Abstract
Huntington disease (HD) is an autosomal inherited disorder that causes the deterioration of brain cells. The polyglutamine (polyQ) expansion of huntingtin (Htt) is implicated in the pathogenesis of HD via interaction with an RNA splicing factor, Htt yeast two-hybrid protein A/forming-binding protein 11 (HYPA/FBP11). Besides the pathogenic polyQ expansion, Htt also contains a proline-rich region (PRR) located exactly in the C terminus to the polyQ tract. However, how the polyQ expansion influences the PRR-mediated protein interaction and how this abnormal interaction leads to the biological consequence remain elusive. Our NMR structural analysis indicates that the PRR motif of Htt cooperatively interacts with the tandem WW domains of HYPA through domain chaperoning effect of WW1 on WW2. The polyQ-expanded Htt sequesters HYPA to the cytosolic location and then significantly reduces the efficiency of pre-mRNA splicing. We propose that the toxic gain-of-function of the polyQ-expanded Htt that causes dysfunction of cellular RNA processing contributes to the pathogenesis of HD.
Keywords: Huntington Disease, NMR, Protein Structure, Protein-Protein Interactions, RNA Processing, RNA Splicing, HYPA/FBP11, Huntingtin, PolyQ Expansion, Tandem WW Domains
Introduction
Huntington disease (HD)2 is a dominantly inherited disorder caused by the expansion of polyglutamine (polyQ) in exon 1 of the huntingtin (Htt) protein (1). The involvement of the exon 1 of Htt gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mouse model R6/2 (2). The normal Htt exon 1 consists of a polyQ repeat of some 20 glutamines and a proline-rich region (PRR) of 40 amino acid residues and also has an additional 17 residues in the N terminus and 13 residues in the C terminus. Numerous proteins have been reported to interact with Htt (3, 4). These Htt-interacting proteins are engaged in transcription, trafficking, endocytosis, signaling, and metabolism, in which many proteins interact with Htt through the PRR region (4). This is well consistent with a recent study on the intrabody gene therapy (5). The intrabody treatment toward PRR of Htt confers significantly beneficial effects in different kinds of mouse models, whereas that toward the N-terminal 17 residues does not exhibit any benefit.
The WW domain-containing proteins, such as Htt yeast two-hybrid proteins (HYPA, HYPB, and HYPC), interact with Htt protein through its PRR region, and these meet the genetic criteria for direct involvement in HD pathology (6). HYPA, a human homolog of mouse forming-binding protein 11 (FBP11), is a component of mammalian mRNA splicing U1 complex (7) and is able to enhance the splicing efficiency in mammalian cells (8). Actually, the HYPA level was also found to be significantly reduced in the stratum of mouse HD model (6). Furthermore, mutation of the splicing factors or change in their stoichiometry can play important roles in diseases, such as Alzheimer disease and amyotrophic lateral sclerosis (9, 10). Thus, interaction between Htt and HYPA might be associated with the pathogenesis of HD. The proline-rich motif that is included in the large Htt protein belongs to a particularly abundant group of molecular recognition domains that can interact with SH3, WW, EVH1 domains, and so on (11, 12). Usually these modular domains recognize short linear proline-rich motifs of not more than 10 residues (11, 12). The PRR motif of Htt consists of almost 40 amino acid residues, whereas HYPA contains two WW domains in a tandem array (see Fig. 1A). This is reminiscent of a complicated mechanism underlying their specific interactions that may consequently impact on the cellular RNA processing.
FIGURE 1.
The tandem WW domains of HYPA have an interdomain chaperoning effect and enhanced interaction with the N-terminal Htt. A, shown are schematic architectures of the N-terminal fragment of Htt18Q (1–171), the N terminus of HYPA (1–423), and the mouse homolog of HYPA, FBP11. The fragment of HYPA also includes a nuclear localization signal (NLS) sequence. B, GST pulldown and Western blotting experiments for the interactions of various WW domains with Htt18Q are shown. The SDS-PAGE with Coomassie staining shows the loading of GST and GST fusion proteins (lower). C, GST shown are pulldown and Western blotting experiments for the interactions of various WW domains with Htt100Q. The Htt100Q protein shows a smear in the gels. D, shown is an overlay plot of the 15N,1H HSQC spectra of 2WW (red), WW1 (cyan), and WW2 (orange). The proteins (∼200 μm) were dissolved in 10 mm sodium phosphate buffer at pH 6.0 for NMR experiments.
Our previous work elucidated the specific binding of Htt-PRR with SH3 and WW domains (13). However, how the expanded polyQ tract influences the interaction of Htt with HYPA and consequently impacts on the cellular function of HYPA are largely unknown. In this report, we applied structural and molecular biology approaches to study the interaction between PRR motif of Htt and the tandem WW domains of HYPA and the effect of polyQ expansion on the function of HYPA in cells. We propose that the toxic gain-of-function of the Htt mutants that causes dysfunction of cellular RNA processing is likely an alternative pathogenic way for HD.
EXPERIMENTAL PROCEDURES
Construction of Plasmids
The cDNA encoding for the N-terminal 171 residues of Htt was cloned into a vector of pcDNA3.0 with a FLAG tag in the N terminus. The wild type and mutant Htt contains 18 and 100 repeated Gln residues denoted as Htt18Q and Htt100Q, respectively. The PRR-deleted mutant Htt100QΔP was cloned by ligation of N-terminal and C-terminal Htt100Q. Although many efforts tried, we have not got the clone of full-length HYPA. To mimic the full-length protein, we cloned the DNA fragments for the N-terminal residues 1–423 (6) and the nuclear localization signal sequence (residues 872–879, KKKSKKRR) into pcDNA3.1 vector (with Myc/His tag). The mouse FBP11 gene was from Fulengen. The cDNAs for the tandem WW domains (residues 91–178, and renumbered as 3–90) and the two single domains (3–43, 52–84) were subcloned into pGBTNH (14) and pGEX-4T-3. Mutation of PYYY to TYYY in WW2 was produced according to the corresponding sequence of WW1 and denoted as P67T for the sake of structural analysis. Several mutants in WW1 and WW2 domains or the linker regions were produced and named as W38A, W79A, W38A/W79A, GS-Sub, and Su(dx)-Linker, respectively.
Peptide Synthesis and Protein Purification
The peptides corresponding to the sequence of Htt PRR (PP1, PP3, PP12, PP23, and PP35) were obtained from solid-phase synthesis and analyzed by electrospray mass spectrometry. The plasmids encoding proteins were transformed to Escherichia coli BL21 (DE3). The proteins were purified by Ni2+-NTA affinity column followed by gel filtration chromatography. 15N/13C-Labeled proteins were prepared by using the M9 minimal medium containing 15NH4Cl and/or d-[13C6]glucose as the sole nitrogen and/or carbon resource, respectively.
NMR Spectroscopy and Structure Determination
The sample containing ∼1 mm 15N/13C-labeled 2WW-P67T protein in a phosphate buffer (10 mm, pH 6.0) was used for the structural determination by NMR as our previous study (13). All data acquisition was carried out at 25 °C on a Varian INOVA 600 spectrometer. For the backbone and side-chain assignments, spectra of HNCO, HNCACB, CBCA(CO)NH, H(CCO)NH, C(CO)NH, and HCCH-TOCSY were obtained. NOE restraints were obtained from 15N- and 13C-edited NOE spectra. The NMR data were processed by using NMRPipe (15) and analyzed with SPARKY (16). Backbone dihedral restraints were derived from TALOS program (17). Hydrogen bond restraints for the backbone consistent with NOE patterns were also added. The structures were calculated using ARIA2.0 (18), assessed by PROCHECK (19), and displayed by MOLMOL (20). 15N relaxation data were acquired using the standard pulse sequences (21).
Cell Culture and Immunofluorescence Microscopy
HEK 293T cells were transfected by FuGENE HD transfection reagent (Roche Applied Science) following the manufacturer's instructions. Cells were harvested 48 h after transfection. For fractionating the cytosolic and nuclear proteins, procedures were strictly followed according to the instruction manual (Beyond Time). The antibodies used were as follows: anti-FLAG (Sigma) and anti-Myc (Cell signaling) antibodies, mouse polyclonal anti-HYPA antibody (Abnova), and mouse polyclonal anti-tubulin (Sigma) or rabbit polyclonal anti-SP1 antibody. For confocal microscopy, HEK 293T cells were grown on glass coverslips for 48 h after transfection. Images were obtained on a Leica TCS SP2 confocal microscope (Leica Microsystems).
Pre-mRNA Splicing Efficiency and Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Alternative Splicing Assays
Pre-mRNA splicing was assayed by recording the luciferase and β-galactosidase activities accordingly (22, 23). Cells were lysed at 48 h after transfection, and the activities of luciferase and β-galactosidase on hydrolyzing the substrates (Promega) were measured using GLOMAX Luminometer (Promega). HEK 293T cells were transfected with the reporter plasmid containing minigene TG13T5 (23). The cells were harvested at 48 h after transfection, and the total RNA was extracted with TRIzol reagent (Invitrogen). cDNA was synthesized by retro-transcription from 1 μg of total RNA with ReverTra Ace-α (TOYOBO). Secondary PCR was carried out by using the following primers: Bra-2, 5′-TAGGATCCGGTCACCAGGAAGTTGGTTAAATCA-3′; a2-3, 5′-CAACTTCAAGCTCCTAAGCCACTGC-3′. For small RNA-mediated interference, the sequences of the small RNAs were as follows: siHYPA1, 5′-GAAGAGUGCACCACAACAUTT-3′; siHYPA2, 5′-GUGUGGAAGUAUCCAGUAATT-3′; control siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′.
RESULTS
Cooperative Interaction of the Tandem WW Domains of HYPA with the PRR Motif of Htt
It was reported that Htt interacts with HYPA through the N-terminal PRR and the tandem WW domains (Fig. 1A) (24). Our previous work identified that the first and third portions of Htt PRR specifically interact with the tandem WW domains (13). To better understand how the tandem WW domains of HYPA interact with the Htt PRR motif, we used GST pulldown experiments to characterize the interactions between N-terminal Htt and different GST-fused WW domains (Fig. 1B). Although not strictly quantitative, GST pulldown consistently shows that the tandem WW domains (2WW) interact with the N-terminal fragment (residues 1–171) of normal Htt (Htt18Q) stronger than the individual WW1 and WW2 domains, whereas the binding of WW2 with Htt18Q is weaker than that of WW1. A similar result is also obtained from the polyQ-expanded Htt (Htt100Q), which shows stronger binding affinity with 2WW than the single WW domains (Fig. 1C). Interestingly, only Htt100Q but not Htt18Q can be precipitated by HYPA in a co-immunoprecipitation experiment (supplemental Fig. S1A), although both Htt18Q and Htt100Q exhibit interaction with HYPA in the GST pulldown (supplemental Fig. S1B). This inconsistency is probably due to the different sensitivities of these two biochemical techniques under different experimental conditions. We further characterize the structural properties of different WW domains by NMR spectroscopy. The WW1 domain shows a wide dispersion of chemical shifts in both 1H and 15N dimensions of the HSQC spectrum (supplemental Fig. S1C), indicating that WW1 forms a well folded structure. However, the HSQC spectrum of the WW2 domain exhibits few resonance peaks and narrow dispersion of the chemical shifts (supplemental Fig. S1D), suggesting that WW2 is unstructured in solution. The unfolded WW2 domain may be the major cause for its weak binding with Htt18Q (Fig. 1B). We recorded the HSQC spectrum of 2WW under the same conditions used for WW1 and WW2. The spectrum shows wide chemical-shift dispersion and relatively sharp line widths of the resonances, indicating that both domain moieties in 2WW are well folded in solution (supplemental Fig. S1E). The overlay plot of the three spectra shows that most resonances of WW2 do not overlap with those in 2WW (Fig. 1D). This indicates that the WW1 domain may have a chaperoning effect on WW2, which means folding of WW1 is beneficial to the folding of the WW2 moiety. This chaperoning effect of WW1 on WW2 may enhance the interaction of 2WW with Htt18Q, as compared with single WW1 and WW2. Thus, the domain chaperoning effect provides implication that the tandem WW domains of HYPA cooperatively interact with the N-terminal fragment of Htt.
Solution Structure of the Tandem WW Domains of HYPA
WW domains are small protein modules composed of ∼40 amino acid residues (25). For structural analysis, we made a mutation of Pro-67 in WW2 to Thr residue, which leads to improvement of the NMR spectra in the WW2 moiety (supplemental Fig. S2A) but no considerable difference of the chemical shifts in 2WW (supplemental Fig. S2B). Thus, the mutation is considered to have little effect on both its structure and binding ability (see below). We elucidated the solution structure of the tandem WW domains (2WW-P67T) by NMR techniques (a summary of the experimental restraints and structural model statistics is shown in supplemental Table S1). Fig. 2A shows the superposition of 10 lowest-energy structures of 2WW-P67T. Each WW domain folds as a triple-stranded antiparallel β-sheet and is connected by a short α-helical linker (Fig. 2B). The structure was refined very well by using only short-range restraints as NOEs, hydrogen bonds, and dihedral angles (supplemental Table S1). This structure is somehow similar to that of its yeast homologue Prp40 (26), which was elucidated based on residual dipolar coupling (RDC) restraints besides NOEs. Especially, the structure includes a rigid helical linker that is close to WW2, implying that the helix contributes to the chaperoning effect on the folding of WW2. This structure is different from those of the tandem WW domains of another splicing factor FBP21 (supplemental Fig. S2D) (27) and the Notch signaling suppressor Su(dx) (supplemental Fig. S2E) (28); both structures contain disordered linkers. Compared with the tandem WW domains of Prp40, that has a long helical linker (supplemental Fig. S2F), 2WW of HYPA contains a shorter α-helical linker, and the binding pockets of the WW domains point toward each other. The heteronuclear 15N-{1H} NOEs provides further evidence that 2WW of HYPA tumbles as a unit fold due to the similar motions of the linker region with the two domains (supplemental Fig. S2C). In comparison of the WW1 and WW2 units in 2WW of HYPA, it gives a backbone r.m.s.d. of 1.88 Å (supplemental Fig. S2G), whereas the backbone r.m.s.d. is up to 1.9 Å when comparing the structure of WW1 unit in 2WW with that of the isolated WW1 domain from this protein (Fig. 2C) (29).
FIGURE 2.
Solution structure of the tandem WW domains of HYPA as determined by NMR spectroscopy. A, backbone superposition of the 10 lowest-energy structures of 2WW is shown. B, shown is a ribbon representation of the structure of 2WW. C, a comparison of the three-dimensional structures of WW1 in the context of 2WW (cyan) and the isolated WW1 (PDB ID 1YWJ, purple) is shown. The backbone traces of the structures in two types of WW1 are superimposed. D, fluorescence titration for the binding of the 2WW mutants with different interdomain linkers with DNS-labeled PRR (Htt-PP35) is shown. black, P67T; red, GS-Sub; blue, Su(dx)-Linker. The sequences with different linkers are shown below the titration curves.
Role of the Helical Linker of 2WW in Interaction with Proline-rich Peptides
Because the structure of the linker region between the two WW domains in HYPA is significantly different from any known structures of tandem WW domains (26–28), we focused on the linker region by substitution with other sequences to investigate the influence on the interaction with a 35-residue PRR peptide of Htt (Htt-PP35). Substitution with DSGGS (GS-Sub) is designed to destroy the helical linker, whereas substitution with the linker from Su(dx) (Su(dx)-Linker) is to insert an extended region. By using fluorescence titration with dansyl chloride (DNS)-labeled Htt-35 peptide (Fig. 2D), both the linker mutations present decreases of the binding affinities with Htt-PP35 (Table 1). This demonstrates that the helical linker plays an important role in cooperative interaction with Htt PRR, and the two WW domains of HYPA act as an ensemble structurally for the specific interaction.
TABLE 1.
Binding affinities (KD) of 2WW and its mutants with Htt-PP35 peptide as determined by fluorescence titration
| WT | W38A | W79A | P67T | GS-Suba | Su(dx)-Linkera | |
|---|---|---|---|---|---|---|
| KD (μm) | 10.6 ± 2.8 | 51.6 ± 14.9 | 524 ± 107 | 29.3 ± 5.3 | 83.4 ± 11.1 | 94.0 ± 6.1 |
a The linker substitution mutants were generated based on the P67T mutant of 2WW.
The Tandem WW Domains of HYPA Cooperatively Interacts with Htt PRR
In general, WW domains recognize short proline-rich motifs typically of not more than 10 amino acid residues (11, 12). However, the PRR motif of Htt contains almost 40 residues. Thus, the experiment is designed to understand how 2WW interacts with the long PRR motif. By using NMR and fluorescence titrations, we elucidated the interaction between the tandem WW domains of HYPA and Htt PRR. Because the middle portion of Htt PRR does not contribute to bind with the 2WW domains (13), we performed chemical shift perturbation on the interactions of the 15N-labeled 2WW with several peptides based on the first and third portions (Fig. 3A). As in the literatures (11, 25), Htt-PP1 and Htt-PP3 bind to both WW domains on the hydrophobic sites formed by Thr-17/Lys-58, Glu-18/59, His-19/Tyr-60, Tyr-27/69, Tyr-28/70, Ser-36/77, and Trp-38/79 (Fig. 3, B and C). These residues show large chemical shift changes, but the linker residues remain unperturbed. Besides the large chemical shift changes in the domain regions, Htt-PP35 titration also causes dramatic chemical shift changes in the linker region (residues 42–53), suggesting that the linker region is involved in the interaction (Fig. 3D). The elongated peptides Htt-PP12 and Htt-PP23 can also cause similar effects on the linker (supplemental Fig. S3). Accordingly, we speculate that the tandem WW domains of HYPA interact with full-length Htt-PRR in a bipartite manner. We also determined the dissociation constants of different WW domains with different peptides by NMR titration (Table 2). During Htt-PP35 titration, the chemical shift changes of the corresponding residues in both WW1 and WW2 moieties are almost the same (Fig. 3D). Based on the titration curves, we calculated the binding affinities with a stoichiometry of 1:2 for the binding of 2WW with Htt-PP1 or Htt-PP3. In the tandem array, the WW2 moiety has increased affinities with Htt-PP1 and Htt-PP3, respectively, whereas WW1 does not (Table 2). This provides further evidence for the interdomain chaperoning effect between WW1 and WW2. Moreover, the affinity for 2WW binding with Htt-PP35 is dramatically increased (Table 1). Comparison of the binding affinities of W38A and W79A mutants with wild-type 2WW suggests a more important role of the WW2 moiety in tandem WW domains. Taken together, these results further demonstrate that the tandem WW domains of HYPA cooperatively interact with Htt PRR in a bipartite manner; that is, both two WW domains of HYPA and two peptide segments of Htt PPR are all engaged in the cooperative interaction.
FIGURE 3.
Chemical shift perturbation analysis of the interactions between the tandem WW domains and various PRR peptides. A, the PRR sequence of Htt shows the synthetic PRR peptides for the titration. Htt-PP35, Htt PRR peptide with 35 residues; Htt-PP1, the first portion of Htt PRR sequence; Htt-PP3, the third portion of Htt PRR sequence. B, shown is a diagram of the chemical shift changes (Δδ) of 2WW upon titration with Htt-PP1 against residue number. The horizontal line denotes the mean of chemical shift changes. C, as in B, titration with Htt-PP3. D, as in B, titration with Htt-PP35. The negative data represent the disappearance of the resonance peaks due to line broadening. All the titrations are in the peptide/2WW molar ratios of 8:1.
TABLE 2.
Binding affinities (KD) of the WW domains with various Htt-PRR peptides as determined by NMR titration
| KD (μm) | PP1 | PP3 | PP12 | PP23 | PP35 |
|---|---|---|---|---|---|
| WW1 | 683 ± 110 | 282 ± 77 | |||
| WW2 (P67T) | 1661 ± 319 | 1435 ± 216 | |||
| 2WW (P67T) | 772 ± 219 | 236 ± 131 | 308 ± 167 | 185 ± 45 | ∼10 |
PolyQ Expansion Alters the Interaction of Htt with HYPA and Cellular Distribution of HYPA
Expanded polyQ tract can cause Htt to interact abnormally with a variety of proteins to gain its toxic function (4). The WW domain-containing proteins may be also engaged in the disease pathogenesis (6). To gain insight into the effects of the polyQ expansion on HYPA interaction and cellular distribution, we performed co-immunoprecipitation and microscopic experiments. As a control, HYPA cannot be precipitated by normal Htt18Q protein (Fig. 4A), but a different situation occurs in the case of the isolated 2WW (Fig. 1B). However, HYPA can be precipitated by polyQ-expanded Htt100Q (Fig. 4A). Compared with wild-type HYPA, the amounts of HYPA mutants in the 2WW region precipitated by Htt100Q are reduced, in the order W38A, W79A, and W38A/W79A. This result suggests that in the context of HYPA, the tandem WW domains can access to the full-length PRR motif in the polyQ-expanded form of Htt in a bipartite manner. Normally, HYPA is localized in nuclear speckle (30), whereas Htt18Q is dominantly localized in cytosol and the polyQ-expanded Htt100Q is localized both in cytosol and nucleus (31). To directly observe the co-localization of Htt and HYPA in cells, we applied confocal fluorescence microscopy imaging (Fig. 4B). In most HEK 293T cells overexpressed with Htt18Q and HYPA, as usual, Htt18Q is mostly localized in cytosol, whereas HYPA is in the nucleus (first row). To our surprise, when Htt100Q overexpressed with HYPA, these two proteins are co-localized mostly in the cytosol (second row), suggesting that HYPA is sequestered to the cytosol by Htt100Q but not by Htt18Q. Abnormal Htt100Q can also sequester W38A and W79A mutants of HYPA to the cytosol but to less extent (Fig. 4, B and C). To further examine whether Htt100Q can redistribute endogenous HYPA, we prepared nuclear and cytosolic extracts from HEK 293T cells transfected with mock vector, Htt18Q, or Htt100Q (Fig. 4D). Western blotting indicates that the endogenous HYPA is decreased in the nuclear fraction when transfected with Htt100Q but not with Htt18Q, whereas the cytosolic fraction is still undetectable due to a small amount of the endogenous HYPA and low sensitivity of the polyclonal antibody we used. These results demonstrate that polyQ expansion enhances interaction with HYPA and triggers cellular redistribution of HYPA. This abnormal interaction can be considered to be a partial contribution of the polyQ-expanded Htt to the pathogenesis of HD.
FIGURE 4.
Interaction with polyQ-expanded Htt leads to cellular redistribution of HYPA protein. A, co-immunoprecipitation of HYPA (IP) and its mutants with Htt18Q and Htt100Q is shown. FLAG-Htt18Q or Htt100Q and Myc-HYPA or its mutants were overexpressed in HEK 293T cells, and the cell lysates were subjected to co-immunoprecipitation experiments. IP, anti-FLAG antibody. The asterisks indicate the heavy chain of the antibodies. HYPA cannot interact with Htt18Q but can with Htt100Q. As compared with wild-type HYPA, all mutations including W38A, W79A, W38A/W79A, and GS-Sub exhibited reduced binding affinities with Htt100Q. B, co-localization of Htt and HYPA as visualized by confocal fluorescence microscopy imaging is shown. FLAG-Htt18Q or Htt100Q (red) and Myc-HYPA or its mutants (green) were both overexpressed in HEK 293T cells. Both Htt18Q and Htt100Q are mostly localized in cytosol, whereas HYPA is in the nucleus. Overexpression of Htt100Q can sequester HYPA to the cytosol location, and it can also sequester W38A and W79A to cytosol, but at a less extent. Scale bar = 10 μm. C, statistic analysis of the cytosolic localizations of HYPA and its mutants is shown. The data are represented as the mean ± S.E. (n = 6). *, p < 0.001. D, overexpression of Htt100Q reduces endogenous nucleus-localized HYPA. Western blotting of nuclear and cytosolic extracts from HEK 293T cells transfected with vector, Htt18Q, or Htt100Q is shown. The endogenous HYPA is reduced in the nuclear fraction when transfected with Htt100Q. Tubulin and SP1 are, respectively, cytosolic and nuclear protein markers. Both Htt18Q and Htt100Q are mostly localized in cytosol.
PolyQ-expanded Htt Causes Post-transcriptional Dysfunction of HYPA
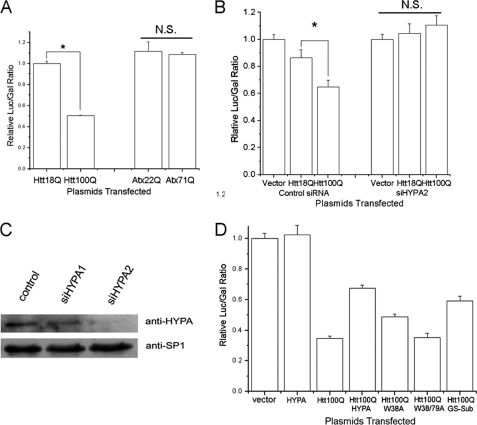
HYPA is one of the constitutive components of splicesome U1 complex that can enhance the efficiency of RNA splicing in mammalian cells (7, 8). Because HYPA could be sequestered by polyQ-expanded Htt, investigating the biological consequences of the abnormal interaction and redistribution is critical to understanding the pathogenesis of HD caused by mutant Htt. To examine whether Htt100Q can influence pre-mRNA splicing, we performed in vivo splicing assays after transient transfection of vector, Htt18Q, or Htt100Q. The splicing efficiency was quantified by using pTN23 reporter gene (22). Unspliced transcripts yield only β-galactosidase, whereas spliced transcripts express a fusion protein with both luciferase and β-galactosidase activities (supplemental Fig. S4). Thus, the ratio of luciferase over β-galactosidase activity stands quantitatively for the pre-mRNA splicing efficiency. As a result, transfection of Htt100Q in HEK 293T cells can significantly reduce the pre-mRNA splicing efficiency, as compared with transfection of Htt18Q (Fig. 5A). As a control, transfection of polyQ expanded ataxin-3 (Atx371Q) has no significant effect on the pre-mRNA splicing, as in the case of normal ataxin-3 (Atx322Q). When the endogenous HYPA is knocked down by RNA interference, the reducing effect of Htt100Q on pre-mRNA splicing efficiency does not exist (Fig. 5, B and C), as compared with control siRNA, in which Htt100Q still has considerable effect on the splicing. This provides a line of evidence that HYPA is downstream of the toxic gain-of-function pathway of mutant Htt. On the other hand, co-transfection of HYPA with Htt100Q can partially alleviate the decreased effect caused by Htt100Q (Fig. 5D, fourth column). In addition, mutations in the tandem WW domains exhibit a considerable effect on the recovery, especially the double mutant W38A/W79A that fails to alleviate the decreased effect (Fig. 5D, sixth column). This result is consistent with the finding from structural analysis that Htt interacts with HYPA through a bipartite manner.
FIGURE 5.
PolyQ-expanded Htt causes dysfunction of HYPA in pre-mRNA splicing. A, Htt100Q specifically reduces pre-mRNA splicing efficiency. The reporter plasmid pTN23 was co-transfected with mock vector, Htt18Q, or Htt100Q in HEK 293T cells, and the enzymatic activities were assayed. The splicing efficiency is assessed using the ratios of luciferase/β-galactosidase (Luc/Gal) activities. Ataxin-3 (Atx3) is a negative control in which polyQ expansion (Atx371Q) has no effect on the pre-mRNA splicing as compared with normal Atx3 (Atx322Q). The data are represented as the mean ± S.E. (n = 3) and normalized to vector control. *, p < 0.01. N.S., not significant. B, the reducing effect of Htt100Q on pre-mRNA splicing efficiency is HYPA-dependent. The reporter plasmid pTN23 was co-transfected with mock vector, Htt18Q, or Htt100Q and control siRNA or siHYPA2. *, p < 0.05. C, the protein level of HYPA in the nuclear extract after siRNA mediated knockdown is shown. D, overexpression of HYPA can partially rescue pre-mRNA splicing dysfunction caused by Htt100Q. The plasmids co-transfected with pTN23 are: 1, vector; 2, HYPA; 3, Htt100Q; 4, Htt100Q + HYPA; 5, Htt100Q + W38A; 6, Htt100Q + W38A/W79A; 7, Htt100Q + GS-Sub.
Both PolyQ Expansion and the PRR Motif Are Important for the Redistribution of Full-length FBP11 and the Dysfunction of Pre-mRNA Processing
Because the PRR motif of Htt is responsible for the interaction with HYPA and its polyQ expansion is the major cause of the cellular redistribution and pre-mRNA splicing repression, we repeated the experiments on PRR-deleted Htt (Htt100QΔP) and mouse full-length FBP11. Confocal fluorescence microscopy imaging shows that, like HYPA (Fig. 4B), FBP11 can also be sequestered to the cytosol by Htt100Q but not by Htt18Q (Fig. 6A, second row). Htt100QΔP considerably alleviates this effect (Fig. 6A, third row). Meanwhile, the pre-mRNA splicing efficiency is recovered by Htt100QΔP to a level by Htt18Q, as compared with that caused by polyQ-expanded Htt (Fig. 6B). Again, FBP11 can partially rescue the splicing repression by Htt100Q as HYPA does (Fig. 5D).
FIGURE 6.
Htt PRR is important to the redistribution of full-length FBP11 and also the dysfunction of pre-mRNA processing. A, shown is co-localization of Htt or its mutants with FBP11 visualized by confocal fluorescence microscopy imaging. FLAG-Htt18Q, Htt100Q, or Htt100QΔP (red) and FBP11-Myc (green) were both transfected into HEK 293T cells. Scale bar = 10 μm. B, effects of Htt mutants on the splicing efficiency are shown. The data are represented as the mean ± S.E. (n = 3) and normalized to vector control. *, p < 0.001. C, redistribution of FBP11 caused by Htt100Q alters alternative splicing of the reporter minigene in a PRR-dependent manner. HEK 293T cells were transfected with a CFTR minigene construct as a reporter. The upper bands correspond to exon 9 inclusion, whereas the lower bands correspond to exon 9 skipping. The ratios from the densities of lower bands over upper bands stand for alternative splicing efficiencies. Transient transfection of Htt100Q increases exon 9 inclusion, whereas co-transfection with FBP11 can rescue the increased exon 9 inclusion caused by Htt100Q. Htt100QΔP has no effect on exon 9 inclusion compared with Htt18Q.
Although alternative splicing can provide cells the capacity of tuning their transcriptome and proteome, misregulation of splicing may cause diseases (9). HYPA is a potential regulator of the splicing of human CFTR exon 9. We further applied a reporter, namely TG13T5, that is composed of CFTR exon 9 hybrid minigene (32). The reporter CFTR minigene was co-transfected with various vectors into HEK 293T cells, and the splicing efficiencies were assayed by the band densities. As a result, Htt100Q significantly increased exon 9 inclusion (Fig. 6C, fourth lane) as compared with Htt18Q, which does not perform such action. Co-transfection with FBP11 can slightly alleviate the effect caused by Htt100Q. Deletion of the PRR motif almost abolishes the increased effect of Htt100Q on exon 9 inclusion (Fig. 6C, third lane), supporting the previous finding that the PRR motif of Htt mediates the interaction with HYPA/FBP11. These results from in vivo pre-mRNA splicing assays suggest that polyQ-expanded Htt may also cause post-transcriptional dysfunction in cells via the abnormal interaction with HYPA/FBP11.
DISCUSSION
Bipartite Interaction between PRR of Htt and 2WW of HYPA
The proline-rich motifs are involved in lots of critical protein complexes in transcription and signal transduction pathways (33). Among the 20 amino acids, proline is unique in its N substitution (11, 12). WW domain is one of the most well characterized modular domains that recognize short proline-rich motifs (11, 25). In some cases, two or more WW domains are arrayed in a tandem way. The tandem WW domains of Prp40, the yeast homolog of HYPA, have a well ordered long helix that links two WW domains as an ensemble (26). This spatial orientation may also contribute to the adapter role for HYPA to cooperatively link different parts of the splicing machinery. However, there is currently no experimental evidence for the cooperative interaction between these tandem WW domains and other PRR-containing proteins. Other solution structures of the tandem WW domains come from Su(dx) (28) and FBP21 (27); both structures show no strictly spatial arrangement between the two WW domains. The binding of a Notch PY peptide to the fourth WW domain of Su(dx) is autoinhibited by the proximal third WW domain. When the third WW domain binds to another WBP1 PY peptide, inhibition of the fourth WW domain is relieved (34). How this autoinhibition arises remains elusive. A previous study showed that the tandem WW domains of FBP21 also interact with the proline-rich region of SIPP1 in a length-dependent manner (27). An FBP21 mutant with substitution of a shorter linker between the two WW domains can still interact with SIPP1 but with relatively lower affinity. Our study gives rise to similar result that the binding affinity of HYPA would be significantly reduced when the linker region is mutated or substituted (Figs. 2D, 4A, and 5D). However, unlike FBP21, the linker region between the WW domains of HYPA is rather rigid. The bipartite mode of the PP1 and PP3 moieties in Htt-PRR and the tandem WW domains in HYPA significantly enhance their binding affinity. The first WW domain of HYPA is well ordered (29, 35–37), whereas it may have a chaperoning effect on the folding of the secondary WW domain. Our results indicate that the two WW domains work as a unit to interact with Htt-PRR. Actually the linker helix is closer to the WW2 domain than WW1 (Fig. 2C). Thus, the linker acts as a bridge both for the folding of WW2 and the cooperative interaction with PRR. This chaperoning effect of the domain folding provides a possibility that the tandem WW domains of HYPA act as an ensemble for bipartite cooperative interaction with the full-length PRR motif of Htt.
PolyQ Expansion of Htt Enhances the Bipartite Interaction with HYPA
HD is thought to be caused by polyQ expansion in the N terminus of Htt (1). Although the exact mechanism remains unknown, it is generally accepted that change of the biochemical properties in the Htt protein can account for both the loss and gain of interactions, and these abnormal interactions altogether contribute to the HD phenotype. Thus, characterizing the mutant Htt is very important for understanding the HD pathology. Htt is a very large protein, and most research concentrates on the N-terminal part that contains a polyQ tract and a PRR motif. As known, the aggregation rates of polyQ peptides increase with the length of polyQ tract (38, 39). The interplay between the flanking sequences and the polyQ tract offers a challenging opportunity for us to understand the HD pathogenesis. The N-terminal 17 amino acid residues are proposed to trigger a complex aggregation of Htt (40), and the PRR motif of Htt may adopt different conformations under the influence of the N-terminal polyQ tract (41). The polyproline after polyQ in synthetic peptides reduces both the formation rate and stability of the amyloid-like aggregates (42). An in vivo study also showed that PRR region can determine the formation of Htt bodies (43), and deletion of PRR can unmask the toxic properties in yeast (44). Flanking sequences around polyQ can alter toxicity in yeast (45). The GST-fused 2WW has higher affinity to Htt18Q as compared with single WW1 or WW2 domain (Fig. 1B), suggesting that both WW domains are engaged in the cooperative interaction. However, in the context of HYPA, the interaction has been weakened, whereas polyQ-expanded Htt can still interact with HYPA, and both WW domains are actually involved (Figs. 4, A and B, and 5D). Although it is well known that aggregation-prone proteins may expose their hydrophobic regions, which can lead to abnormal unspecific interaction (46), this is not the case in the interaction between Htt and HYPA. Deletion of the PRR motif in Htt can abolish cytosolic distribution of FBP11 and suppress dysfunction of pre-mRNA processing (Fig. 6). The aggregation-prone property of mutant Htt also contributes to the enhanced interaction. Thus, both the aggregation-prone property and the long PRR region of Htt confer the gain-of-interaction between Htt and HYPA.
A Possible Alternative Pathogenesis for HD
Htt is engaged in development and anti-apoptosis; it also facilitates vesicular transport and controls neuronal gene transcription (47). When polyQ expansion occurs in Htt protein, it readily gets aggregation and abnormal protein-protein interactions and even affects ubiquitin-proteasome system, metabolism, and mitochondrial function besides loss of normal function (48, 49). Htt mutant can also abnormally interact with short polyQ-containing proteins, such as co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). Depletion of CBP may cause loss of nuclear location and CBP-regulated gene transcription disorder (50). The present research demonstrates that polyQ-expanded Htt can enhance the interaction with HYPA and sequester HYPA protein to the cytosol location (Fig. 4, A and B). Endogenous HYPA is reduced in the mutant Htt-transfected cell models (Fig. 4D) and in the HD mouse striatum (6). It was also reported that mutations in yeast spliceosomal components show differences in the splicing defects toward particular pre-mRNA substrates (51). The spliceosomal components can also affect alternative splicing in Drosophila; different protein components may affect different exons and target different pre-mRNAs (52). Moderate changes in expression of splicing factors can have significant effects on alternative splicing and may cause diseases (9). The reduction of HYPA may lead to a splicing defect of a group of pre-mRNAs. Actually, mutant Htt can lead to lower efficiency of pre-mRNA splicing and change of the alternative splicing of the reporter gene (Figs. 5 and 6). Taken together, we propose that the polyQ expansion might alter biophysical properties of the Htt protein and in turn leads to enhanced interaction with HYPA. This abnormal interaction causes redistribution of HYPA from its original nuclear location to the cytosol, impairs the function of spliceosome, and affects alternative splicing, which may provide an alternative way for the pathogenesis of HD. Because the exon 1 region of Htt is enough to generate HD phenotype (2), the PRR motif is critical for the toxicity of polyQ-expanded Htt. Thus, blocking the exposed PRR region might be a promising therapeutic strategy for treating the disease. Still, more experimental research is required to further explore the splicing dysfunction in animal models.
Supplementary Material
Acknowledgments
We thank Dr. Z. H. Qin, Soochow University School of Medicine, for providing the plasmids of Htt18Q and Htt100Q, Dr. I. C. Eperon, University of Leicester, UK, for providing the plasmids of pTN23 and pTN24, and Drs. E. Buratti and F. E. Baralle, International Center for Genetic Engineering and Biotechnology, Italy, for the minigene TG13T5. We also thank Drs. J. Y. Hui and Z. F. Li, Shanghai Institutes for Biological Sciences, for informative suggestion and discussion.
This work was supported by National Basic Research Program of China Grants 2006CB910305 and 2006CB806508 and National Natural Science Foundation of China Grants 30670431 and 10979070.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S4.
The atomic coordinates and structure factors (code 2L5F) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- HD
- Huntington disease
- Htt
- huntingtin
- PRR
- proline-rich region
- HYPA
- huntingtin yeast two-hybrid protein A
- HSQC
- heteronuclear single quantum correlation
- CFTR
- cystic fibrosis transmembrane conductance regulator
- NOE
- nuclear Overhauser effect
- DNS
- dansyl chloride.
REFERENCES
- 1. The Huntingtons Disease Collaborative Research Group (1993) Cell 72, 971–983 [DOI] [PubMed] [Google Scholar]
- 2. Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S. W., Bates G. P. (1996) Cell 87, 493–506 [DOI] [PubMed] [Google Scholar]
- 3. Kaltenbach L. S., Romero E., Becklin R. R., Chettier R., Bell R., Phansalkar A., Strand A., Torcassi C., Savage J., Hurlburt A., Cha G. H., Ukani L., Chepanoske C. L., Zhen Y., Sahasrabudhe S., Olson J., Kurschner C., Ellerby L. M., Peltier J. M., Botas J., Hughes R. E. (2007) PLoS Genet. 3, e82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li S. H., Li X. J. (2004) Trends Genet 20, 146–154 [DOI] [PubMed] [Google Scholar]
- 5. Southwell A. L., Ko J., Patterson P. H. (2009) J. Neurosci. 29, 13589–13602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Passani L. A., Bedford M. T., Faber P. W., McGinnis K. M., Sharp A. H., Gusella J. F., Vonsattel J. P., MacDonald M. E. (2000) Hum. Mol. Genet. 9, 2175–2182 [DOI] [PubMed] [Google Scholar]
- 7. Wahl M. C., Will C. L., Lührmann R. (2009) Cell 136, 701–718 [DOI] [PubMed] [Google Scholar]
- 8. Lin K. T., Lu R. M., Tarn W. Y. (2004) Mol. Cell. Biol. 24, 9176–9185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper T. A., Wan L., Dreyfuss G. (2009) Cell 136, 777–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dredge B. K., Polydorides A. D., Darnell R. B. (2001) Nat. Rev. Neurosci. 2, 43–50 [DOI] [PubMed] [Google Scholar]
- 11. Zarrinpar A., Bhattacharyya R. P., Lim W. A. (2003) Sci. STKE 2003, RE8. [DOI] [PubMed] [Google Scholar]
- 12. Kay B. K., Williamson M. P., Sudol M. (2000) FASEB J. 14, 231–241 [PubMed] [Google Scholar]
- 13. Gao Y. G., Yan X. Z., Song A. X., Chang Y. G., Gao X. C., Jiang N., Zhang Q., Hu H. Y. (2006) Structure 14, 1755–1765 [DOI] [PubMed] [Google Scholar]
- 14. Bao W. J., Gao Y. G., Chang Y. G., Zhang T. Y., Lin X. J., Yan X. Z., Hu H. Y. (2006) Protein Expr. Purif. 47, 599–606 [DOI] [PubMed] [Google Scholar]
- 15. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 16. Goddard T. D., Kneller D. G. (2008) SPARKY 3, University of California, San Francisco, CA [Google Scholar]
- 17. Cornilescu G., Delaglio F., Bax A. (1999) J. Biomol. NMR 13, 289–302 [DOI] [PubMed] [Google Scholar]
- 18. Rieping W., Habeck M., Bardiaux B., Bernard A., Malliavin T. E., Nilges M. (2007) Bioinformatics 23, 381–382 [DOI] [PubMed] [Google Scholar]
- 19. Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 20. Koradi R., Billeter M., Wüthrich K. (1996) J. Mol. Graph. 14, 51-55 [DOI] [PubMed] [Google Scholar]
- 21. Farrow N. A., Muhandiram R., Singer A. U., Pascal S. M., Kay C. M., Gish G., Shoelson S. E., Pawson T., Forman-Kay J. D., Kay L. E. (1994) Biochemistry 33, 5984–6003 [DOI] [PubMed] [Google Scholar]
- 22. Nasim M. T., Eperon I. C. (2006) Nat. Protoc. 1, 1022–1028 [DOI] [PubMed] [Google Scholar]
- 23. Che M. X., Jiang Y. J., Xie Y. Y., Jiang L. L., Hu H. Y. (2011) FASEB J., in press [Google Scholar]
- 24. Faber P. W., Barnes G. T., Srinidhi J., Chen J., Gusella J. F., MacDonald M. E. (1998) Hum. Mol. Genet. 7, 1463–1474 [DOI] [PubMed] [Google Scholar]
- 25. Macias M. J., Wiesner S., Sudol M. (2002) FEBS Lett. 513, 30–37 [DOI] [PubMed] [Google Scholar]
- 26. Wiesner S., Stier G., Sattler M., Macias M. J. (2002) J. Mol. Biol. 324, 807–822 [DOI] [PubMed] [Google Scholar]
- 27. Huang X., Beullens M., Zhang J., Zhou Y., Nicolaescu E., Lesage B., Hu Q., Wu J., Bollen M., Shi Y. (2009) J. Biol. Chem. 284, 25375–25387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fedoroff O. Y., Townson S. A., Golovanov A. P., Baron M., Avis J. M. (2004) J. Biol. Chem. 279, 34991–35000 [DOI] [PubMed] [Google Scholar]
- 29. Kato Y., Hino Y., Nagata K., Tanokura M. (2006) Proteins 63, 227–234 [DOI] [PubMed] [Google Scholar]
- 30. Mizutani K., Suetsugu S., Takenawa T. (2004) Biochem. Biophys. Res. Commun. 313, 468–474 [DOI] [PubMed] [Google Scholar]
- 31. DiFiglia M., Sapp E., Chase K. O., Davies S. W., Bates G. P., Vonsattel J. P., Aronin N. (1997) Science 277, 1990–1993 [DOI] [PubMed] [Google Scholar]
- 32. Buratti E., Dörk T., Zuccato E., Pagani F., Romano M., Baralle F. E. (2001) EMBO J. 20, 1774–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neduva V., Russell R. B. (2007) Sci. STKE 2007, pe1. [DOI] [PubMed] [Google Scholar]
- 34. Jennings M. D., Blankley R. T., Baron M., Golovanov A. P., Avis J. M. (2007) J. Biol. Chem. 282, 29032–29042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato Y., Nagata K., Takahashi M., Lian L., Herrero J. J., Sudol M., Tanokura M. (2004) J. Biol. Chem. 279, 31833–31841 [DOI] [PubMed] [Google Scholar]
- 36. Pires J. R., Parthier C., Aido-Machado R., Wiedemann U., Otte L., Böhm G., Rudolph R., Oschkinat H. (2005) J. Mol. Biol. 348, 399–408 [DOI] [PubMed] [Google Scholar]
- 37. Kato Y., Miyakawa T., Kurita J., Tanokura M. (2006) J. Biol. Chem. 281, 40321–40329 [DOI] [PubMed] [Google Scholar]
- 38. Chen S., Ferrone F. A., Wetzel R. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11884–11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scherzinger E., Sittler A., Schweiger K., Heiser V., Lurz R., Hasenbank R., Bates G. P., Lehrach H., Wanker E. E. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 4604–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thakur A. K., Jayaraman M., Mishra R., Thakur M., Chellgren V. M., Byeon I. J., Anjum D. H., Kodali R., Creamer T. P., Conway J. F., Gronenborn A. M., Wetzel R. (2009) Nat. Struct. Mol. Biol. 16, 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim M. W., Chelliah Y., Kim S. W., Otwinowski Z., Bezprozvanny I. (2009) Structure 17, 1205–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhattacharyya A., Thakur A. K., Chellgren V. M., Thiagarajan G., Williams A. D., Chellgren B. W., Creamer T. P., Wetzel R. (2006) J. Mol. Biol. 355, 524–535 [DOI] [PubMed] [Google Scholar]
- 43. Qin Z. H., Wang Y., Sapp E., Cuiffo B., Wanker E., Hayden M. R., Kegel K. B., Aronin N., DiFiglia M. (2004) J. Neurosci. 24, 269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dehay B., Bertolotti A. (2006) J. Biol. Chem. 281, 35608–35615 [DOI] [PubMed] [Google Scholar]
- 45. Duennwald M. L., Jagadish S., Muchowski P. J., Lindquist S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11045–11050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiti F., Dobson C. M. (2006) Annu. Rev. Biochem. 75, 333–366 [DOI] [PubMed] [Google Scholar]
- 47. Cattaneo E., Zuccato C., Tartari M. (2005) Nat. Rev. Neurosci. 6, 919–930 [DOI] [PubMed] [Google Scholar]
- 48. Shao J., Diamond M. I. (2007) Hum. Mol. Genet. 16, R115–R123 [DOI] [PubMed] [Google Scholar]
- 49. Li X. J., Friedman M., Li S. (2007) Trends Genet. 23, 531–533 [DOI] [PubMed] [Google Scholar]
- 50. Nucifora F. C., Jr., Sasaki M., Peters M. F., Huang H., Cooper J. K., Yamada M., Takahashi H., Tsuji S., Troncoso J., Dawson V. L., Dawson T. M., Ross C. A. (2001) Science 291, 2423–2428 [DOI] [PubMed] [Google Scholar]
- 51. Pleiss J. A., Whitworth G. B., Bergkessel M., Guthrie C. (2007) PLoS Biol. 5, e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park J. W., Parisky K., Celotto A. M., Reenan R. A., Graveley B. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15974–15979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.