Abstract
Rift Valley fever virus (RVFV), which belongs to the genus Phlebovirus, family Bunyaviridae, is a negative-stranded RNA virus carrying a single-stranded, tripartite RNA genome. RVFV is an important zoonotic pathogen transmitted by mosquitoes and causes large outbreaks among ruminants and humans in Africa and the Arabian Peninsula. Human patients develop an acute febrile illness, followed by a fatal hemorrhagic fever, encephalitis or ocular diseases. A viral nonstructural protein, NSs, is a major viral virulence factor. Past studies showed that NSs suppresses the transcription of host mRNAs, including interferon-β mRNAs. Here we demonstrated that the NSs protein induced post-transcriptional downregulation of dsRNA-dependent protein kinase, PKR, to prevent phosphorylation of eIF2α and promoted viral translation in infected cells. These two biological activities of the NSs most probably have a synergistic effect in suppressing host innate immune functions and facilitate efficient viral replication in infected mammalian hosts.
Keywords: Rift Valley fever virus, PKR, NSs protein, interferon, transcriptional suppression
Introduction
Rift Valley fever virus (RVFV) causes a mosquito-borne endemic disease, Rift Valley fever, which is a febrile illness resulting in a high rate of abortions in ruminants, such as sheep, goat and cattle, and is an acute febrile illness followed by fatal hemorrhagic fever, encephalitis or ocular diseases in humans. RVFV is distributed in sub-Saharan African countries and has also caused large outbreaks in Madagascar, Saudi Arabia and Yemen.[1-4] After the period of acute febrile illness, fewer than 1% of the patients develop a hemorrhagic syndrome with petechiae, sclera icterus and hypotension.[2] If the patients develop these symptoms, they usually die in 5 to 10 days.[2] Some patients develop neurological symptoms or retinal vasculitis.[2, 5]
RVFV, which belongs to the genus Phlebovirus, family Bunyaviridae, is a negative-stranded RNA virus[6] carrying a single-stranded, tripartite RNA genome composed of S, M and L segments. The S-segment encodes the N protein and a nonstructural protein, NSs, and expresses these proteins by using an ambi-sense strategy in infected cells. The M-segment encodes two major envelope glycoproteins, Gn and Gc; a nonstructural protein, NSm; and a minor structural protein, the 78kD protein; those 4 proteins are synthesized from different AUGs within the same open reading frame (ORF).[7] Finally, the L segment encodes L protein, a viral RNA-dependent RNA polymerase.
RVFV NSs protein is not essential for virus replication in cell culture [8], while it works as a major viral virulence factor in infected animals.[9] Over the past several years, significant progress has been made toward an understanding of the biological functions of RVFV NSs protein. Namely, the NSs protein suppresses host innate immune functions and manipulates host translational controls to promote efficient virus replication in infected mammalian hosts. The present report summarizes recent major progress in studies of RVFV, including the development of RVFV reverse genetics systems, and an increased understanding of the biological functions of the RVFV NSs protein.
RVFV NSs inhibits cellular general transcription
Experiments using an RVFV mutant Clone 13 (C13) provided evidence that the NSs protein contributes to virus virulence. C13 was a plaque-cloned mutant virus from a wild-type (wt) RVFV strain, 74HB59.[10] C13 was found to lack approximately 70% of the NSs ORF, whereas other genes were nearly identical to the wt virus. Bouloy et al. isolated reassortent viruses between C13 and wt RVFV, ZH548, and found that the reassortent viruses carrying the C13 S-segment were highly attenuated in mice.[9]
Subsequently, Le May et al reported an interesting biological function of NSs.[11] They found that a reduction in host mRNA synthesis occurred in cells infected with wt RVFV, but not in C13-infected cells. Expression of the wt NSs, but not the C13 NSs, also resulted in the suppression of reporter gene expression. Further studies showed that the NSs interacts with the p44 subunit of TFIIH, an essential transcriptional factor of cellular RNA polymerase II.[11] Further, it was found that NSs forms a characteristic filamentous structure in the nucleus of infected cells,[12] and that p44 subunits co-localize with filamentous structures.[11] Their data suggested that the binding of NSs to p44 interferes with the assembly of TFIIH in nucleus, resulting in the inhibition of cellular transcription.
RVFV NSs inhibits the induction of interferon (IFN)-β
The suppression of general host cellular transcription occurs after 8 h post RVFV infection in cell cultures, whereas the suppression of IFN-β mRNA synthesis occurs as early as 3 h post RVFV infection.[11, 13] These data suggest that RVFV may exert suppression of IFN-β mRNA synthesis by using a mechanism that differs from that of suppression of general host mRNA synthesis. IFN synthesis is regulated by specific transcription factors, including interferon regulatory factor (IRF-3), NF-kB, and AP-1, while NSs does not suppress the nuclear translocation of IRF-3 nor inactivates NF-κB and AP-1 functions.[13] Le May et al. showed that the NSs interacts with Sin3A-associated protein 30 (SAP30) through the transcription factor YY1, and maintains the repressor complex of SAP30, YY1 and Sin3A-associated co-repressor factors on IFN-β promoter.[14] Their data suggested that NSs, SAP30 and Sin3A-associated factors are recruited on the IFN-β promoter through YY1, while inhibiting CBP recruitment, histone acetylation, and transcriptional activation of the IFN-β promoter. Thus NSs not only suppresses host general transcription, but also specifically suppress IFN-β transcription early in infection.
Significant levels of IFN-α are induced in monkeys that are lethally infected with RVFV.[15] These data mean that although NSs can suppress synthesis of IFN-α/β mRNAs in infected cells, type I IFN is, in fact, produced in RVFV-infected mammalian hosts. It is likely that uninfected cells may acquire antiviral status by exposure to type I IFN in infected mammalian hosts. Also uninfected bystander cells may take up virus-specific structural moieties (e.g., double-stranded (ds) RNAs and viral single-stranded (ss) RNAs) which are released from infected cells, recognize them by host pattern recognition receptors, such as TLR3 or TLR7,[16] and activate signaling pathways that induce antiviral status. One may wonder whether the NSs' host transcription suppression activity alone is sufficient for the virus to efficiently replicate in the cells that have been in an antiviral status prior to infection. It is conceivable that RVFV may have developed another major defense mechanism that inactivates the host antiviral function early in infection.
Establishment of RVFV reverse genetics systems
To further understand the functions of RVFV proteins, including NSs, perform detailed analyses of replication strategies of RVFV, explore RVFV-host interactions, identify viral factors affecting RVFV pathogenesis and pursue vaccine development, we have developed a reverse genetics system of MP-12 strain of RVFV.[8] MP-12 is an attenuated strain of RVFV [17] and, unlike C13, it has an intact NSs gene; there is a single amino-acid substitution between MP-12 NSs gene and the NSs gene of ZH548, a parental wt virus of MP-12. Experiments using wt RVFV must be performed in a high-level biocontainment lab, e.g., BSL-4 labs at most institutions in the US, whereas MP-12 can be handled in a BSL-2 lab. Wt RVFV is a select agent in the US, whereas MP-12 is not. Thus, a detailed characterization of MP-12 and its mutants would be substantially easier to undertake than would that of wt RVFV and its mutant viruses.
To develop a T7 RNA polymerase-driven reverse genetics system of MP-12, we constructed three RNA-expression plasmids, each of which encoded full-length antiviral-sense RNA from the S, M or L-segments between the T7 promoter and hepatitis delta virus ribozyme sequence. Also a cassette consisting of an encephalomyocarditis virus internal ribosome entry site and N gene was cloned downstream of the T7 promoter and upstream of the T7 terminator to generate an N-protein-expression plasmid. The L gene replaced the N gene in the L-protein-expression plasmid. For the expression of viral proteins encoded in the M gene, an M gene was cloned into a eukaryotic expression vector pCAGGS. Co-transfection of these plasmids into BHK/T7-9 cells that stably express T7 RNA polymerase resulted in the production of MP-12.[8] Like reverse genetics systems of Bunyamwera virus[18] and La Crosse virus[19], transfection of only three RNA expression plasmids often results in recovery of infectious MP-12. Although the expression of NSs promotes the replication of RVFV minigenome RNA,[20] NSs expression inhibits the recovery of the virus.[8] After our success in developing an RVFV reverse genetics systems, others also reported development of similar T7 RNA polymerase-driven RVFV reverse genetics systems, including one for wt RVFV.[21] These RVFV reverse genetics systems use BHK-derived cell lines, and the importance of a compromised RIG-I pathway in BHK cells for the successful recovery of RVFV from plasmids was pointed out.[21] Moreover, the development of RVFV reverse genetic systems driven by RNA polymerase I was also reported.[21, 22]
Using the MP-12 reverse genetics system, we have generated various MP-12-derived mutant viruses, including MP-12 expressing C13-type NSs (rMP12-C13type), one lacking NSs (rMP12-NSdel), another expressing Renilla luciferase (rMP12-rLuc), and an additional one expressing green fluorescent protein (rMP12-GFP).[8] Consistent with the finding that NSs suppresses the induction of IFN-β,[13] rMP12-C13type and rMP12-NSdel induce significant amounts of IFN-β mRNA in IFN-competent MRC-5 cells, and MP-12 mutants lacking the NSs gene fail to replicate efficiently in MRC-5 cells.[8] In contrast, MP-12 mutants lacking the NSs gene replicated efficiently in IFN-incompetent Vero cells.[8] The luciferase and GFP genes in the rescued viruses were retained without mutations after 10 passages in Vero E6 cells, demonstrating that NSs is not required for viral replication in IFN-incompetent cells and NSs ORF can be replaced by a foreign gene in RVFV. Our subsequent study showed that the expression of a 78kD protein and NSm protein, both of which are encoded at the 5′ region of M gene ORF, is not necessary for RVFV replication[23] and that NSm suppresses virus-induced apoptosis.[24] Others reported that the NSm affects the virulence of RVFV.[25] These studies clearly illuminate the usefulness of RVFV reverse genetics systems for further analyses of this important pathogen.
A novel function of RVFV NSs to suppress the host innate immune system
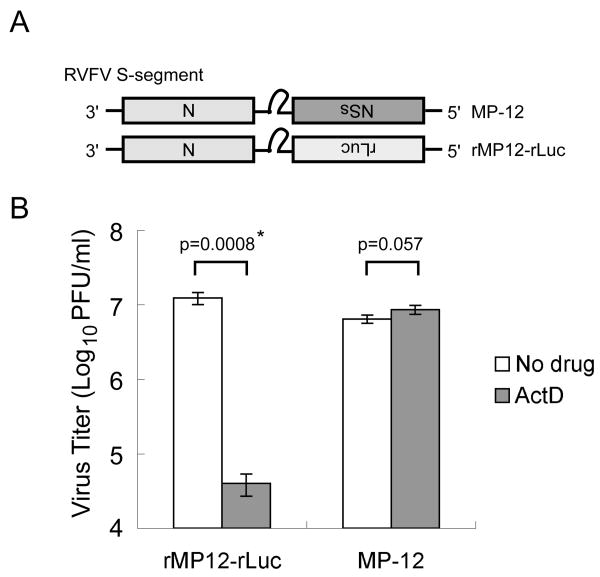
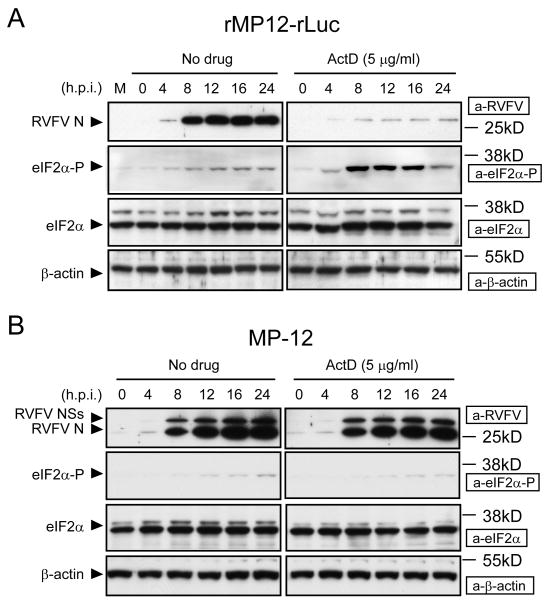
We hypothesized that, when combined, RVFV replication and NSs-induced host transcription suppression likely induces a cellular environment unsuitable for viral replication. To secure efficient RVFV replication, the NSs protein, in turn, alters this putative, virally unfriendly cellular environment to one that supports efficient viral replication. To test this possibility, we examined the replication of RVFV lacking the NSs gene in the presence of a host transcription inhibitor, actinomycin D (ActD) or α-amanitin, drugs which mimick the host transcriptional suppressive activities of the NSs. As shown in Fig. 1, MP-12 replicated more efficiently than did rMP12-rLuc in the presence of ActD,[26] which suggested to us that RVFV NSs has an alternative function other than that of cellular transcriptional suppression to support efficient viral replication under transcriptional shutoff. rMP12-rLuc replication in the presence of ActD, but not in the absence of ActD, resulted in an accumulation of phosphorylated eIF2α and poor accumulation of N protein (Fig.2); phosphorylation of eIF2α causes the suppression of translation initiation.[27] In contrast to rMP12-rLuc, an efficient accumulation of N protein occurred in MP-12-infected cells, both in the presence and absence of ActD, and an accumulation of phosphorylated eIF2α did not occur in rMP-12-infected cells (Fig. 2). These data suggested that NSs suppressed the accumulation of phosphorylated eIF2α, which was induced by the replication of MP-12 lacking NSs in the presence of transcription inhibitor.
Figure 1.
Effects of ActD on the replication of MP-12 and MP-12 lacking the NSs gene. The Fig is adapted from Ikegami et al.[26] (A) Schematic representations of the S segments of MP-12 and rMP12-rLuc. (B) Type I IFN-deficient VeroE6 cells were mock-treated or independently infected with MP-12 and rMP12-rLuc at an moi of 3, immediately treated with ActD (5 μg/ml) or left untreated, and culture fluids were harvested at 16 h.p.i. Virus titers of MP-12 and rMP12-rLuc were measured by a plaque assay. The virus replication of rMP12-rLuc was significantly reduced in the presence of ActD (*p<0.001; Student's t-test). Data are expressed as mean +/- standard deviation of three independent experiments.
Figure 2.
Status of eIF2α phosphorylation in rMP12-rLuc-infected cells and MP-12-infected cells in the presence of transcriptional inhibitors. The Fig is adapted from Ikegami et al.[26] VeroE6 cells were mock infected (M) or infected with rMP12-rLuc (A) or MP-12 (B) at an moi of 3, and then immediately treated with ActD or left untreated (No drug). Samples were harvested at the indicated time points post infection for Western blot analysis. RVFV N protein, NSs proteins, phosphorylated eIF2α, total eIF2α, and β-actin are shown by arrowheads. The data are representative of three independent experiments.
Among four kinases that phosphorylate eIF2α[28, 29], dsRNA-dependent protein kinase (PKR) phosphorylates eIF2α in response to dsRNA or 5′-triphosphated single-strand RNA. PKR is ubiquitously expressed in many tissues, yet it is highly induced following stimulation with IFN-α/β,[30] and suppresses viral translation in response to viral replication. To test whether PKR was involved in eIF2α phosphorylation in the cells infected with RVFV-lacking NSs in the presence of ActD, we created a recombinant MP-12 expressing a dominant-negative form of PKR, PKRΔE7[26, 31] in place of the NSs (rMP12-PKRΔE7). In the presence of ActD, the replication of rMP12-PKRΔE7 did not induce the accumulation of phosphorylated eIF2α, and rMP12-PKRΔE7 efficiently replicated as did MP-12 (Fig.3).[26] The results suggested to us that viral replication in the presence of transcriptional shutoff induces the accumulation of phosphorylated eIF2α via PKR, and MP-12 NSs inhibits the PKR-mediated phosphorylation of eIF2α.
Figure 3.
Role of PKR in eIF2α phosphorylation in infected cells under transcriptional suppression. The Fig is adapted from Ikegami et al.[26] (A) Schematic representations of S segments of MP-12 and rMP12-PKRΔE7. (B) VeroE6 cells were independently infected with MP-12, rMP12-rLuc and rMP12-PKRΔE7 at an moi of 3, or were mock infected. Cells were immediately treated with ActD (Act) or 50 μg/ml of α-amanitin (Ama), or were untreated. Cell extracts were prepared at 16 h.p.i. for Western blot analysis. Western blot analysis showing the accumulation of eIF2α, phosphorylated eIF2α, N protein, NSs protein, Flag-PKRΔE7 and β-actin in infected VeroE6 cells.
The PKR-like ER-localized eIF2α kinase PERK is another kinase that phosphorylates eIF2α.[28] We tested the effect of NSs on PERK-mediated eIF2α phosphorylation by treating MP-12-infected cells with thapsigargin, which induces endoplasmic reticulum stress and activates PERK,[32] and found that MP-12 infection failed to inhibit the thapsigargin-induced eIF2α phosphorylation (data not shown), which suggested to us that the NSs did not suppress PERK activity.
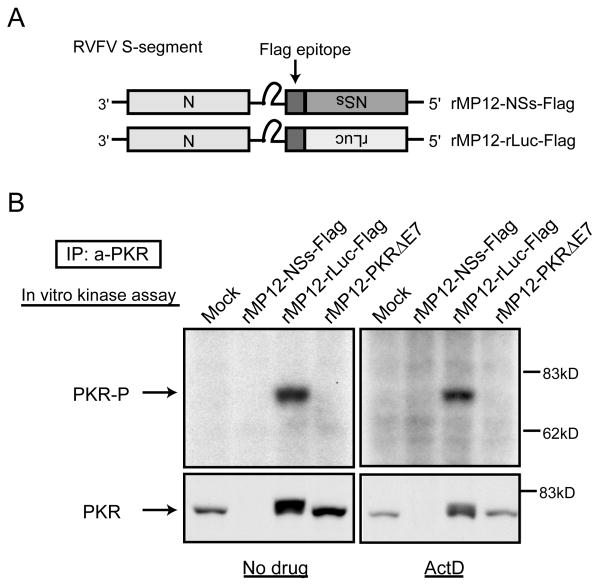
We used an immunoprecipitation (IP)-kinase assay to test the activation status of PKR in cells infected with rMP-12-Flag or rMP12-rLuc-Flag in the presence of ActD. In this assay, PKR was immunoprecipitated from the cell extracts of infected cells by anti-PKR antibody, and the PKR bound to the protein A beads was incubated in an appropriate buffer in the presence of [γ-32P]ATP.[33] If activated PKR is immunoprecipitated, it undergoes autophosphorylation. Autophosphorylated PKR is detected by separating the PKR in SDS-PAGE following autoradiography of the gel. Autophosphorylated PKR was detected in cells infected with rMP12-rLuc-Flag, but not in those infected with rMP12-NSs-Flag (Fig. 4). To our surprise, Western blot analysis of the precipitates by anti-PKR antibody showed that no PKR was immunoprecipitated from the extracts of rMP12-NSs-Flag-infected cells (Fig. 4), which led us to suggest that NSs downregulated PKR expression.
Figure 4.
Autophosphorylation of PKR in infected cells. The Fig is adapted from Ikegami et al.[26] (A) Schematic representations of RVFV S segments of rMP12-NSs-Flag and rMP12-rLuc-Flag. (B) 293 cells were mock-infected or infected with rMP12-NSs-Flag, rMP12-rLuc-Flag or rMP12-PKRΔE7 at an moi of 3, and, then, cells were mock-treated (No drug) or immediately treated with ActD. A cytoplasmic fraction was collected at 16 h.p.i. and the IP-kinase assay of PKR was performed as described previously.[26] A portion of the samples were used for Western blot analysis by using anti-PKR monoclonal antibody to show the abundance of immunoprecipitated PKR (bottom panel).
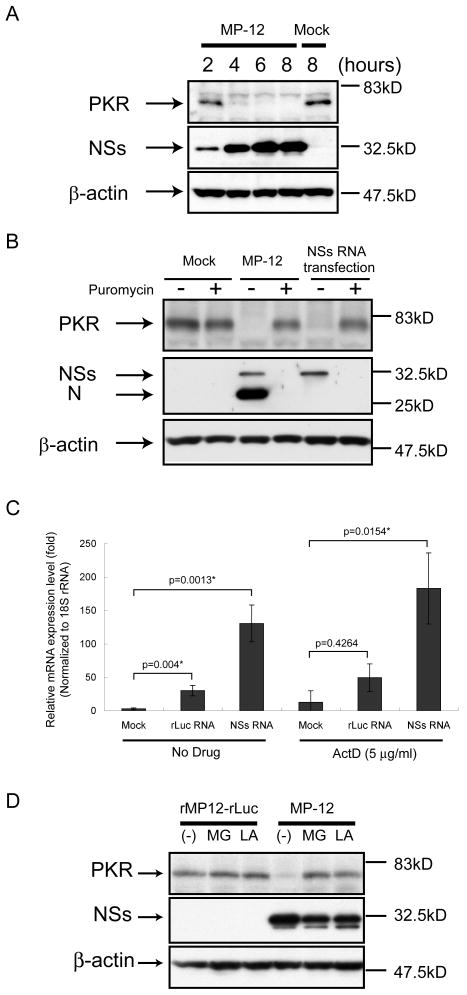
PKR was downregulated as early as 4 h p.i. in MP-12-infected cells, and NSs expression alone induced the downregulation of PKR (Fig. 5). In contrast, the suppression of cellular translation by treating cells with puromycin (100 μg/ml) for 16 h did not induce significant downregulation of PKR (Fig.5B).[26] Real-time PCR analysis showed that the abundance of PKR mRNA in NSs-expressing cells was greater than that in mock-treated cells and cells expressing rLuc protein, both in the presence and absence of ActD (Fig.5C), a finding that revealed NSs-mediated reduction of PKR abundance was not due to the reduced abundance of PKR mRNA. These data strongly suggested that NSs promotes PKR downregulation at the posttranscriptional level. Proteasome inhibitors, such as MG132 or lactacyctin, interfered with PKR downregulation in MP-12-infected cells (Fig.5D),[26] which suggested to us that RVFV NSs induced the downregulation of PKR by degradation through proteasomes in infected cells.
Figure 5.
Analysis of NSs-induced PKR downregulation. The Fig is adapted from Ikegami et al.[26] (A) 293 cells were mock infected (Mock) or infected with MP-12 (MP-12) at an moi of 3. Whole-cell lysates were collected at 2, 4, 6 and 8 h.p.i. Anti-PKR antibody, anti-NSs antibody and anti-β-actin antibody were used to detect PKR, NSs and β-actin, respectively. (B) 293 cells were mock infected (Mock) or infected with MP-12 at an moi of 3 or transfected with in vitro-synthesized RNA transcripts encoding NSs. Cells were then mock-treated or treated with 100μg/ml of puromycin. Cell extracts were harvested at 16 h.p.i. or 16 h post transfection, and the abundance of PKR and viral proteins were analyzed by Western blotting with anti-PKR antibody (top panel), anti-RVFV antibody (middle panel) or anti-β-actin antibody (bottom panel). (C) 293 cells were mock-transfected or transfected with in vitro-synthesized RNA transcripts encoding MP-12 NSs or rLuc. Cells were mock-treated or treated with 5 μg/ml of ActD. Total RNA was harvested at 8 h post transfection, and analyzed by real-time PCR. The relative abundance of PKR mRNA of each sample was calculated by the ΔΔCT method based on the abundance of 18S ribosomal RNA. The data shown in the graph (mean +/- standard deviation) were obtained from three independent experiments. The p value was determined by Student's t-test (*: p<0.05). (D) 293 cells were infected with rMP12-rLuc or MP-12 at an moi of 3, and, then, treated with 10 μM of MG132 (MG) or 50 μM of lactacystin (LA) or they were mock treated (-). Whole-cell lysates were collected at 8 h.p.i. and the abundance of PKR, NSs and β-actin was examined by Western blot analysis.
Studies from our group and those of others revealed that RVFV NSs has two distinct biological functions: NSs suppresses transcription of host mRNAs,[11] including type I IFN mRNAs,[13] and also downregulates PKR.[26] Because NSs-mediated downregulation of PKR was important for efficient viral translation under the conditions of host mRNA transcription induced by ActD, we suspect that NSs-mediated downregulation of PKR is also important in RVFV-infected cells where host mRNA synthesis is inhibited by the NSs in the nucleus.
NSs is expressed early in virus infection
The RVFV S-segment is an ambi-sense genome; NSs mRNA encoding NSs protein is synthesized from antiviral-sense RNA, while N mRNA encoding the N protein is synthesized from viral-sense RNA. This strategy of the NSs expression predicts that NSs protein synthesis occurs after synthesis of anti-viral sense S-segment from the incoming viral-sense S segment. One may wonder how RVFV could accumulate a sufficient abundance of NSs protein that efficiently suppresses IFN-β transcription and downregulates PKR as early as 3-4 h p.i. We observed that RVFV particles carry both viral-sense and antiviral-sense S-segments, and NSs mRNA is transcribed immediately after infection from an incoming antiviral sense S-segment prior to viral RNA replication.[34] A rapid accumulation of NSs in infected cells most probably is crucial for the virus to suppress host innate immune functions.
Unanswered questions
We demonstrated that NSs promotes PKR downregulation at posttranscriptional level. However, we noted several unanswered questions in our studies. One of them is why a significant accumulation of phosphorylated eIF2α occurred only in cells in which replication of RVFV lacking NSs was combined with treatment by transcriptional inhibitors; only a low level of phosphorylated eIF2α accumulation occurred in rMP-12-rLuc-infected cells in the absence of ActD (Fig. 2). One likely mechanism relates to the eIF2α dephosphorylation step, wherein phosphorylation of eIF2α at Serine 51 induces a rapid synthesis of activating transcription factor (ATF)-4 mRNA, which can be translated in the presence of phosphorylated eIF2α.[35] Expressed ATF4 then induces GADD34 expression[36] and GADD34 protein interacts with type 1 protein serine/threonine phosphatase, PP1, and this complex dephosphorylates eIF2α to resume cellular translation[37]. rMP12-rLuc replication in transcriptionally active cells probably induced PKR activation and eIF2α phosphorylation, the latter of which then induced GADD34 upregulation and subsequent eIF2α dephosphorylation, allowing efficient viral translation. In the presence of ActD, rMP12-rLuc replication induced PKR activation, whereas the transcription inhibitors would prevent GADD34 upregulation and subsequent eIF2α dephosphorylation, causing an accumulation of phosphorylated eIF2α, which inhibited viral translation.
Another main unanswered question is how NSs promoted PKR downregulation. It was reported that treatment of a macrophage cell line with IFN-γ induced PKR degradation.[38] Because NSs induced PKR degradation in ActD-treated cells, and ActD treatment completely inhibited an accumulation of host mRNAs, including IFN-β mRNA,[26] it is highly unlikely that IFN-γ was involved in the NSs-induced PKR downregulation. PKC activation also potentially induces PKR downregulation.[39] Treatment of MP-12-infected cells with a PKC inhibitor, GÖ6983, did not inhibit NSs-mediated PKR downregulation,[26] which implied that PKC was not involved in it.
Acknowledgments
The work was supported by a grant from NIAID to SM and CJP through the Western Regional Center of Excellence for Biodefense and Emerging Diseases Research, U54 AI057156, NIH-NIAID-DMID-02-24 Collaborative Grant on Emerging Diseases. Additional funding was via a McLaughlin postdoctoral fellowship (TI and WK), and from the Sealy Center for Vaccine Development at The University of Texas Medical Branch (TI).
References
- 1.Morvan J, Saluzzo JF, Fontenille D, et al. Rift Valley fever on the east coast of Madagascar. Res Virol. 1991;142:475–482. doi: 10.1016/0923-2516(91)90070-j. [DOI] [PubMed] [Google Scholar]
- 2.Peters CJ. Handbook Series of Zoonoses, Section B: Viral Zoonoses. Vol. 1. Boca Raton, FL: CRC Press; 1981. pp. 403–420. [Google Scholar]
- 3.Balkhy HH, Memish ZA. Rift Valley fever: an uninvited zoonosis in the Arabian Peninsula. Int J Antimicrob Agents. 2003;21:153–157. doi: 10.1016/s0924-8579(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 4.Meegan JM. The Rift Valley fever epizootic in Egypt 1977-78. 1. Description of the epizootic and virological studies. Trans R Soc Trop Med Hyg. 1979;73:618–623. doi: 10.1016/0035-9203(79)90004-x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hazmi A, Al-Rajhi AA, Abboud EB, et al. Ocular complications of Rift Valley fever outbreak in Saudi Arabia. Ophthalmology. 2005;112:313–318. doi: 10.1016/j.ophtha.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Schmaljohn C. Fields Virology. 4th. Lippincott, Williams & Wilkins; 2001. Bunyaviridae: the viruses and their replication; pp. 1581–1602. [Google Scholar]
- 7.Gerrard SR, Nichol ST. Synthesis, proteolytic processing and complex formation of N-terminally nested precursor proteins of the Rift Valley fever virus glycoproteins. Virology. 2007;357:124–133. doi: 10.1016/j.virol.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikegami T, Won S, Peters CJ, et al. Rescue of infectious rift valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J Virol. 2006;80:2933–2940. doi: 10.1128/JVI.80.6.2933-2940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouloy M, Janzen C, Vialat P, et al. Genetic evidence for an interferon-antagonistic function of rift valley fever virus nonstructural protein NSs. J Virol. 2001;75:1371–1377. doi: 10.1128/JVI.75.3.1371-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller R, Saluzzo JF, Lopez N, et al. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg. 1995;53:405–411. doi: 10.4269/ajtmh.1995.53.405. [DOI] [PubMed] [Google Scholar]
- 11.Le May N, Dubaele S, Proietti De Santis L, et al. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell. 2004;116:541–550. doi: 10.1016/s0092-8674(04)00132-1. [DOI] [PubMed] [Google Scholar]
- 12.Yadani FZ, Kohl A, Prehaud C, et al. The carboxy-terminal acidic domain of Rift Valley Fever virus NSs protein is essential for the formation of filamentous structures but not for the nuclear localization of the protein. J Virol. 1999;73:5018–5025. doi: 10.1128/jvi.73.6.5018-5025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billecocq A, Spiegel M, Vialat P, et al. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J Virol. 2004;78:9798–9806. doi: 10.1128/JVI.78.18.9798-9806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le May N, Mansuroglu Z, Leger P, et al. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 2008;4:e13. doi: 10.1371/journal.ppat.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrill JC, Jennings GB, Johnson AJ, et al. Pathogenesis of Rift Valley fever in rhesus monkeys: role of interferon response. Arch Virol. 1990;110:195–212. doi: 10.1007/BF01311288. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 17.Caplen H, Peters CJ, Bishop DH. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66:2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- 18.Lowen AC, Noonan C, McLees A, et al. Efficient bunyavirus rescue from cloned cDNA. Virology. 2004;330:493–500. doi: 10.1016/j.virol.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Blakqori G, Weber F. Efficient cDNA-based rescue of La Crosse bunyaviruses expressing or lacking the nonstructural protein NSs. J Virol. 2005;79:10420–10428. doi: 10.1128/JVI.79.16.10420-10428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikegami T, Peters CJ, Makino S. Rift valley fever virus nonstructural protein NSs promotes viral RNA replication and transcription in a minigenome system. J Virol. 2005;79:5606–5615. doi: 10.1128/JVI.79.9.5606-5615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habjan M, Penski N, Spiegel M, et al. T7 RNA polymerase-dependent and -independent systems for cDNA-based rescue of Rift Valley fever virus. J Gen Virol. 2008;89:2157–2166. doi: 10.1099/vir.0.2008/002097-0. [DOI] [PubMed] [Google Scholar]
- 22.Billecocq A, Gauliard N, Le May N, et al. RNA polymerase I-mediated expression of viral RNA for the rescue of infectious virulent and avirulent Rift Valley fever viruses. Virology. 2008;378:377–384. doi: 10.1016/j.virol.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Won S, Ikegami T, Peters CJ, et al. NSm and 78-kilodalton proteins of Rift Valley fever virus are nonessential for viral replication in cell culture. J Virol. 2006;80:8274–8278. doi: 10.1128/JVI.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Won S, Ikegami T, Peters CJ, et al. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J Virol. 2007;81:13335–13345. doi: 10.1128/JVI.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bird BH, Albarino CG, Nichol ST. Rift Valley fever virus lacking NSm proteins retains high virulence in vivo and may provide a model of human delayed onset neurologic disease. Virology. 2007;362:10–15. doi: 10.1016/j.virol.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Ikegami T, Narayanan K, Won S, et al. Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog. 2009;5:e1000287. doi: 10.1371/journal.ppat.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gale M, Jr, Tan SL, Katze MG. Translational control of viral gene expression in eukaryotes. Microbiol Mol Biol Rev. 2000;64:239–280. doi: 10.1128/mmbr.64.2.239-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 29.Garcia MA, Gil J, Ventoso I, et al. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhen KL, Samuel CE. Isolation of the interferon-inducible RNA-dependent protein kinase Pkr promoter and identification of a novel DNA element within the 5′-flanking region of human and mouse Pkr genes. Virology. 1997;227:119–130. doi: 10.1006/viro.1996.8306. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Koromilas AE. Dominant negative function by an alternatively spliced form of the interferon-inducible protein kinase PKR. J Biol Chem. 2001;276:13881–13890. doi: 10.1074/jbc.M008140200. [DOI] [PubMed] [Google Scholar]
- 32.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 33.Gunnery S, Mathews MB. RNA binding and modulation of PKR activity. Methods. 1998;15:189–198. doi: 10.1006/meth.1998.0623. [DOI] [PubMed] [Google Scholar]
- 34.Ikegami T, Won S, Peters CJ, et al. Rift Valley fever virus NSs mRNA is transcribed from an incoming anti-viral-sense S RNA segment. J Virol. 2005;79:12106–12111. doi: 10.1128/JVI.79.18.12106-12111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding HP, Calfon M, Urano F, et al. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864–34873. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- 37.Novoa I, Zeng H, Harding HP, et al. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maggi LB, Jr, Heitmeier MR, Scheuner D, et al. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO. 2000;19:3630–3638. doi: 10.1093/emboj/19.14.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Chase BI, Whitmore M, et al. Double-stranded RNA-dependent protein kinase (PKR) is downregulated by phorbol ester. FEBS. 2005;272:1568–1576. doi: 10.1111/j.1742-4658.2005.04572.x. [DOI] [PubMed] [Google Scholar]