubMed] [Google Scholar]
- 63. Silva J. L., Foguel D., Royer C. A. (2001) Trends Biochem. Sci. 26, 612–618 [DOI] [PubMed] [Google Scholar]
- 64. Friede M., Aguado M. T. (2005) Adv. Drug Deliv. Rev. 57, 325–331 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.