Abstract
Reports suggest that excessive ceramide accumulation in mitochondria is required to initiate the intrinsic apoptotic pathway and subsequent cell death, but how ceramide accumulates is unclear. Here we report that liver mitochondria exhibit ceramide formation from sphingosine and palmitoyl-CoA and from sphingosine and palmitate. Importantly, this activity was markedly decreased in liver from neutral ceramidase (NCDase)-deficient mice. Moreover, the levels of ceramide were dissimilar in liver mitochondria of WT and NCDase KO mice. These results suggest that NCDase is a key participant of ceramide formation in liver mitochondria. We also report that highly purified liver mitochondria have ceramidase, reverse ceramidase, and thioesterase activities. Increased accessibility of palmitoyl-CoA to the mitochondrial matrix with the pore-forming peptide zervamicin IIB resulted in 2-fold increases in palmitoyl-CoA hydrolysis by thioesterase. This increased hydrolysis was accompanied by an increase in ceramide formation, demonstrating that both outer membrane and matrix localized thioesterases can regulate ceramide formation. Also, ceramide formation might occur both in the outer mitochondrial membrane and in the mitochondrial matrix, suggesting the existence of distinct ceramide pools. Taken together, these results suggest that the reverse activity of NCDase contributes to sphingolipid homeostasis in this organelle in vivo.
Keywords: Fatty acid, Fatty acid Metabolism, Liver Metabolism, Mitochondria, Sphingolipid, Ceramidase, Palmitoyl - CoA, Thioesterase
Introduction
In recent years the sphingolipid ceramide (1) has gained appreciation as a bioactive lipid that modulates apoptotic/necrotic cellular processes (2, 3). Although increased ceramide at the plasma membrane can amplify a primary death signal by clustering receptors in ceramide-rich platforms (4) or indirectly change the ratio/activity of proapoptotic/antiapoptotic members of BCL-2 family proteins at the outer mitochondrial membrane (for review, see Ref. 5), evidence also suggests a direct action of ceramide on mitochondria. Specifically, the selective hydrolysis of mitochondrial sphingomyelin to ceramide by the targeting of bacterial sphingomyelinase to mitochondria results in apoptosis (6). These studies underscore the physiological significance of the mitochondrial ceramide and sphingomyelin pools (7–12). Functionally, ceramides directly suppress respiratory chain activity (13, 14), which is followed by a burst of reactive oxygen species production (15), modulate permeability transition pore activity (16–21), and permeabilize the mitochondrial outer membrane for cytochrome c acting alone (14, 22–25) or in cooperation with Bax (26, 27). Moreover, many paradigms of cell death triggered by TNF-α (15, 28), Fas ligation (29), ischemia/reperfusion (30, 31), etoposide (9), UV (9), and ionizing radiation (29, 32, 33) are associated with the increase in mitochondrial ceramide, suggesting that this might be a general phenomenon. Given these intimate and direct connections between ceramide and mitochondria, understanding the source and metabolism of ceramides in these organelles is key with respect to basic biology and clinical applications.
Two possibilities may account for ceramide increases in mitochondria. First, ceramide preformed in the ER2 can be transferred to mitochondria by catalyzed exchange via membrane contacts (34). In contrast, mitochondria could be a specialized compartment of sphingolipid metabolism with their own subset of biosynthetic and degradative enzymes. Recent studies identified two novel sphingomyelinases, which hydrolyze sphingomyelin to ceramide, and phosphocholine in mitochondria from zebrafish (10) and mouse tissues (35). Notably, in yeast the mammalian neutral sphingomyelinase ortholog Isc1p associates with mitochondria in the post-diauxic phase of yeast growth and regulates mitochondrial sphingolipid metabolism (36, 37). Ceramide synthase (CerS), which condenses (dihydro)sphingosine with acyl-CoA to form ceramide, has been partially purified from bovine liver mitochondria-enriched fractions (38). Ceramide synthase activity was confirmed in highly purified rat liver mitochondria (39); however, the mechanism behind this activity is unclear because of the decreased (or opposing) sensitivity of the reaction to fumonisin B1, which inhibits conventional ER CerSs (40). A recent report described association of several CerS isoforms, including CerS1, CerS2, and CerS6, with purified mouse brain mitochondria (30). No such association was found in HeLa cells (33), suggesting that this might be a cell type/tissue-specific event.
On the catabolic side of ceramide metabolism, ceramidases (CDase) catalyze the cleavage of the N-acyl linkage of ceramide to generate sphingosine and free fatty acid (FFA) (41). A conventional view suggests that ceramide content is balanced by ceramide producing (CerSs and sphingomyelinases) and ceramide degrading (CDases) enzyme activities (1). Intriguingly, purified neutral CDase (NCDase) catalyzes both the hydrolysis and synthesis of ceramide (42, 43). In contrast to CerS activity, this later reaction is CoA-independent and fumonisin B1-insensitive. Reverse CDase activity in rat liver mitochondria at neutral pH has been described (39); however, this pathway and the enzyme involved have not been defined. Also, the originally proposed localization of overexpressed human NCDase in mitochondria (44) is being re-examined (45). Here we report that highly purified rat/mice liver mitochondria have ceramidase, reverse ceramidase, and thioesterase (which hydrolyzes palmitoyl-CoA to CoA and fatty acid) activities. Moreover, mitochondria from NCDase-deficient mice have significantly decreased formation of ceramide from sphingosine and palmitoyl-CoA (or palmitate) compared with mitochondria from WT mice, indicating that NCDase participates in ceramide formation in liver mitochondria and that ceramide formation may occur from sphingosine and palmitoyl-CoA from coupled activities of a mitochondrial thioesterase and NCDase catalyzing the reverse reaction.
EXPERIMENTAL PROCEDURES
Animals and Reagents
Male C57BL/6 mice (8–12 weeks old) and male Sprague-Dawley rats (250–300 g) were from Charles River Laboratories (Wilmington, MA). NCDase KO mice were generated in the laboratory of Dr. Richard Proia (46) (NIDDK, NIH) and transferred to the animal facility of MUSC. The mice were C57BL/6 background and were backcrossed for five additional generations with the same background at MUSC. Males (8–12 weeks old) were used for this study. Animals were fasted overnight before experimentation. Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at MUSC and followed the NIH guidelines for experimental animal use. 17C-sphingosine was from Avanti Polar Lipids (Alabaster, AL). NBD-C12 fatty acid was obtained from Molecular Probes (Eugene, OR), and urea-C6-ceramide and d-MAPP were provided by the Lipidomics Core Facility MUSC (Charleston, SC). Percoll PLUS was from GE Healthcare. Nordihydroguaiaretic acid (NDGA) was purchased from EMD Chemicals (Gibbstown, NJ). Channel-former peptide zervamicin IIB (47) was isolated from the biomass of the fungus-producer Emericellopsis salmosynnemata strain 336 IMI 58330 as described in Balashova et al. (48). All other chemicals were purchased from Sigma.
Antibodies
The antibody against mouse NCDase (46) was a generous gift from Dr. Richard Proia (NIDDK, NIH). Antibodies against VDAC (mitochondrial marker) were supplied by EMD Chemicals. Rat and mouse VDAC was assessed using anti-porin (Ab-5) rabbit polyclonal antibody (#PC548) and anti-VDAC1 rabbit polyclonal antibody (#AP1059) accordingly. The rabbit polyclonal anti-LAMP-2 (lysosomal marker), mouse monoclonal anti-α1 subunit of the sodium/potassium ATPase (plasma membrane marker), and the rabbit polyclonal anti-calnexin (ER marker) antibodies were purchased from Abcam (Cambridge, MA). Secondary horseradish peroxidase-conjugated antibodies were supplied by Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
Preparation of Mitochondria from Mouse and Rat Liver
Liver mitochondria were isolated and purified as described in Hovius et al. (49) and Graham (50) with some modifications. Briefly, livers from two rats (or 5 mice) were homogenized in isolation medium containing 250 mm mannitol, 1 mm EDTA, 0.1% BSA (w/v, essentially fatty acid free), and 10 mm HEPES (pH 7.4 adjusted by KOH). Homogenization was performed in a motor-driven Potter-Elvehjem homogenizer using 8 up-and-down strokes of the pestle, with rotations of 800 rpm. Homogenate was centrifuged at 600 × gmax for 5 min in a Sorvall SA-600 rotor to pellet the nucleus and unbroken cells. Pellet was discarded, and supernatant was centrifuged once more at 600 × gmax for 5 min. To pellet mitochondria, supernatant from the previous step was centrifuged at 9300 × gmax for 10 min in a Sorvall SA-600 rotor. The mitochondrial pellet was suspended in isolation medium and centrifuged at 9300 × gmax for 10 min once more. The resulting pellet was suspended in 4 ml (rats) or 2 ml (mice) of isolation medium and loaded by 2–3 ml atop 20 ml of 30% (v/v) Percoll PLUS in 225 mm mannitol, 1 mm EDTA, 0.1 BSA (w/v), and 25 mm HEPES (pH 7.4 adjusted by KOH). The gradient was centrifuged for 30 min at 95,000 × gmax in a Beckman Ti 50.2 rotor. The mitochondrial band was collected from the lower part of the gradient, suspended in isolation buffer without BSA, and spun for 10 min at 6300 × gmax in a Sorvall SA-600 rotor. This step was repeated once more. The mitochondrial pellet from the last centrifugation was suspended in a minimal volume of isolation buffer without BSA.
Preparation of Microsomes from Rat Liver
Microsomes were isolated according the Hovius et al. (49) from the supernatant of the first crude mitochondrial pellet (see above). Supernatant was centrifuged at 27,000 × gmax for 10 min in a Sorvall SA-600 rotor. Microsomes were pelleted from the resulting supernatant by centrifugation at 105,000 × gmax for 1 h in a Beckman Ti 50.2 rotor. The pellet was resuspended in isolation buffer without BSA.
Protein Determination
Microsomal and mitochondrial protein concentration was quantified with a Bicinchoninic acid assay kit (Pierce) using BSA as a standard.
Cytochrome c Reductase Assay
Eukaryotic NADPH-cytochrome c reductase is a marker of the endoplasmic reticulum. This activity in mitochondria and microsomes was assayed as described by the manufacturer by a cytochrome c reductase (NADPH) assay kit (Sigma; #CY0100) with slight modifications. Briefly, 50 μg of mitochondria or microsomes were added to 1 ml of working solution consisting of 300 mm potassium phosphate buffer (pH 7.8), 50 μm cytochrome c, 1 mm potassium cyanide, and 1 μg of antimycin A at 25 °C. After recording the base-line absorbance at 550 nm, the reaction was started by the addition of 100 μm NADPH. The rate of cytochrome c reduction was calculated using extinction coefficient 21.1 mm−1cm−1).
Mitochondrial Incubation Medium
Unless otherwise specified, incubations of isolated mitochondria were conducted at 37 °C using 1 mg/ml protein in medium containing 250 mm sucrose, 5 μm rotenone, and 10 mm HEPES (pH 7.4 adjusted by KOH). Incubations were conducted in a water-jacketed cell equipped with magnetic stirring.
Lipid Delivery
17C-sphingosine, palmitate, and urea-C6-ceramide were delivered to the mitochondrial suspension as an ethanol solution (final concentration <1%). Delivery of sphingosine and palmitate as a complex with BSA as described in Lahiri et al. (51) and Schulze et al. (52) does not provide a substantial increase in the rate of ceramide production compared with ethanol delivery under our experimental conditions (results not shown).
Measurement of Mitochondrial Permeabilization
Inner membrane permeabilization was assayed by measurements of mitochondrial swelling. Mitochondrial swelling was measured by changes in absorbance at 520 nm using a Brinkmann PC 900 probe colorimeter and a fiber optic probe.
Measurement of Mitochondrial Thioesterase Activity
Palmitoyl-CoA hydrolase activity of mitochondria (0.5 mg/ml) was measured by release of thiol groups (CoA) (53) in mitochondrial incubation medium supplemented with 300 μm 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) at 37 °C. Formation of thiol groups was monitored at 412 nm in a PerkinElmer Life Sciences split beam spectrophotometer. The reaction was initiated after 5 min of preincubation (with/without zervamicin IIB) by the addition of palmitoyl-CoA to the sample cuvette. The amount of thiol released was calculated using the molar absorption coefficient ϵ412 = 13,600 m−1cm−1 (54). The amount of thiols released calculated from absorbance changes in the presence of DTNB were corrected for changes in light scattering induced by palmitoyl-CoA in control runs.
Western Blot Analysis
Mitochondria or tissue samples were lysed in a buffer containing 50 mm Tris-HCl (pH 7.4), 5 mm EDTA, 150 mm NaCl, 1% Triton X-100, 1 mm Na3VO4, and 10 mm NaF supplemented with a protease inhibitor mixture (Roche Applied Science). After 1 h on ice, lysates were centrifuged at 15,000 × g for 10 min to remove insoluble material. Protein samples were then prepared by boiling lysates in reducing SDS sample buffer. 30 μg of total proteins from each lysate was loaded onto 4–20% gradient SDS-polyacrylamide gels, subjected to electrophoresis, and then transferred to polyvinylidene difluoride membranes and blocked with 5% nonfat dry milk in TBS-T buffer (10 mm Tris, 150 mm NaCl, and 0.2% Tween 20 (pH 8.0)) overnight at 4 °C. The blots were subsequently probed with appropriate primary antibodies followed by horseradish peroxidase-labeled secondary antibody. Immunoreactive bands were visualized using n ECL chemiluminescence kit (Pierce). Where indicated Western blots were quantified using Quantity One software (Bio-Rad).
Ceramide Synthase Activity Assay
Ceramide synthase activity was monitored by the formation of ceramide from sphingosine and palmitoyl-CoA or palmitate. To enhance the sensitivity of the assay, we employed a novel synthetic 17C-shingosine instead of natural 18C-sphingosine as a substrate (55), and 17C16:0-ceramide formed in the reaction was measured using tandem MS (56). Mitochondria were incubated with/without inhibitors for 5 min in mitochondrial incubation medium, and ceramide formation was initiated by addition of substrates and allowed to progress for 15 min. The reaction was terminated, and ceramides were extracted by the addition of 0.5 ml of the sample to 2 ml of the ethyl acetate/isopropyl alcohol/water (60:30:10%, v/v/v) solvent system. The lipid extracts were fortified with internal standards, dried under a stream of nitrogen gas, and reconstituted in 100 μl of methanol for electrospray ionization tandem mass spectrometry analysis of ceramides, which was performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer, operating in a multiple reaction-monitoring, positive-ionization mode.
The samples were injected onto the HP1100/TSQ 7000 liquid chromatography/MS system and gradient-eluted from the BDS Hypersil C8, 150 × 3.2-mm, 3-μm particle size column with a 1.0 mm methanolic ammonium formate, 2 mm aqueous ammonium formate mobile-phase system. The peaks for the target analytes and internal standards were collected and processed with the Xcalibur software system. Calibration curves were constructed by plotting peak area ratios of synthetic standards, representing each target analyte, to the corresponding internal standard. The target analyte peak area ratios from the samples were similarly normalized to their respective internal standard and compared with the calibration curves using a linear regression model.
Ceramidase Activity Assay
Enzyme activity was measured using detergent/lipid mixed micelles assay (57) with d-erythro-C12-NBD-ceramide (58) as a substrate with some modifications (59). An aliquot of liver mitochondria from WT C57BL/6 mice, NCDase knock-out mice, or rat was prepared as previously described, and 25 μg of protein was subjected to NCDase activity assays. The activity was measured using detergent/lipid mixed micelles with d-erythro-C12-NBD-ceramide as a substrate at a concentration of 50 μm or 1.08 mol % in a 100 mm Tris-HCl buffer (pH 7.4) containing 0.3% (w/v) Triton X-100 final concentration, with a total volume of 100 μl. The reaction mixture was incubated at 37 °C for 1 h. The reaction was terminated by the addition of 300 μl of chloroform/methanol (1:1, v/v), and the organic phase was spotted on TLC plate, which was then developed with chloroform, methanol, 25% ammonia (90:20:0.5, v/v/v). Conversion of NBD-C12-ceramide to NBD-dodecanoic acid was detected by spotting the organic phase on a TLC plate. Fluorescent-labeled products were analyzed and quantified with a PhosphorImager (Storm 860) system. Results were quantified by densitometric analysis using ImageQuant software.
Statistical Analysis
Where indicated, values were expressed as the mean value of the treatment groups ± S.D. Data were analyzed for statistically significant differences between groups using the two-tailed Student's t test. Statistical significance was ascribed to the data when p < 0.05.
RESULTS
Mitochondria Form Ceramide from Sphingosine and Palmitoyl-CoA at Isotonic Conditions
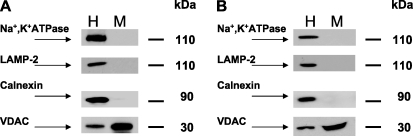
Rat liver mitochondria were isolated by differential and Percoll gradient centrifugation according to Hovius et al. (49) and had negligible contamination with the major cellular membrane compartments as assessed by Western blot using antibodies against plasma membrane marker protein (Na+, K+ATPase), lysosomes (LAMP-2), and endoplasmic reticulum (calnexin) (Fig. 1, panel A). The outer mitochondrial membrane marker VDAC was enriched in the mitochondrial fraction 4–5 times as compared with homogenate. ER marker enzyme activity NAHPH:cytochrome c reductase was decreased from 223.0 ± 4.9 (ER) to 8.8 ± 0.3 (mitochondria, n = 3) nmol/min/mg protein, indicating that these mitochondrial protein fractions contain ∼4% of ER proteins. This is in accord with the 4.3% contamination reported for the isolation method employed in the original study (49). Mitochondrial preparations from mouse liver were of similar quality with respect to contamination (Fig. 1, panel B).
FIGURE 1.
Percoll-purified mitochondria display minimal contamination with other cellular membrane compartments. Panel A, shown are rat liver fractions. Panel B, shown are mouse liver fractions. A Western blot for Na+, K+ATPase (plasma membrane marker), LAMP-2 (lysosomal marker), calnexin (ER marker), and VDAC (mitochondrial marker) was performed by loading an equal amount (30 μg) of homogenate (H) or mitochondrial (M) protein per lane, except rat VDAC was assessed using protein loading of 15 μg per lane. The blot is representative of three independent experiments.
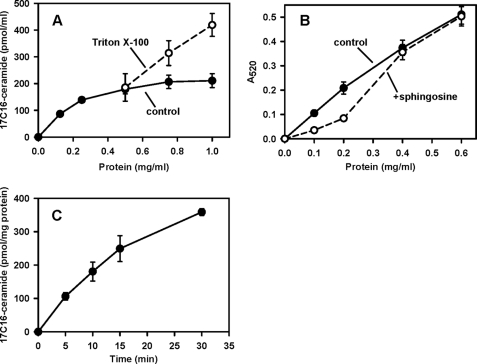
It was previously reported that osmotically lysed (in 25 mm potassium phosphate buffer) purified rat liver mitochondria form ceramide from sphingosine and palmitoyl-CoA in a FB1-insensitive manner (39), and this activity was attributed to the presence of mitochondria-specific CerS. We assessed mitochondrial ceramide formation at isotonic conditions (∼300 mosmol/liter) when the intactness of the mitochondrial inner and outer membranes was preserved and observed that they readily form ceramide from sphingosine and palmitoyl-CoA. In these experiments we utilized sphingosine and palmitoyl-CoA at 15 and 50 μm, respectively, concentrations optimal for CerS activity measurements in the ER (60). Ceramide formation versus protein concentration showed near linear dependence in the range of 0–0.25 mg/ml and then plateaued in the range 0.8–1 mg/ml (Fig. 2, panel A). However, utilization of mitochondria in the low concentration range is questionable because at the conditions employed sphingosine induces decreases in mitochondrial absorbance (indicative of mitochondrial lysis/swelling) at protein values up to 0.4 mg/ml (Fig. 2, panel B). This might produce artifacts related to solubilization of membrane-bound enzymes. Thus, we utilized mitochondria at 1 mg/ml. Although some responses due to effects on Vmax in the nonlinear range might be missed under these conditions, we prefer these conditions in the light of preserved mitochondrial integrity. At a mitochondrial protein concentration of 1 mg/ml, 50 μm palmitoyl-CoA did not affect mitochondrial integrity under our experimental conditions; however, higher concentrations resulted in progressive mitochondrial destabilization. At 1 mg of protein/ml, the reaction rate was linear within 15 min (Fig. 2, panel C). These conditions were chosen for further experiments. Importantly, mitochondrial lysis by Triton X-100 resulted in a considerable increase in ceramide synthesis within the mitochondrial protein concentration range of 0.75–1 mg/ml. Such an increase might indicate either stimulation of enzyme activity by detergent or exposure of latent ceramide synthase activity of the mitochondrial matrix, an issue addressed in subsequent experiments. Stimulation of ceramide synthase activity by Triton X-100 is unusual because in yeast this detergent suppresses CerS activity of the ER (61). In rat liver ER, membrane solubilization by high concentration of palmitoyl-CoA was suggested to be responsible for inhibition of CerS activity (60). We, therefore, investigated other potential mechanisms of ceramide production in liver mitochondria.
FIGURE 2.
Ceramide is produced from sphingosine and palmitoyl-CoA by purified rat liver mitochondria at isotonic conditions. Panel A, ceramide formation as a function of protein concentration is shown. Mitochondria at the indicated amounts were incubated as described under “Experimental Procedures” in the presence of 50 μm palmitoyl-CoA and 15 μm sphingosine for 15 min before termination of the reaction by addition of ethyl acetate/isopropyl alcohol extraction mixture. Where indicated, Triton X-100 (final concentration 0.5%) was added simultaneously with mitochondria. Panel B, absorption of mitochondrial suspension as a function of protein concentration in the absence or in the presence of 15 μm sphingosine is shown. Absorption was measured 15 min after sphingosine addition. Panel C, time course of ceramide formation is shown. Mitochondria (1 mg/ml) were incubated as described under “Experimental Procedures” in the presence of 50 μm palmitoyl-CoA and 15 μm sphingosine for the indicated times before termination of the reaction by the addition of ethyl acetate/isopropyl alcohol extraction mixture. Results represent the average ±S.D. (n = 3).
Inhibitor of Neutral Ceramidase Suppresses Ceramide Formation from Sphingosine and Palmitoyl-CoA
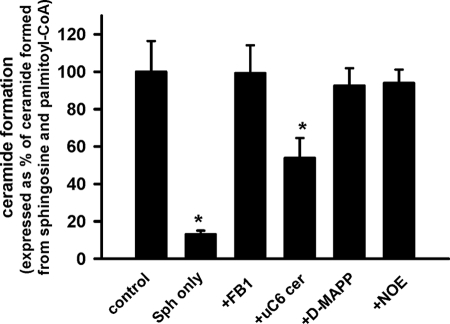
To assess which enzyme of ceramide metabolism is involved in mitochondrial ceramide formation, we tested inhibitors of ceramidases and a selective inhibitor of conventional ER CerS, FB1. Urea-ceramides are competitive inhibitors of neutral ceramidase (62). Fig. 3 shows that urea-C6-ceramide substantially (46%) suppressed ceramide formation at 50 μm. Further increases in urea-C6-ceramide concentrations did not result in increased inhibition, presumably because of poor compound solubility in aqueous solutions. Inhibitors of alkaline ceramidase d-MAPP (20 μm) (63) and acid ceramidase N-oleoylethanolamine (100 μm) (64) failed to inhibit ceramide formation. The CerS inhibitor in the ER, FB1, at concentrations that maximally suppress CerS activity (50 μm) (43) also had no effect, confirming the original observation of Bionda et al. (39). Thus, NCDase may contribute not only to ceramide cleavage but also to mitochondrial ceramide production.
FIGURE 3.
Effect of inhibitors of sphingolipid metabolism on ceramide production from sphingosine and palmitoyl-CoA by rat liver mitochondria. Mitochondria (1 mg/ml) were incubated as described under “Experimental Procedures” in the presence of 50 μm palmitoyl-CoA and 15 μm sphingosine, except that in column 2 palmitoyl-CoA was omitted. After 15 min the reaction was terminated by the addition of ethyl acetate/isopropyl alcohol extraction mixture. CerS inhibitor FB1 (50 μm), NCDase inhibitor urea-C6-ceramide (50 μm), acid CDase inhibitor N-oleoylethanolamine (NOE, 100 μm), and the inhibitor of alkaline CDase d-MAPP (20 μm) were added simultaneously with mitochondria and incubated for 5 min before substrates addition. Results are expressed as a % of the ceramide formed in the presence of palmitoyl-CoA and sphingosine and represent the average ± S.D. *, p < 0.05, n = 3.
Inhibitors of Mitochondrial Thioesterases Suppress Ceramide Formation from Sphingosine and Palmitoyl-CoA
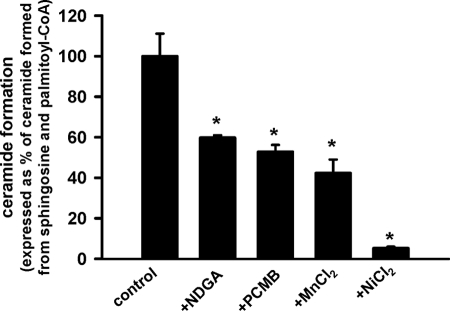
Ceramide synthesis by reverse ceramidase activity normally proceeds with free fatty acid and not fatty acyl-CoA. Mitochondrial thioesterases, however, can hydrolyze acyl-CoAs to CoA and free fatty acid and, therefore, could potentially lead to generation of substrate for reverse ceramidase activity. We, therefore, tested a set of mitochondrial thioesterase inhibitors on ceramide production using our experimental conditions. NDGA, an inhibitor of purified mitochondrial thioesterase Acot2 (MTE-I) (65), suppressed ceramide formation by 40% at 100 μm (Fig. 4). Similarly, another inhibitor of purified Acot2, p-chloromercuribenzoic acid (66), at 40 μm suppressed ceramide formation by 47%. Further increases in NDGA or p-chloromercuribenzoic acid concentrations induced progressive destabilization of mitochondrial membranes. It has also been reported that Mn2+ and Ni2+ suppress activity of partially purified mitochondrial thioesterase (67, 68). In our experiments, Mn2+ and Ni2+ employed at 1.5 mm decreased ceramide formation by 58 and 95%, respectively (Fig. 4). These data suggest that coupled activities of neutral ceramidase and thioesterase are essential for ceramide formation from sphingosine and palmitoyl-CoA by rat liver mitochondria.
FIGURE 4.
Effect of thioesterase inhibitors on ceramide production from sphingosine and palmitoyl-CoA by rat liver mitochondria. Mitochondria (1 mg/ml) were incubated as described under “Experimental Procedures” in the presence of 50 μm palmitoyl-CoA and 15 μm sphingosine for 15 min before the reaction was terminated by the addition of ethyl acetate/isopropyl alcohol extraction mixture. Where indicated, NDGA (100 μm), p-chloromercuribenzoic acid (PCMB; 40 μm), MnCl2 (1.5 mm), and NiCl2 (1.5 mm) were added simultaneously with mitochondria and incubated for 5 min before substrate addition. In experiments with MnCl2 and NiCl2, EDTA was omitted from incubation medium. Results are expressed as a % of ceramide formed in the presence of palmitoyl-CoA and sphingosine and represent the average ±S.D. *, p < 0.05, n = 3.
NCDase Is Present in Purified Mitochondrial Fractions from Rat Liver and Mouse
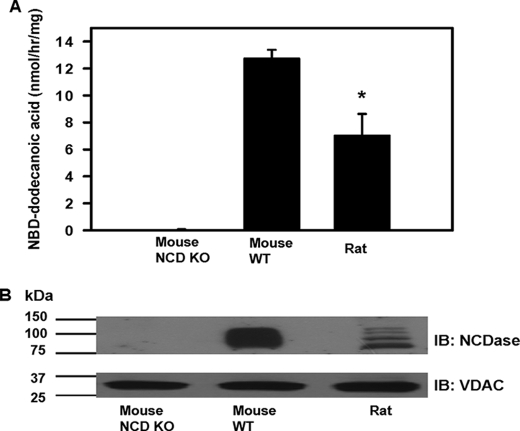
To determine definitely whether purified mitochondria contain NCDase, we measured hydrolysis by mitochondria of d-erythro-C12-NBD-ceramide as a substrate. Fig. 5, panel A, demonstrates the presence of ceramidase activity in mitochondria both from WT mouse and rat at neutral pH. Ceramidase activity of rat liver mitochondria was 45% less than activity found in WT mouse liver mitochondria. Mitochondria from NCDase KO mouse had negligible activity. Western blot analysis of mitochondrial fractions confirmed the presence of NCDase in the WT mouse and rat mitochondria (Fig. 5, panel B). At the same time, ceramidase was virtually absent in NCD KO mouse mitochondria. These data prove the presence of neutral ceramidase in liver mitochondria and suggest that this enzyme is a major carrier of mitochondrial ceramidase activity at neutral pH.
FIGURE 5.
Liver mitochondria from WT mouse and rat display ceramidase activity at neutral pH and contain NCDase. Panel A, mitochondria from WT mouse, NCDase KO mouse, and rat were treated and subjected to neutral ceramidase activity assay as described under “Experimental Procedures” using d-erythro-C12-ceramide as a substrate. The results represent the average ±S.D. *, p < 0.05, n = 3. Panel B, Western blot (IB) analysis shows the presence of NCDase in WT mouse and rat mitochondria but not in mitochondria from NCD KO mouse. VDAC was used as a loading control. The blot is representative of three independent experiments.
Rat Liver Mitochondria Produce Ceramide by a Reverse Ceramidase Reaction
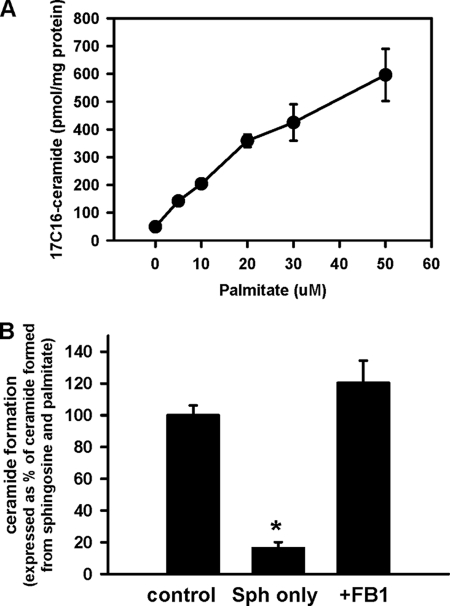
The only feasible method of ceramide formation with the participation of ceramidase is via reverse ceramidase activity, e.g. formation of ceramide from sphingosine and FFA. Indeed, such an activity was shown for the purified neutral ceramidase from liver (42) and brain (43) in a Triton X-100 mixed micelle assay. Importantly, ceramidase did not catalyze fatty acid transfer from acyl-CoA. Because purification of the membrane resident enzyme and utilization of detergent may produce artifacts in substrate specificity, we tested whether intact mitochondria can produce ceramide via the reverse ceramidase reaction with sphingosine and palmitate used as substrates. Fig. 6, panel A, shows that mitochondria produce ceramide from palmitate in a dose-dependant manner and the rate of ceramide formation is comparable with that observed in the presence of palmitoyl-CoA. Ceramide formation from palmitate and sphingosine was FB1-insensitive (Fig. 6, panel B), a finding in agreement with previously reported data for purified neutral ceramidase (43).
FIGURE 6.
Dose-response curve (A) and FB1 sensitivity (B) of ceramide formation from sphingosine and palmitate by rat liver mitochondria. Panel A, mitochondria (1 mg/ml) were incubated with 15 μm sphingosine and the indicated amounts of palmitate at the conditions described in “Experimental Procedures” for 15 min before the reaction was terminated by addition of ethyl acetate/ isopropyl alcohol extraction mixture. Results represent the average ±S.D., n = 3. In panel B, where indicated, 50 μm palmitate, 15 μm sphingosine, and 50 μm FB1 (simultaneously with mitochondria) were added to incubation medium. Results are expressed as a % of the ceramide formed in the presence of palmitate and sphingosine (Sph) and represent the average ±S.D., n = 3.
Knock Out of NCDase Suppresses Liver Mitochondria Ceramide Formation
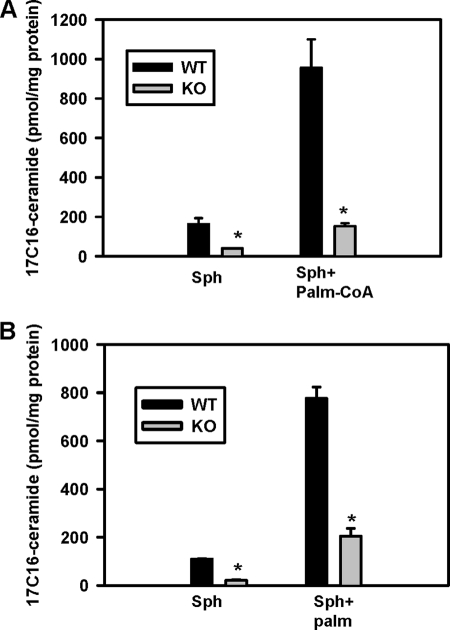
NCDase KO mice have a normal life span and do not show obvious abnormalities (46). To establish the involvement of NCDase in mitochondrial ceramide formation from sphingosine and palmitoyl-CoA/or palmitate, we studied liver mitochondria from NCDase KO mice. Ceramide formation in KO mouse liver mitochondria was substantially suppressed both at the basal level (sphingosine alone, Fig. 7, panel A and B, 75–78%, respectively) and in the presence of palmitoyl-CoA (Fig. 7, panel A, 84.7%) or palmitate (Fig. 7, panel B, 74.2%). Moreover, analysis of ceramide species in these KO mice liver mitochondria revealed a decrease in total ceramide, and of certain ceramide subspecies (supplemental Fig. S1). These data strongly suggest that NCDase may have a role in mitochondrial ceramide production and may contribute to the overall ceramide profile of liver mitochondria at basal conditions in vivo.
FIGURE 7.
Knock-out of NCDase suppresses formation of ceramide from sphingosine and palmitoyl-CoA (panel A) or palmitate (panel B) in mouse liver mitochondria. Mitochondria (1 mg/ml) were incubated as indicated with 50 μm palmitoyl-CoA, 50 μm palmitate (Palm), and 15 μm sphingosine (Sph) under conditions described under “Experimental Procedures” for 15 min before the reaction was terminated by addition of ethyl acetate/isopropyl alcohol extraction mixture. The results represent the average ± S.D. *, p < 0.05, n = 3.
Purified Mitochondria Have an Active Palmitoyl-CoA Hydrolytic Activity
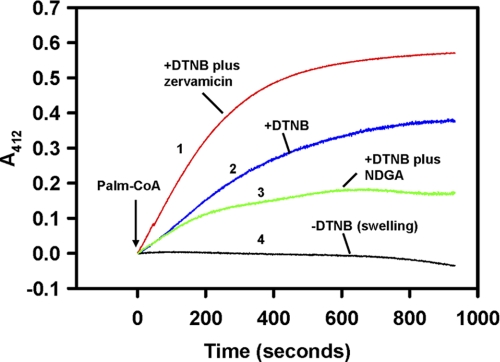
NCDase does not utilize palmitoyl-CoA in a reverse reaction (42, 43), so we postulated that the initial step is the hydrolysis of palmitoyl-CoA by mitochondrial-resident thioesterase to form palmitate and CoA. To evaluate this, we measured thioesterase activity using DTNB to trap CoA liberated from hydrolysis of palmitoyl-CoA. The addition of palmitoyl-CoA to mitochondria induced release of CoA and, hence, palmitate (Fig. 8, trace 2). Because palmitoyl-CoA is impermeable for the mitochondrial inner membrane, hydrolysis of palmitoyl-CoA reflects operation of thioesterase attached to the outer membrane or the enzyme trapped in intermembrane space. To assess the potential contribution of matrix thioesterase to mitochondrial palmitate production, we permeabilized the inner membrane by the pore-forming peptide, zervamicin IIB (47). The addition of zervamicin IIB (Fig. 8, traces 1 versus 2) increased the rate of palmitoyl-CoA hydrolysis by 2-fold (from 6.4 ± 0.2 to 14.7 ± 0.03 nmol/min/mg of protein, n = 3), suggesting that, at defined conditions, matrix thioesterase can effectively contribute to the mitochondrial pool of FFAs. Thioesterase inhibitor NDGA at 100 μm partially suppressed thioesterase activity (Fig. 8, traces 3 versus 2).
FIGURE 8.
Liver mitochondria from rat display thioesterase activity, which is enhanced in the presence of pore-forming peptide zervamicin IIB. Rat mitochondrial thioesterase activity was assayed as described under “Experimental Procedures.” Traces are corrected for the initial rapid drop in absorbance caused by the addition of palmitoyl-CoA, which reflects interaction of this compound with the mitochondrial membranes (96). Trace 1, thioesterase activity in the presence of pore-forming peptide zervamicin IIB (20 μg/ml); trace 2, no pretreatment; trace 3, suppression of thioesterase activity by 100 μm of NDGA. The trace was corrected for the time-dependant change in absorbance induced by simultaneous presence of NDGA and palmitoyl-CoA; trace 4, control for absorbance changes for traces 1 and 2 induced by palmitoyl-CoA in the absence of DTNB. The plot is representative of three independent experiments.
Permeabilization of the Inner Mitochondrial Membrane Increases Ceramide Production
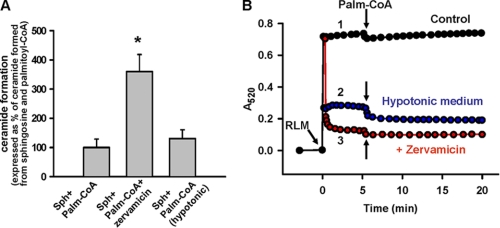
To determine the potential contribution of the mitochondrial matrix thioesterase to ceramide formation, mitochondria were treated with zervamicin IIB in the presence of sphingosine and palmitoyl-CoA. Zervamicin IIB treatment increased mitochondrial ceramide production by 3-fold (Fig. 9, panel A), which coincided with increased hydrolysis of palmitoyl-CoA (Fig. 8). Evaluation of the absorbance of the mitochondrial suspension as an indicator of mitochondrial volume showed drastic mitochondrial swelling in the presence of zervamicin IIB (Fig. 9, panel B). This swelling originates from the entry into the mitochondrial matrix of sucrose, which was used as an osmotic support. Increased ceramide production observed in the presence of zervamicin IIB might originate either from increased palmitate formation or from the stretching of the mitochondrial inner membrane, which in turn activates ceramidase. To exclude the later possibility, ceramide production was measured in a hypotonic medium (10 mm of HEPES (pH 7.4) adjusted by KOH). At these conditions, the mitochondrial inner membrane is completely unfolded (69). As depicted in Fig. 9, panel B, trace 2, mitochondrial absorbance at hypotonic conditions was similar to that observed with zervamicin IIB. Also, hypotonic conditions did not increase ceramide production similar to zervamicin IIB (Fig. 9, panel A). Therefore, the increased ceramide production in the presence of zervamicin IIB is likely due to acceleration of palmitoyl-CoA diffusion into the mitochondrial matrix. These results indicate that both cytosol-accessible thioesterase and enzyme localized in the mitochondrial matrix are potential sources of free palmitate, which can be subsequently utilized by ceramidase in a reverse reaction. This idea is in line with the ability of the thioesterase inhibitors to suppress ceramide production from sphingosine and palmitoyl-CoA (Fig. 4).
FIGURE 9.
Permeabilization in the mitochondrial inner membrane with pore-forming peptide zervamicin IIB increases ceramide production from palmitoyl-CoA and sphingosine in rat liver mitochondria. Mitochondria (RLM, 1 mg/ml) were incubated as described under “Experimental Procedures” in the presence of 50 μm palmitoyl-CoA (Palm-CoA) and 15 μm sphingosine (Sph) for 15 min before the reaction was terminated by addition of ethyl acetate/isopropyl alcohol extraction mixture. Where indicated, zervamicin IIB (20 μg/ml) was added simultaneously with mitochondria. In experiments where mitochondrial swelling was induced by hypotonic treatment, the medium consisted of 10 mm HEPES, 5 μm rotenone, and 500 μm EDTA (pH 7.4 adjusted by KOH). Panel A, ceramide production by mitochondria is shown. Panel B, absorbance of mitochondrial suspension at 520 nm is shown.
DISCUSSION
Ceramide is considered to be the central hub of sphingolipid metabolism (1), a trigger of cell death pathways associated with mitochondrial dysfunction (2, 3, 5, 70, 71). The prevailing paradigm depicts ceramidases as attenuating the levels of ceramide by decomposing it to sphingosine and FFA (41, 72, 73). Contrary to this view, in the present study we identified a novel pathway of ceramide production in intact liver mitochondria from sphingosine and palmitoyl-CoA controlled by NCDase. This pathway is provided by the coupled activities of mitochondrial thioesterase, which hydrolyzes palmitoyl-CoA to CoA and palmitate, and NCDase, which utilizes sphingosine and released palmitate in a reverse reaction to produce ceramide.
The presence of NCDase in liver mitochondria is a novel observation. Overexpressed in HEK293 cells, rat and mouse NCDase tagged with myc or fused with GFP at the C terminus are mainly localized to the plasma membrane, with additional redistribution in ER/Golgi compartments (45, 74). It was reported that the human enzyme localizes exclusively in mitochondria when overexpressed as a GFP fusion protein in HEK293 or MCF7 cells (44). However, this interpretation was questioned and attributed to mislocalization of enzyme due to GFP fusion to the N terminus (45). Examination of subcellular redistribution of endogenous enzyme revealed its tissue-specific nature (75). In rat kidney, NCDase was localized to the apical membranes of proximal tubules, distal tubules, and collecting ducts by confocal microscopy. By Percoll density gradient centrifugation of kidney membranes, NCDase was recovered in the fractions with a plasma membrane marker enzyme. In contrast, in liver hepatocytes, NCDase was redistributed across the cytoplasm as punctuate subcellular vesicles reminiscent of mitochondria, or lysosomes, with only partial co-localization with the marker for lysosomal/late endosomal compartments. By density centrifugation of total heavy membranes (20,000 × g/10 min post nuclei pellet), the liver enzyme was found in fractions with high β-galactosidase activity, a lysosome marker. However, because of similar buoyant densities of lysosomes and mitochondria, the presence of NCDase in mitochondria has not been excluded. This consideration suggests that NCDase might localize to multiple cellular compartments.
Liver mitochondrial preparations utilized in our experiments contain negligible contamination with lysosomes (Fig. 1) yet display ceramidase activity at neutral pH (Fig. 5, panel A) and the presence of NCDase as assessed by Western blot (Fig. 5, panel B). The stronger signal observed on Western blot with WT mouse liver mitochondria versus rat liver mitochondria might reflect more ceramidase present in this preparation. This interpretation coincides with lower ceramidase activity of rat liver mitochondrial fractions. However, different affinity of antibodies raised against the mouse enzyme can contribute to this phenomenon. Multiple ceramidase bands resolved in rat liver mitochondrial fraction might indicate some post-translational modifications, such as a different degree of glycosylation (42). Localization of NCDase in mitochondria shown in the present study agrees with the report of Bionda et al. (39) regarding the presence of reverse ceramidase reaction in Triton X-100-lysed, highly purified mitochondria. However, the magnitude of this reaction, relevance to intact mitochondria, and the carrier of this activity were not addressed.
Besides NCDase, acid and presumably alkaline ceramidases can catalyze reverse ceramidase reactions in mammalian cells (76, 77). Our experiments suggest that intact liver mitochondria not only catalyze formation of ceramide from sphingosine and palmitate, but also this reaction is higher (rat mitochondria; Fig. 6A) or comparable (mouse mitochondria, Fig. 7B) to the formation of ceramide from sphingosine and palmitoyl-CoA, suggesting a physiological relevance. Moreover, the reverse reaction is drastically suppressed in mitochondria from NCDase-deficient mice, suggesting that NCDase is the primary carrier of this activity at neutral pH in liver mitochondria (Fig. 7B). Remarkably, mitochondria from NCDase-deficient mice had 85% less activity in ceramide formation from sphingosine and palmitoyl-CoA compared with mitochondria from WT mice. Less pronounced suppression of ceramide production by the NCDase inhibitor urea-C6-ceramide (46%, Fig. 3) as compared with what is seen in NCDase KO mice could be related to poor solubility of this compound in aqueous solutions. Alternatively, this compound may only be a partial inhibitor of the reverse ceramidase activity as opposed to its inhibitory action of the ceramide hydrolytic activity. The lack of effect of the acid ceramidase inhibitor N-oleoylethanolamine and the alkaline ceramidase inhibitor d-MAPP would likely exclude the contribution of the reverse ceramidase activity of these enzymes in ceramide production. With respect to the contribution of CerS to the remaining 15% of NCDase-unrelated ceramide production, the question is more complicated. In our experiments, mitochondrial ceramide production from sphingosine and palmitoyl-CoA had minimal sensitivity to the CerS inhibitor FB1 (Fig. 3). However, resistance of C18-ceramide production to FB1 was observed in HEK-293T cells overexpressing CerS1 (78). Overall, our data suggest that in liver mitochondrial fractions, ceramide appears to be generated to a significant degree by reverse NCDase activity.
Such control of ceramide formation by NCDase is unusual because highly purified neutral ceramidases from the heavy membrane fraction of mouse liver (42) and rat brain (43) (150,000- and 20,000-fold purification, respectively) do not utilize acyl-CoA as a source of fatty acid in the reverse ceramidase reaction. However, earlier studies of Yavin and Gatt (79) provide evidence that partially purified (>200-fold) ceramidase from rat brain readily forms ceramide from oleyl-CoA as well as from oleic acid. An investigation of this phenomenon revealed the presence of acyl-CoA-hydrolyzing activity in ceramidase preparation, and the authors concluded that acyl-CoAs are not direct substrates for reverse ceramidase activity but should be first decomposed to CoA and FFA. Such co-purification of ceramidase and thioesterase activities and their coupled operation in reverse ceramidase reaction with acyl-CoA as a substrate suggested metabolic and possibly physical interaction between these two enzymes. We found that a similar type of functional coupling occurs in liver mitochondria in the time course of ceramide formation from sphingosine and palmitoyl-CoA. Indeed, ceramide formation was suppressed by both NCDase inhibitor urea-C6-ceramide and the inhibitors of thioesterase (Figs. 3 and 4). Moreover, the presence of a partial reaction of this coupling was also demonstrated; e.g. hydrolysis of palmitoyl-CoA to CoA and palmitate (Fig. 8) and the formation of ceramide with palmitate as a substrate (Figs. 6 and 7).
The hydrolysis of the thioester bond within long chain acyl-CoA is catalyzed by a family of thioesterases (Acots) (80, 81), which balance intracellular levels of acyl-CoAs, fatty acids, and CoA. Acots are localized in major intracellular compartments including cytosol, ER, peroxisomes, and mitochondria. From 13 members of the mammalian Acot family, four are associated with mitochondria, namely, Acot2 (MTE-I), Acot9, (MTE-2), Acot10, and Acot13 (81). Acot2 is thought to be localized preferentially in mitochondrial matrix (82), which is the major subcompartment of thioesterase activity in liver mitochondria (67). However, part of the activity is redistributed to the intermembrane space and the outer membrane. Indeed, up-regulation of Acot2 is accompanied by the increased hydrolysis of palmitoyl-CoA, which is impermeable to the inner membrane (83). Such dual redistribution of Acot activity agrees with our data showing active palmitoyl-CoA hydrolysis by intact mitochondria (Fig. 8), which can be doubled when permeability of the inner membrane is increased by the pore-forming peptide zervamicin IIB, allowing the passage of water soluble palmitoyl-CoA into the matrix space. Increased ceramide formation in the presence of zervamicin IIB strongly suggests that both cytosolic and mitochondrial matrix pools of palmitoyl-CoA can serve as a source of palmitate for reverse ceramidase and that up-regulation of mitochondrial Acot might be a regulatory event for this reaction. Indeed, diabetes or peroxisome proliferators up-regulate Acot2, which is matched by a severalfold increase in FFA export from mitochondria (83, 84). At the same time, the increase in ceramide production as a result of zervamicin IIB-facilitated diffusion of palmitoyl-CoA into the matrix can also potentially indicate the presence of NCDase in the matrix space. The spatial requirements for metabolic coupling between the NCDase and the thioesterase remain unknown. As mentioned above, available data support dual localization of thioesterase activity both in the mitochondrial matrix (palmitoyl-CoA inaccessible pool) and in the intermembrane space (palmitoyl-CoA accessible pool). Our unpublished data suggest that NCDase resides preferentially in the intermembrane space. It implies that NCDase and part of thioesterases are localized in the same compartment. However, partial localization of NCDase to the matrix space together with Acot2 is not excluded. Moreover, considering two above-mentioned partial reactions of ceramide production (thioesterase and reverse ceramidase activities) with FFA as an intermediate, it is not necessary that NCDase and thioesterase be localized in the same compartment. This is because FFAs undergo fast diffusion (flip-flop) across lipid bilayer membranes (85, 86). It is possible, for example, that FFA produced by thioesterase in the matrix space could be readily accessible to NCDase in the intermembrane space. This also implies that there is no need for physical interaction of these enzymes because of the fast lateral and transverse diffusion of FFA. However, it does not exclude some local (microdomain) coupling. The coupling of reverse ceramidase and thioesterase activities is of particular interest in a physiological context. In the cytoplasm, the concentration and the amount of FFA are highly buffered. In isolated hepatocytes, no FFA accumulation in the cytoplasm can be detected despite the increased uptake caused by FFA elevation in the medium (87). This is attributed to immediate activation of FFA to acyl-CoAs with the subsequent incorporation into phospholipids, triglycerides, or utilization in β-oxidation in mitochondria or peroxisomes. It seems, then, that the acyl-CoA (free, or as a complex with acyl-CoA-binding protein, ACBP (88)), is a transportable form of fatty acids, and thioesterases can locally raise the concentration of FFA, possibly for delivery to specific reactions. Such a mechanism was shown to operate in hormone-induced steroidogenesis, when arachidonic acid (AA) released by phospholipase A2 from plasma membrane phospholipids or cholesterol esters should be first re-esterified to AA-CoA by acyl-CoA synthase 4 and than finally presented for subsequent metabolism as FFA after AA-CoA hydrolysis by mitochondrial Acot2 (65, 89, 90). This underscores the importance of compartment-specific coupled operation of mitochondrial reverse ceramidase and thioesterase reactions observed in our study.
In the context of apoptosis, the interplay between acyl-CoA-producing reactions and acyl-CoA consumption in mitochondrial β-oxidation may represent another point of metabolic regulation of ceramide formation. In permeabilized hepatocytes, the pro-apoptotic molecule tBid suppresses mitochondrial carnitine palmitoyltransferase-1 (91), an enzyme involved in the passage of fatty acids to the mitochondrial matrix. This suppression resulted in accumulation of palmitoyl-CoA outside the mitochondria, which could affect ceramide synthesis. Indeed, inhibition of palmitoyltransferase-1 in intact cells by etomoxir enhanced ceramide production and augmented palmitate-induced apoptosis (92). An interesting idea is that in the time course of apoptosis a tBid-induced local raise in palmitoyl-CoA in the mitochondria produces ceramide via thioesterase and reverse ceramidase, which in turn potentiates insertion of Bax into the outer membrane.
Despite convincing evidence as to the ability of purified ceramidases or mitochondria resident ceramidase (our studies) to catalyze a reverse reaction, little is known regarding its physiological significance. Recent published work from our group suggests that the reverse activity of alkaline ceramidases 1 and 2 may be involved in a compensatory mechanism to sustain long-chain ceramides after down-regulation of CerS2 in SMS-KCNR neuroblastoma and MCF-7 breast cancer cells (77). Similarly, in the yeast Saccharomyces cerevisiae, sphingolipid reduction by inactivation of ER CerS (lag1Δlac1Δ deletion strain) can be corrected by overexpression of ceramidases (93). In our experiments, knock-out of NCDase substantially changed the ceramide profile of mouse liver mitochondria (supplemental Fig. S1). Two major ceramides, C22- and C24-, were decreased by 55 and 38%, respectively. Although the amount of some ceramides was not changed (C24:1-, C14-, C20:1-, C26:1-) or increased (C18-, C18:1-, C20:4-), the overall result of these changes translated to a 25% decrease of total ceramide. The particular changes of the ceramide profile in mitochondria of NCDase KO mice might be of physiological adaptive importance, but this role remains to be established. The lack of changes in sphingoid bases might indicate their increased utilization by the sequence of the reactions catalyzed by sphingosine kinase and sphingosine-1-phosphate lyase.
As for substrate specificity of the reverse ceramidase activity of NCDase in a Triton X-100/FFA mixed micelle assay, purified NCDase from rat brain showed Vmax values in descending order from myristate (C14:0) to lignocerate (C24:0), whereas Km values were all comparable (43). Thus, catalytic efficiency (Vmax/Km) was highest with myristate (highest synthesis rate) and lowest with lignocerate. The difference in the ceramide profile of NCDase KO mice observed in our study from the above results with purified enzyme indicate that more complex regulatory mechanisms are likely involved in vivo. These may include (a) the difference in substrate specificity of the thioesterase, which provides FFA for further metabolism (80), as compared with the substrate specificity of NCDase, (b) availability of the particular type of acyl-CoAs in the cytoplasm for mitochondrial thioesterase, and (c) activation of compensatory mechanisms, such as activation/increased expression of other ceramide-producing enzymes when one of them is down-regulated (77, 94), or increase of ceramide transfer from ER to mitochondria (33, 34, 95). All these mechanisms could potentially shape the ceramide profile of mitochondria after NCDase knock out. The 25% decrease of total ceramide in mitochondria from NCDase KO mice should be considered as the lower estimate of the contribution of the reverse ceramidase reaction to the mitochondrial ceramide pool because of the possibility of compensatory responses. Overall, it is evident that the reverse ceramidase reaction catalyzed by NCDase contributes to the ceramide steady state of liver mitochondria in vivo.
In summary, these studies suggest that NCDase is a key participant of ceramide formation from sphingosine and acyl-CoA in liver mitochondria. We provide evidence that the reaction occurs in two steps. First, palmitoyl-CoA is hydrolyzed by mitochondria by thioesterase to palmitate and CoA, and the NCDase condenses palmitate and sphingosine to form ceramide in a reverse ceramidase reaction. This ceramide formation pathway seems to be predominant in isolated liver mitochondria at basal conditions compared with the reaction catalyzed by mitochondrial CerSs and can contribute to establishing the ceramide profile of liver mitochondria in vivo. The presence of dissimilar pathways of ceramide formation in mitochondria versus the ER indicates that these compartments may represent distinct units of cellular metabolic control.
Supplementary Material
Acknowledgments
We thank Kathy Wiita-Fisk for administrative assistance, Stefka Spassieva for critical reading of the manuscript, and Christian Frezza for valuable advice related to isolation of mouse mitochondria. We thank Dr. Richard Proia for generously providing anti-NCDase antibody. We thank Dr. Jennifer G. Schnellmann for help with preparation of the manuscript. Measurement of sphingolipids was conducted by the Lipidomics Core of MUSC in a facility constructed with support from the National Institutes of Health Grant C06 RR018823 from the Extramural Research Facilities Program of the National Center for Research Resources.
This work was supported, in whole or in part, by National Institutes of Health Grants AG16583 (to L. M. O.), CA87584 (to Y. A. H.), and P20RR17677-04 (to T. I. G.). This work was also supported by the Russian Federal Target Program “Scientific and Science-Educational Personnel of Innovative Russia” (project NK-602P/19, state contract P1159) (to T. V. O.). This manuscript is based upon work supported in part by a MERIT Awards by the Office of Research and Development, Department of Veterans Affairs, Ralph H. Johnson Veterans Affairs Medical Center, Charleston, South Carolina (to L. M. O. and T. I. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ER
- endoplasmic reticulum
- CDase
- ceramidase
- NCDase
- neutral ceramidase
- CerS
- ceramide synthase
- D-MAPP
- d-erythro-2-(N-myristolyamino)-1-phenyl-1-propanol
- DTNB
- 5,5′-dithiobis-(2-nitrobenzoic acid)
- FB1
- fumonisin B1
- FFA
- free fatty acid
- NDGA
- nordihydroguaiaretic acid
- VDAC
- voltage-dependent anion channel
- MUSC
- Medical University of South Carolina
- NBD
- 7-nitrobenz-2-oxa-1,3-diazol-4-yl
- Acot
- acyl-coenzyme A thioesterase.
REFERENCES
- 1. Hannun Y. A., Obeid L. M. (2008) Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 2. Kolesnick R. N., Krönke M. (1998) Annu. Rev. Physiol. 60, 643–665 [DOI] [PubMed] [Google Scholar]
- 3. Morales A., Lee H., Goñi F. M., Kolesnick R., Fernandez-Checa J. C. (2007) Apoptosis 12, 923–939 [DOI] [PubMed] [Google Scholar]
- 4. Stancevic B., Kolesnick R. (2010) FEBS Lett. 584, 1728–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novgorodov S. A., Gudz T. I. (2009) J. Cardiovasc. Pharmacol. 53, 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birbes H., El Bawab S., Hannun Y. A., Obeid L. M. (2001) FASEB J. 15, 2669–2679 [DOI] [PubMed] [Google Scholar]
- 7. Ardail D., Popa I., Alcantara K., Pons A., Zanetta J. P., Louisot P., Thomas L., Portoukalian J. (2001) FEBS Lett. 488, 160–164 [DOI] [PubMed] [Google Scholar]
- 8. Tserng K. Y., Griffin R. (2003) Anal. Biochem. 323, 84–93 [DOI] [PubMed] [Google Scholar]
- 9. Dai Q., Liu J., Chen J., Durrant D., McIntyre T. M., Lee R. M. (2004) Oncogene 23, 3650–3658 [DOI] [PubMed] [Google Scholar]
- 10. Yabu T., Shimuzu A., Yamashita M. (2009) J. Biol. Chem. 284, 20349–20363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andreyev A. Y., Fahy E., Guan Z., Kelly S., Li X., McDonald J. G., Milne S., Myers D., Park H., Ryan A., Thompson B. M., Wang E., Zhao Y., Brown H. A., Merrill A. H., Raetz C. R., Russell D. W., Subramaniam S., Dennis E. A. (2010) J. Lipid Res. 51, 2785–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monette J. S., Gómez L. A., Moreau R. F., Bemer B. A., Taylor A. W., Hagen T. M. (2010) Biochem. Biophys. Res. Commun. 398, 272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gudz T. I., Tserng K. Y., Hoppel C. L. (1997) J. Biol. Chem. 272, 24154–24158 [DOI] [PubMed] [Google Scholar]
- 14. Di Paola M., Cocco T., Lorusso M. (2000) Biochemistry 39, 6660–6668 [DOI] [PubMed] [Google Scholar]
- 15. García-Ruiz C., Colell A., Marí M., Morales A., Fernández-Checa J. C. (1997) J. Biol. Chem. 272, 11369–11377 [DOI] [PubMed] [Google Scholar]
- 16. Pastorino J. G., Tafani M., Rothman R. J., Marcinkeviciute A., Hoek J. B., Farber J. L., Marcineviciute A. (1999) J. Biol. Chem. 274, 31734–31739 [DOI] [PubMed] [Google Scholar]
- 17. Novgorodov S. A., Szulc Z. M., Luberto C., Jones J. A., Bielawski J., Bielawska A., Hannun Y. A., Obeid L. M. (2005) J. Biol. Chem. 280, 16096–16105 [DOI] [PubMed] [Google Scholar]
- 18. Novgorodov S. A., Gudz T. I., Obeid L. M. (2008) J. Biol. Chem. 283, 24707–24717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szalai G., Krishnamurthy R., Hajnóczky G. (1999) EMBO J. 18, 6349–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy S. S., Madesh M., Davies E., Antonsson B., Danial N., Hajnóczky G. (2009) Mol. Cell 33, 377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arora A. S., Jones B. J., Patel T. C., Bronk S. F., Gores G. J. (1997) Hepatology 25, 958–963 [DOI] [PubMed] [Google Scholar]
- 22. Siskind L. J., Kolesnick R. N., Colombini M. (2002) J. Biol. Chem. 277, 26796–26803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siskind L. J., Kolesnick R. N., Colombini M. (2006) Mitochondrion 6, 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghafourifar P., Klein S. D., Schucht O., Schenk U., Pruschy M., Rocha S., Richter C. (1999) J. Biol. Chem. 274, 6080–6084 [DOI] [PubMed] [Google Scholar]
- 25. Di Paola M., Zaccagnino P., Montedoro G., Cocco T., Lorusso M. (2004) J. Bioenerg. Biomembr. 36, 165–170 [DOI] [PubMed] [Google Scholar]
- 26. Kashkar H., Wiegmann K., Yazdanpanah B., Haubert D., Krönke M. (2005) J. Biol. Chem. 280, 20804–20813 [DOI] [PubMed] [Google Scholar]
- 27. Ganesan V., Colombini M. (2010) FEBS Lett. 584, 2128–2134 [DOI] [PubMed] [Google Scholar]
- 28. Birbes H., Luberto C., Hsu Y. T., El Bawab S., Hannun Y. A., Obeid L. M. (2005) Biochem. J. 386, 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsko C. M., Hunter O. C., Rabinowich H., Lotze M. T., Amoscato A. A. (2001) Biochem. Biophys. Res. Commun. 287, 1112–1120 [DOI] [PubMed] [Google Scholar]
- 30. Yu J., Novgorodov S. A., Chudakova D., Zhu H., Bielawska A., Bielawski J., Obeid L. M., Kindy M. S., Gudz T. I. (2007) J. Biol. Chem. 282, 25940–25949 [DOI] [PubMed] [Google Scholar]
- 31. Martínez-Abundis E., Correa F., Pavón N., Zazueta C. (2009) FEBS J. 276, 5579–5588 [DOI] [PubMed] [Google Scholar]
- 32. Deng X., Yin X., Allan R., Lu D. D., Maurer C. W., Haimovitz-Friedman A., Fuks Z., Shaham S., Kolesnick R. (2008) Science 322, 110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mesicek J., Lee H., Feldman T., Jiang X., Skobeleva A., Berdyshev E. V., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2010) Cell. Signal. 22, 1300–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stiban J., Caputo L., Colombini M. (2008) J. Lipid Res. 49, 625–634 [DOI] [PubMed] [Google Scholar]
- 35. Wu B. X., Rajagopalan V., Roddy P. L., Clarke C. J., Hannun Y. A. (2010) J. Biol. Chem. 285, 17993–18002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaena de Avalos S., Okamoto Y., Hannun Y. A. (2004) J. Biol. Chem. 279, 11537–11545 [DOI] [PubMed] [Google Scholar]
- 37. Kitagaki H., Cowart L. A., Matmati N., Vaena de Avalos S., Novgorodov S. A., Zeidan Y. H., Bielawski J., Obeid L. M., Hannun Y. A. (2007) Biochim. Biophys. Acta 1768, 2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimeno H., Soeda S., Sakamoto M., Kouchi T., Kowakame T., Kihara T. (1998) Lipids 33, 601–605 [DOI] [PubMed] [Google Scholar]
- 39. Bionda C., Portoukalian J., Schmitt D., Rodriguez-Lafrasse C., Ardail D. (2004) Biochem. J. 382, 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levy M., Futerman A. H. (2010) IUBMB Life 62, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mao C., Obeid L. M. (2008) Biochim. Biophys. Acta 1781, 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tani M., Okino N., Mitsutake S., Tanigawa T., Izu H., Ito M. (2000) J. Biol. Chem. 275, 3462–3468 [DOI] [PubMed] [Google Scholar]
- 43. El Bawab S., Birbes H., Roddy P., Szulc Z. M., Bielawska A., Hannun Y. A. (2001) J. Biol. Chem. 276, 16758–16766 [DOI] [PubMed] [Google Scholar]
- 44. El Bawab S., Roddy P., Qian T., Bielawska A., Lemasters J. J., Hannun Y. A. (2000) J. Biol. Chem. 275, 21508–21513 [DOI] [PubMed] [Google Scholar]
- 45. Hwang Y. H., Tani M., Nakagawa T., Okino N., Ito M. (2005) Biochem. Biophys. Res. Commun. 331, 37–42 [DOI] [PubMed] [Google Scholar]
- 46. Kono M., Dreier J. L., Ellis J. M., Allende M. L., Kalkofen D. N., Sanders K. M., Bielawski J., Bielawska A., Hannun Y. A., Proia R. L. (2006) J. Biol. Chem. 281, 7324–7331 [DOI] [PubMed] [Google Scholar]
- 47. Sansom M. S., Balaram P., Karle I. L. (1993) Eur. Biophys. J. 21, 369–383 [DOI] [PubMed] [Google Scholar]
- 48. Balashova T. A., Shenkarev Z. O., Tagaev A. A., Ovchinnikova T. V., Raap J., Arseniev A. S. (2000) FEBS Lett. 466, 333–336 [DOI] [PubMed] [Google Scholar]
- 49. Hovius R., Lambrechts H., Nicolay K., de Kruijff B. (1990) Biochim. Biophys. Acta 1021, 217–226 [DOI] [PubMed] [Google Scholar]
- 50. Graham J. M. (1999) in Current Protocols in Cell Biology (Bonifacino J. S., Dasso M., Harford J. B., Lippincott-Schwartz J., Yamada K. M. eds) Vol. 1, pp. 3.4.6–3.4.22, John Wiley & Sons, Inc., New York [Google Scholar]
- 51. Lahiri S., Lee H., Mesicek J., Fuks Z., Haimovitz-Friedman A., Kolesnick R. N., Futerman A. H. (2007) FEBS Lett. 581, 5289–5294 [DOI] [PubMed] [Google Scholar]
- 52. Schulze H., Michel C., van Echten-Deckert G. (2000) Methods Enzymol. 311, 22–30 [DOI] [PubMed] [Google Scholar]
- 53. Berge R. K., Farstad M. (1979) Eur. J. Biochem. 96, 393–401 [DOI] [PubMed] [Google Scholar]
- 54. Means G. E., Feeney R. E. (1971) Chemical Modification of Proteins, p. 220, Holden-Day, San Francisco [Google Scholar]
- 55. Spassieva S., Bielawski J., Anelli V., Obeid L. M. (2007) Methods Enzymol. 434, 233–241 [DOI] [PubMed] [Google Scholar]
- 56. Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Methods 39, 82–91 [DOI] [PubMed] [Google Scholar]
- 57. Gatt S. (1966) J. Biol. Chem. 241, 3724–3730 [PubMed] [Google Scholar]
- 58. Tani M., Okino N., Mitsutake S., Ito M. (1999) J. Biochem. 125, 746–749 [DOI] [PubMed] [Google Scholar]
- 59. Wu B. X., Snook C. F., Tani M., Büllesbach E. E., Hannun Y. A. (2007) J. Lipid Res. 48, 600–608 [DOI] [PubMed] [Google Scholar]
- 60. Hirschberg K., Rodger J., Futerman A. H. (1993) Biochem. J. 290, 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vallée B., Riezman H. (2005) EMBO J. 24, 730–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Usta J., El Bawab S., Roddy P., Szulc Z. M., Yusuf, Hannun A., Bielawska A. (2001) Biochemistry 40, 9657–9668 [DOI] [PubMed] [Google Scholar]
- 63. Bielawska A., Greenberg M. S., Perry D., Jayadev S., Shayman J. A., McKay C., Hannun Y. A. (1996) J. Biol. Chem. 271, 12646–12654 [DOI] [PubMed] [Google Scholar]
- 64. Morales A., París R., Villanueva A., Llacuna L., García-Ruiz C., Fernández-Checa J. C. (2007) Oncogene 26, 905–916 [DOI] [PubMed] [Google Scholar]
- 65. Maloberti P., Lozano R. C., Mele P. G., Cano F., Colonna C., Mendez C. F., Paz C., Podestá E. J. (2002) Eur. J. Biochem. 269, 5599–5607 [DOI] [PubMed] [Google Scholar]
- 66. Svensson L. T., Alexson S. E., Hiltunen J. K. (1995) J. Biol. Chem. 270, 12177–12183 [DOI] [PubMed] [Google Scholar]
- 67. Berge R. K., Farstad M. (1979) Eur. J. Biochem. 95, 89–97 [DOI] [PubMed] [Google Scholar]
- 68. Lee K. Y., Schulz H. (1979) J. Biol. Chem. 254, 4516–4523 [PubMed] [Google Scholar]
- 69. Stoner C. D., Sirak H. D. (1969) J. Cell Biol. 43, 521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Siskind L. J. (2005) J. Bioenerg. Biomembr. 37, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Birbes H., El Bawab S., Obeid L. M., Hannun Y. A. (2002) Adv. Enzyme Regul. 42, 113–129 [DOI] [PubMed] [Google Scholar]
- 72. el Bawab S., Mao C., Obeid L. M., Hannun Y. A. (2002) Subcell. Biochem. 36, 187–205 [DOI] [PubMed] [Google Scholar]
- 73. Osawa Y., Uchinami H., Bielawski J., Schwabe R. F., Hannun Y. A., Brenner D. A. (2005) J. Biol. Chem. 280, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 74. Tani M., Iida H., Ito M. (2003) J. Biol. Chem. 278, 10523–10530 [DOI] [PubMed] [Google Scholar]
- 75. Mitsutake S., Tani M., Okino N., Mori K., Ichinose S., Omori A., Iida H., Nakamura T., Ito M. (2001) J. Biol. Chem. 276, 26249–26259 [DOI] [PubMed] [Google Scholar]
- 76. Okino N., He X., Gatt S., Sandhoff K., Ito M., Schuchman E. H. (2003) J. Biol. Chem. 278, 29948–29953 [DOI] [PubMed] [Google Scholar]
- 77. Spassieva S. D., Mullen T. D., Townsend D. M., Obeid L. M. (2009) Biochem. J. 424, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Venkataraman K., Riebeling C., Bodennec J., Riezman H., Allegood J. C., Sullards M. C., Merrill A. H., Jr., Futerman A. H. (2002) J. Biol. Chem. 277, 35642–35649 [DOI] [PubMed] [Google Scholar]
- 79. Yavin E., Gatt S. (1969) Biochemistry 8, 1692–1698 [DOI] [PubMed] [Google Scholar]
- 80. Hunt M. C., Alexson S. E. (2002) Prog. Lipid Res. 41, 99–130 [DOI] [PubMed] [Google Scholar]
- 81. Kirkby B., Roman N., Kobe B., Kellie S., Forwood J. K. (2010) Prog. Lipid Res. 49, 366–377 [DOI] [PubMed] [Google Scholar]
- 82. Svensson L. T., Engberg S. T., Aoyama T., Usuda N., Alexson S. E., Hashimoto T. (1998) Biochem. J. 329, 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. King K. L., Young M. E., Kerner J., Huang H., O'Shea K. M., Alexson S. E., Hoppel C. L., Stanley W. C. (2007) J. Lipid Res. 48, 1511–1517 [DOI] [PubMed] [Google Scholar]
- 84. Gerber L. K., Aronow B. J., Matlib M. A. (2006) Am. J. Physiol. Cell Physiol. 291, C1198–C1207 [DOI] [PubMed] [Google Scholar]
- 85. Kamp F., Zakim D., Zhang F., Noy N., Hamilton J. A. (1995) Biochemistry 34, 11928–11937 [DOI] [PubMed] [Google Scholar]
- 86. Pillai B. K., Jasuja R., Simard J. R., Hamilton J. A. (2009) J. Biol. Chem. 284, 33296–33304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Renaud G., Bouma M. E., Foliot A., Infante R. (1985) Arch. Int. Physiol. Biochim. 93, 313–319 [DOI] [PubMed] [Google Scholar]
- 88. Faergeman N. J., Knudsen J. (1997) Biochem. J. 323, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Castilla R., Gadaleta M., Castillo A. F., Duarte A., Neuman I., Paz C., Cornejo Maciel F., Podestá E. J. (2008) Endocrinology 149, 3743–3752 [DOI] [PubMed] [Google Scholar]
- 90. Maloberti P., Castilla R., Castillo F., Maciel F. C., Mendez C. F., Paz C., Podestá E. J. (2005) FEBS J. 272, 1804–1814 [DOI] [PubMed] [Google Scholar]
- 91. Giordano A., Calvani M., Petillo O., Grippo P., Tuccillo F., Melone M. A., Bonelli P., Calarco A., Peluso G. (2005) Cell Death Differ. 12, 603–613 [DOI] [PubMed] [Google Scholar]
- 92. Paumen M. B., Ishida Y., Muramatsu M., Yamamoto M., Honjo T. (1997) J. Biol. Chem. 272, 3324–3329 [DOI] [PubMed] [Google Scholar]
- 93. Schorling S., Vallée B., Barz W. P., Riezman H., Oesterhelt D. (2001) Mol. Biol. Cell 12, 3417–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mullen T. D., Spassieva S., Jenkins R. W., Kitatani K., Bielawski J., Hannun Y. A., Obeid L. M. (2011) J. Lipid Res. 52, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang X., Rao R. P., Kosakowska-Cholody T., Masood M. A., Southon E., Zhang H., Berthet C., Nagashim K., Veenstra T. K., Tessarollo L., Acharya U., Acharya J. K. (2009) J. Cell Biol. 184, 143–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brecher P. (1983) Mol. Cell. Biochem. 57, 3–15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.