Abstract
Fibroproliferative scars are an important clinical problem, and yet the mechanisms which regulate scar formation remain poorly understood. This study explored the hypothesis that the epidermis plays a critical role in dictating scar formation, and that those interactions differ in skin and mucosa. Paired skin and vaginal mucosal wounds on NZW rabbits diverged significantly; the cutaneous epithelium exhibited a greater and prolonged response to injury as compared to the mucosa. Microarray analysis of the injured epithelium was performed, and numerous factors were identified which were more strongly upregulated in skin, including several pro-inflammatory cytokines and pro-fibrotic growth factors. Analysis of the underlying mesenchymal tissue demonstrated a fibrotic response in the dermis of the skin but not the mucosal lamina propria, in the absence of a connective tissue injury. To determine if the pro-inflammatory factors produced by the epidermis may have a role in dermal fibrosis, an interleukin-1 receptor antagonist was administered locally to healing skin wounds. In the NZW rabbit model, blockade of interleukin-1 signaling was effective in preventing hypertrophic scar formation. These results support the idea that soluble factors produced by the epithelium in response to injury may influence fibroblast behavior and regulate scar formation in vivo.
Introduction
In nearly all connective tissues, the process of wound repair occurs via four major overlapping stages: homeostasis, inflammation, proliferation/extracellular matrix synthesis, and maturation/remodeling (Tredget et al. 1997). However, the magnitude of the injury response may differ vastly between various tissue types. For example, oral mucosal wounds tend to heal faster and with less inflammation than do equivalent cutaneous wounds (Szpaderska et al. 2003; Mak et al. 2009). Oral mucosal wounds also demonstrate a more highly regulated angiogenic response (Szpaderska et al. 2005), and have a differential expression of key pro-fibrotic growth factors (Eslami et al. 2009) compared to skin, resulting in less scarring than in skin wounds (Wong et al. 2009). Nevertheless, very little is known regarding the cellular and molecular mechanisms that are responsible for the superior wound repair in various mucosal tissues. Although the wound environment has been invoked (Zelles et al. 1995), studies suggest that there are also intrinsic tissue differences which may affect healing in mucosa and skin (Bussi et al. 1995).
Increasing evidence suggests that the epithelium may play a major role in regulating the behavior of the underlying connective tissue following injury. One of the few accepted clinical therapies for the treatment and prevention of cutaneous hypertrophic scarring is occlusion of the epidermis with silicone gel (Tredget et al. 1997; Mustoe 2008), and its mode of action is most likely by increasing the hydration state of the epidermal keratinocytes (Sawada and Sone 1992; Mustoe 2008; Tandara and Mustoe 2008; Gallant-Behm and Mustoe 2010). In vitro investigations have confirmed that hydrated keratinocytes may modulate fibroblast behavior, including collagen synthesis, through the production and release of pro-inflammatory cytokines (Chang et al. 1995; Tandara et al. 2007). Tissue-specific differences in epithelium structure, hydration state, and behavior may therefore have a major impact on the underlying connective tissue. Notably, the mucosal epithelium differs from skin in that it lacks a stratum corneum and does not need to serve functionally as a barrier to water loss. Furthermore, mucosal healing occurs in a fully hydrated environment. We therefore hypothesized that the mucosal epithelium would return to a homeostatic baseline more rapidly than the cutaneous epithelium and would behave differently following injury. In the present study, we demonstrate that the cutaneous and vaginal mucosal epithelium have a differential response to injury, and that increased epidermal production of pro-inflammatory and pro-fibrotic cytokines and growth factors results in dermal fibrosis and scar formation.
Results
Epithelial response to injury is greater in skin than in mucosa
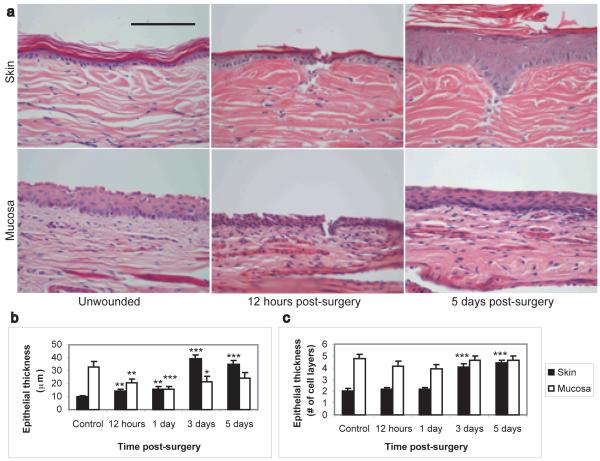
In order to identify tissue specific differences in epithelial behavior following injury, we developed a model of epithelial-restricted injury in the New Zealand White (NZW) rabbit. A grid of shallow incisional wounds, which extended through the entire epithelium but did not significantly damage the underlying connective tissue (30-50μm deep, Figure 1A, 12 hour time point), were created on the skin and vaginal mucosa, and samples were taken at various times post-surgery for histology and molecular analysis (Figure 1). All wounds were completely re-epithelialized between 1 and 2 days post-surgery. However, analysis of the reparative response demonstrated a marked difference in epithelial thickness, which suggests that the skin and mucosa utilized different means to restore the epithelial integrity. Immediately following injury, the mucosal epithelium thinned significantly, without a reduction in the number of epithelial cell layers (Figure 1B, C). This was followed by a return to the normal epithelial thickness following re-epithelialization. These findings indicate that the vaginal mucosa is similar to the gut mucosa in that it undergoes restitution, a process of epithelial cell dedifferentiation and migration to rapidly close a wound, followed by cell proliferation to restore the normal epithelial thickness in the area surrounding the wound site (Basson 2001). In contrast, these results show that the cutaneous epidermis increased in thickness within 12 hours of injury (Figure 1). This suggests that cell proliferation in skin either precedes or occurs at the same time as cell migration. Furthermore, cell proliferation in the epidermis continued after the completion of re-epithelialization and was sustained for many days, resulting in an epidermis that was hypertrophic, four times as thick as unwounded controls, and demonstrated a thickened stratum corneum. Similar persistent thickening was seen in other studies in the mouse (Schierle et al. 2007) and the rat (Kloeters et al. 2008). Thus, the cutaneous epithelium demonstrated an exaggerated response to injury as compared to the mucosal epithelium.
Figure 1. Differential epithelial response to wounding in skin and mucosa.
Representative histology photomicrographs of control and injured skin and vaginal mucosa (A) demonstrate that following injury, the cutaneous epithelium undergoes significant hypertrophy while the mucosal epithelium does not. Measurement of epithelial thickness (B and C) indicates that mucosal wounds heal via restitution, resulting in a transient thinning of the epithelium without a reduction in the number of cell layers. In contrast, cutaneous wounds healed by keratinocyte proliferation, resulting in a significant increase in epidermal thickness following re-epithelialization. Magnification: 40X. Scale bar = 100μm. N=8 wounds per time point. * = p<0.05, ** = p<0.01, *** = p<0.001.
Microarray analysis identifies significant differences in gene expression between skin and mucosal epithelium following injury
A microarray analysis was performed on RNA extracted from the cutaneous and vaginal mucosal epithelium at 12 hours, 1 day, 3 days, and 5 days following injury, as well as unwounded tissue. A custom Agilent 4×44K rabbit microarray was utilized. From a total of 23679 unique probes, 14647 met all preprocessing criteria (see Materials and Methods section for complete details). A principal component analysis was performed (Supplemental Figure 1A), and the largest source of variability in the data was identified as tissue type. A heatmap of the top 50 probes in the first principal component (Supplemental Figure 1B) demonstrates that epithelial gene expression is dramatically different between skin and mucosa at all time points, including prior to injury. In fact, a total of 1083 unique genes were differentially expressed in unwounded skin and mucosa (complete dataset is available online). Of those, 528 unique genes were upregulated in skin, and 555 were upregulated in mucosa. Functional analysis using the Gene Ontology database indicated that the majority of those differentially expressed genes fell into the categories of cellular process, cellular metabolic process, transport, anatomical structure development, and cellular component organization (Supplemental Figure 2A). The second principal component identified was time following injury (heatmap of top 50 probes available in Supplemental Figure 1C). To prevent the tissue specific differences at time 0 from overshadowing the gene expression changes over time in response to injury, the data were then normalized against their respective unwounded tissue prior to any additional analysis. Not surprisingly, the top 50 probes identified in the second principal component included a number of molecules involved in epithelial proliferation and differentiation, inflammation, and extracellular matrix synthesis and reorganization. However, at all time points following injury, the skin responded more vigorously to injury than did the mucosa (Table 1). Prior to complete re-epithelialization (12 hour and 1 day time points), three times more genes were upregulated in skin than in mucosa, as compared to unwounded tissue. Furthermore, in the genes that were differentially expressed between skin and mucosa at each time point, there was a greater upregulation of the top genes in skin than in mucosa. In skin, the most highly upregulated gene (compared to mucosa) was IL-8 (up 859-fold at 3 days). This was closely followed by S100 calcium binding protein A12 (581-fold), prostaglandin-endoperoxide synthase 2 (295-fold), IL-1β (231-fold), vanin 1 (210-fold), CCL4-like-2 (208-fold), serum amyloid A1 (194-fold), S100 calcium binding protein A9 and A8 (191-fold and 153-fold respectively), MMP-1 (141-fold), arginase (103-fold), MMP-3 (88-fold), and apolipoprotein B mRNA editing enzyme, catalytic polypeptide 1 (62-fold). In contrast, there was not a single gene that following injury was upregulated in mucosa more than 50-fold as compared to skin. Also of significance, it was observed that the cutaneous response to injury was prolonged as compared to mucosa. At 5 days after injury, only 34 genes remained upregulated in mucosa (as compared to unwounded tissue), whereas 454 genes remained upregulated in skin. Functional analysis identified many of the genes that remained upregulated in skin after re-epithelialization as belonging to the Gene Ontology groups of cellular process, anatomical structure development, multicellular organismal development, response to stress, inflammatory response, signal transduction, cell migration, and wound healing (Supplemental Figure 2A). These results correlate with the histology findings of increased and prolonged cutaneous keratinocyte activation following injury. Taken together, they indicate that the skin has a greater response to injury and requires a longer period of time to restore homeostasis after injury than does the mucosa.
Table 1. Gene expression changes over time following injury.
| Number of unique genes | ||||
|---|---|---|---|---|
| Comparison | 12 hours | 1 day | 3 days | 5 days |
| Injured vs uninjured skin | 926 | 988 | 495 | 454 |
| Injured vs uninjured mucosa | 261 | 309 | 100 | 34 |
| Injured skin vs injured mucosa |
728 |
892 |
463 |
489 |
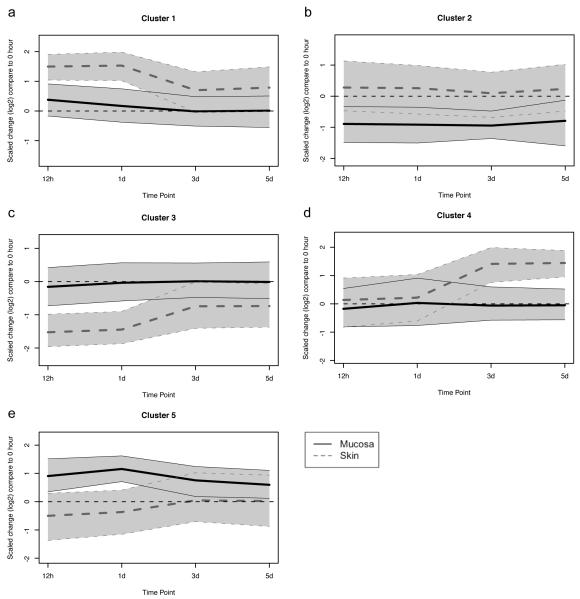
The above investigations have identified a number of major differences between skin and vaginal mucosa, including differences in basal gene expression and the identification of genes that are upregulated in one tissue as compared to another at each different time point following wounding. However, it is also important to identify the genes that have a different temporal pattern of expression between the two tissue types following injury. In order to do this, a K-means cluster analysis was performed on all 1075 unique genes that were differentially expressed between the skin and mucosal wounds at one or more time points, after normalization to unwounded tissue. A total of five clusters of genes were identified (Figure 2 and Supplementary Table 1). Cluster 1 contains a group of genes which are more highly upregulated in skin than in mucosa immediately following wounding, and which persist in their elevation over time (e.g. IL-1α). Cluster 2 contains several genes which are downregulated in mucosa following wounding, but which remain unchanged in skin (e.g. β-catenin). Cluster 3 is the opposite of cluster 1, wherein those genes are significantly downregulated in skin at the early time points following injury, but which are modestly downregulated or are unchanged in mucosa at the same time points (e.g. growth differentiation factor 1). The genes included in cluster 4 are upregulated following re-epithelialization in skin, but remain unchanged in mucosa (e.g. iNOS). Finally, the genes in cluster 5 are upregulated in mucosa and downregulated in skin following injury (e.g. MMP-2). Functional analysis of these clusters was performed. Functionalities that are over-represented in clusters 1 and 4 (up in skin) include cellular process, response to stress, oxidative stress, stimulus, and wounding, inflammatory response, defense response, cellular metabolic process, and response to TGF-β receptor signaling. Functionalities that are over-represented in clusters 3 and 5 (down in skin) include cellular process, response to stress and stimulus, anatomical structure development, organ development, anatomical structure morphogenesis, and regulation of developmental process. Although many of the Gene Ontology categories overlap for these clusters, it is clear that factors that are involved in cell proliferation, inflammation, and cell metabolism are upregulated in skin following wounding, whereas factors that are involved in cellular differentiation, tissue organization, and organ development have a reduced expression in skin following wounding, as compared to mucosa.
Figure 2. Cluster analysis: Different temporal patterns of gene expression are observed in skin and mucosal epithelium following injury.
K-means cluster analysis was performed on 1075 unique genes that were differentially expressed in skin and mucosal wounds compared to unwounded tissue. A value of 0 indicates that expression of that group of genes is the same as unwounded tissue. The bold line indicates the cluster center, and the grey band, the 95% confidence interval. Clusters A and D are upregulated in skin, but not mucosa following injury. Cluster B and C are downregulated in mucosa and skin, respectively, following injury. Cluster E is upregulated in mucosa following injury but is slightly downregulated in skin over the same time course.
Pro-inflammatory, pro-fibrotic signals produced by the epithelium following injury induce a fibrotic response in the underlying connective tissue
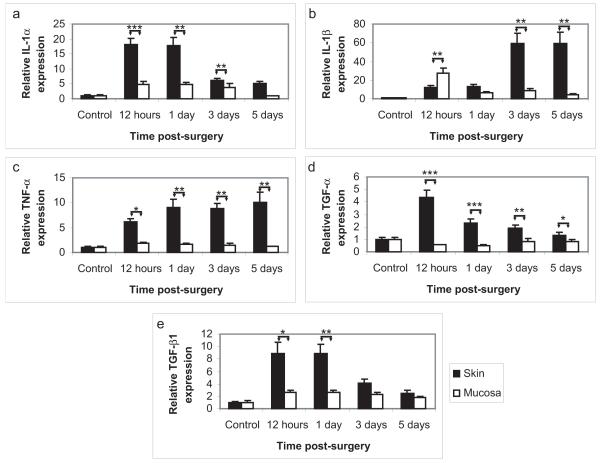
The epithelium has the capacity to interact with the underlying connective tissue through the production of pro-inflammatory cytokines, chemokines, and growth factors (Barrientos et al. 2008). Furthermore, microarray analysis identified that the Gene Ontology functionalities of “inflammation” and “TGF-β receptor signaling” were increased in skin as compared to mucosa following epithelial injury. Therefore, additional investigations were performed in order to elucidate the role of epithelial-induced inflammation in the process of wound healing. Quantitative reverse transcriptase PCR was performed on all epithelial samples utilized in the microarray analysis as well as on several additional samples (N=8 per time point). Several pro-inflammatory cytokines were investigated, including IL-1α, IL-1β, and TNF-α (Figure 3A-C). As observed in the microarray, IL-1α expression was significantly upregulated in both skin and vaginal mucosa following injury (15-fold and 3-fold, respectively at 12 hours), and that upregulation was significantly greater in skin than in mucosa. This was then followed by a significant upregulation in IL-1β in skin following re-epithelialization (42-fold at 3 days). Furthermore, TNF-α expression was elevated in skin but not in mucosa at all time points following injury. Thus, it was confirmed that the cutaneous epithelium produced significantly more pro-inflammatory cytokines and for a significantly longer period of time than did the mucosal epithelium of the same animal, following an identical injury. Pro-fibrotic growth factors are also important for regulating the wound healing process. Therefore, the growth factors TGF-α, and TGF-β were also investigated by Q-RT-PCR (Figure 3D-E). Both factors were significantly upregulated in the cutaneous epithelium but not in the mucosal epithelium, and for TGF-α, this upregulation persisted beyond the time of re-epithelialization. These results validate the microarray findings, and confirm that the pro-inflammatory and pro-fibrotic response of the skin is greater than that of the mucosa, following epithelial injury.
Figure 3. Increased pro-inflammatory/pro-fibrotic gene expression in cutaneous epithelium following injury.
Quantitative reverse transcriptase PCR was performed on all epithelial RNA samples (N=8). Expression of the pro-inflammatory cytokines IL-1α (A), IL-1β (B), and TNF-α (C), as well as the growth factors TGF-α (D), and TGF-β (E) were significantly higher in skin than in mucosa following injury. These results confirm the results of the microarray, including the temporal pattern of IL-1α and β upregulation in skin. Notably, the upregulation of IL-1β and TNF-α in skin was not inhibited by wound closure (day 2), indicating that the epidermal keratinocytes remain highly active for a period of time following re-epithelialization. * = p<0.05, ** = p<0.01, *** = p<0.001.
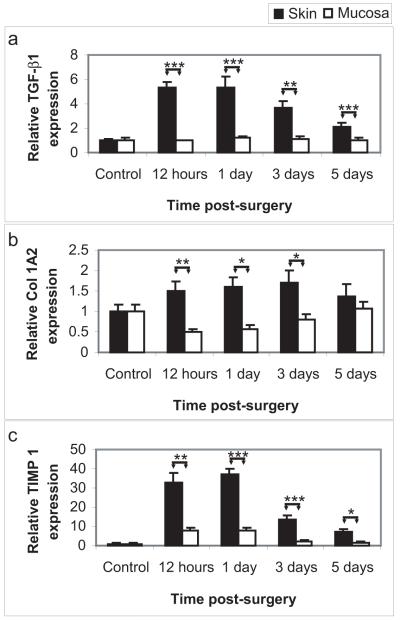
A number of the factors produced by the epithelium have the potential to cross the basement membrane and act on the fibroblasts and other cells of the underlying connective tissue (Barrientos et al. 2008). For example, in the normal circumstance of an injury that affects both the epithelium and connective tissue, epithelial-derived IL-1β and TGF-β have the potential to induce fibroblast expression of TGF-β, thereby stimulating fibroblast proliferation and extracellular matrix synthesis (Barrientos et al. 2008). Therefore, in this study, Q-RT-PCR was performed on RNA extracted from the unwounded connective tissue of the skin (dermis) and vaginal mucosa (lamina propria) that was located beneath the injured epithelium, to determine if an injury restricted to the epithelium may produce a pro-fibrotic response in the connective tissue. Notably, there was indeed a significant upregulation of TGF-β in the skin, but not the mucosa, in the absence of a connective tissue injury. Furthermore, expression of collagen 1a2, one of the major extracellular matrix components in the dermis and lamina propria, and of TIMP-1, the major inhibitor of the matrix metalloproteinases, was also increased in cutaneous but not mucosal connective tissue following an epithelial injury. These results indicate that the increased epidermal keratinocyte activation following injury and the increased production of pro-inflammatory and pro-fibrotic factors by the injured epidermis were sufficient to produce a fibrotic response in the dermis in the absence of dermal injury. Furthermore, the reduced response of the mucosal epithelium to injury was sufficient to restore the epithelial barrier, but did not result in pro-fibrotic changes in the underlying connective tissue. Thus, these findings suggest that the response of the epithelium following injury may in fact regulate the connective tissue’s behavior.
Excessive or prolonged inflammation may induce cutaneous fibrosis and scarring
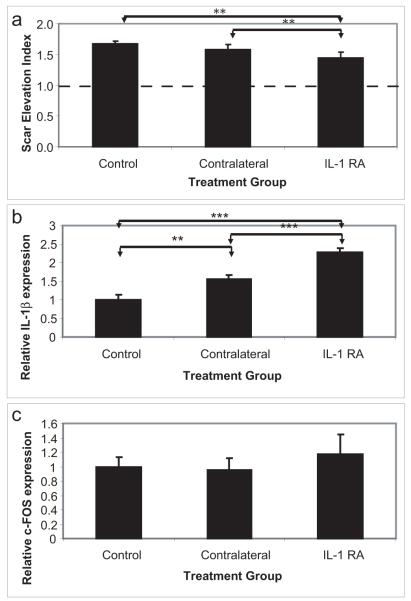
Most injuries to the skin are not restricted to the epithelium, and therefore, some fibroblast proliferation and extracellular matrix synthesis are required for complete repair of the dermis. However, in many cases, the response to wounding is greater than the injury itself, resulting in a scar. In order to determine the role of excessive or prolonged inflammation in the regulation of wound healing and scar formation, we utilized a more invasive wound model that reliably forms clinically-relevant scars. Full thickness punch wounds were created on the ventral ear of New Zealand White rabbits, and were allowed to heal. Due in part to the slow healing (18-20 days to complete re-epithelialization) and due to the long delay in restoring the epithelial integrity (weeks to months after re-epithelialization)(Gallant-Behm and Mustoe 2010), these wounds reproducibly form hypertrophic scars between 28 and 35 days following the initial surgery. In this study, an IL-1 receptor antagonist (Anakinra, Amgen, Thousand Oaks CA), which inhibits both IL-1α and IL-1β signaling, was administered locally to a subset of wounds following complete re-epithelialization. In order to determine whether the recombinant human IL-1RA would function in the rabbit, a multiple sequence alignment of the human and rabbit sequence was performed using Multalin version 5.4.1 (France) (Corpet 1988) (Supplemental Figure 3). It was determined that 16 of the 17 amino acid residues required for the antagonist-receptor interaction (Schreuder et al. 1997) were conserved between rabbit and human. Furthermore, administration of the IL-1RA was effective in reducing the dermal fibrosis in this animal model (Figure 5). There was a compensatory increase in IL-1β expression observed following treatment with the IL-1RA, however, there remained sufficient antagonist in the system to prevent increased downstream signaling (c-FOS expression, Figure 5B, C). Therefore, interference with the inflammatory cascade following complete re-epithelialization was indeed sufficient to reduce the dermal fibrotic response and inhibit scarring.
Figure 5. Interleukin-1 antagonists reduce cutaneous fibrosis following injury.
To determine the role of proinflammatory cytokines in regulating dermal fibrosis, an interleukin-1 receptor antagonist was locally administered in the NZW model of hypertrophic scarring. The scar elevation index (SEI) was measured using histology sections of wounds at day 35 (A). An SEI >1 indicates a raised, hypertrophic scar. Administration of IL-1RA significantly reduced the dermal hypertrophy as compared to both contralateral wounds and control animals. Contralateral and IL-1RA treated wounds both demonstrated a compensatory increase in the expression of IL-1β (B), however, there was no increase in the expression of downstream signaling molecules (C), due to the high local concentration of IL-1RA. N=24-48 wounds. * = p<0.05, ** = p<0.01, *** = p<0.001.
Discussion
In this report we show that the skin and vaginal mucosal epithelium have a differential response to injury, and that the injured epithelium can affect the behavior of the underlying mesenchymal tissue, even in the absence of a connective tissue injury. Given that little is understood about the pathophysiology of hypertrophic scar and keloid formation, the mechanism described here may help to explain how fibroproliferative scars form in some individuals and in some tissues, but not in others. The observations strongly suggest that the epithelium has an impact on the degree of underlying dermal fibrosis, and suggests that reduction in epithelial signaling will result in decreasing fibrosis. These findings are consistent with previous work indicating that occlusive dressings result in decreased cutanous scarring by reducing epithelial cytokine production (Kloeters et al. 2008; Tandara and Mustoe 2008; O’Shaughnessy et al. 2009; Gallant-Behm and Mustoe 2010), while disruption of the stratum corneum water barrier results in increased scarring. These findings are also consistent with very recent work comparing mouse oral mucosal wounds to cutaneous wounds (Chen et al. 2010), and are complementary to previous studies performed after tape stripping, surgical injury, and burn injury in skin (Sextius et al. 2010, Cole et al. 2001, Spies et al. 2002).
Microarray analysis has identified numerous molecular differences in the cutaneous and mucosal epithelial response to injury. One of the major groups of genes which are upregulated in the cutaneous epithelium as compared to mucosa comprises pro-inflammatory cytokines and pro-fibrotic growth factors, including IL-1α, IL-1β, IL-8, TNF-α, and TGF-β1. Although secreted protein levels have not been tested, these factors remain crucial for the regulation of fibroblast proliferation, migration, and extracellular matrix synthesis (Maas-Szabowski and Fusenig 1996; Varga 2002; Tandara et al. 2007; Yano et al. 2008, Kawaguchi et al. 2004, Duncan et al. 1989, Wynn et al. 2008). These factors form a major axis by which the epithelium communicates with the underlying mesenchymal tissue to stimulate tissue repair and fibrosis. Interleukin 1 signaling in particular has been found to be involved in the development of numerous other fibrotic diseases, including subglottic stenosis (upper airway mucosal fibrosis) (Sandaluche et al. 2007), liver fibrosis (Gieling et al. 2009), renal fibrosis (Jones et al. 2009), lung fibrosis (Gasse et al. 2007), inflammatory arthritis (Abdollahi-Roodsaz et al. 2009), systemic sclerosis (Kawaguchi et al. 2006) and cardiac fibrosis during progression to heart failure (Blyszczuk et al. 2009). Interference with interleukin signaling may be an effective therapeutic to prevent or resolve fibrotic diseases. For example, interleukin receptor antagonists have been utilized as therapies in inflammatory and fibrotic conditions such as septic shock, inflammatory arthritis, graft-versus-host disease, inflammatory bowel disease (Arend 1993), and lung fibrosis (Gasse et al. 2007). In the skin, preliminary studies have suggested that IL-1 receptor antagonists can reduce the deep tissue response to injury and may modulate the wound healing process (Thomay et al. 2009). In this study, we have successfully used an IL-1 receptor antagonist to prevent skin fibrosis in a reliable model of hypertrophic scar formation. This suggests that other avenues for the development of future therapeutics for the prevention and treatment of fibroproliferative scars may be found in those factors which are differentially expressed in the skin and mucosal epithelium following injury. For example, TNF neutralizing antibodies and receptor antagonists are currently utilized in clinical practice in rheumatology and may be efficacious in preventing or treating cutaneous fibrosis.
We describe here that the amount of epithelial proliferation differs in the skin and vaginal mucosa following an identical injury. Keratinocyte proliferation and differentiation is highly regulated by extracellular calcium (Ca2+) concentration (Hennings et al. 1980). This study has identified a number of calcium binding proteins of the EF-hand (helix-loop-helix) type which may be involved in the differential epithelial response to injury in skin and mucosa. Microarray analysis identified that expression of the S100 calcium binding proteins A6, 8, 9, 11, and 12 was increased in unwounded mucosa as compared to unwounded skin, yet was elevated in skin wounds at all time points. The S100 proteins are a multifunctional group of calcium sensors that are involved in regulating cell cycle progression and which contribute to the formation of the cornified envelope in epidermal keratinocytes (Donato 1999), as well as in sensing cutaneous epithelial barrier disruption or stress (Sextius et al. 2010, Marionnet et al. 2003). Additionally the S100 proteins have numerous roles in modulating the inflammatory response, including roles in myeloid cell maturation and function, macrophage activation, leukocyte chemotaxis, and fatty acid synthesis and activity (Donato 1999). In particular, secreted S100A8/9 heterodimers and S100A12 (also known as calgranulin C or EN-RAGE) have numerous pro-inflammatory functions including the induction of inflammatory cell recruitment, cytokine synthesis, and may induce collagen synthesis by mesenchymal cells (Yang et al. 2001; Halayko and Ghavami 2009). Furthermore, S100A8/9 and S100A12 may be induced by IL-1β, TNF-α, and TGF-β (Gottsch et al. 1999; Lim et al. 2009), all of which were upregulated in injured cutaneous epithelium compared to mucosa. Therefore, the S100 calcium binding proteins may have a major role in regulating the epithelial response to injury and in the cross-talk between the epithelium and the underlying connective tissue following injury, thereby affecting the magnitude and duration of the tissue repair response in the skin and vaginal mucosa.
In conclusion, we show here that the epithelium plays a major role in the regulation of connective tissue behavior. Keratinocyte proliferation, activation, and production of pro-inflammatory and pro-fibrotic factors are not only involved in the regulation of wound healing, but may also result in connective tissue fibrosis, or scarring. This study has shown that it is possible to interfere with this epithelial-connective tissue interaction, resulting in a clinically relevant improvement in outcome. Thus, understanding the role of the epithelium in wound repair may provide insights into pathways that regulate fibrosis in the skin and other tissues, and may lead to the development of new therapeutics.
Materials and Methods
See the Materials and Methods section in the supplementary information for more details. Briefly, paired skin and mucosal wounds were created on female New Zealand White rabbits according to a Northwestern University IACUC-approved protocol. At various time points after injury, the animals were euthanized and tissue samples were taken for histology and total RNA was extracted from the epithelial and mesenchymal tissue compartments. Quantitative RT-PCR and microarray analysis was performed. K-means clustering and functional analysis was performed on the genes which were differentially expressed over time in the two tissues or which exhibited different temporal expression patterns. To determine the relative role of IL-1 in regulating dermal fibrosis, the New Zealand White rabbit model of hypertrophic scarring was utilized. Following wound re-epithelialization, an IL-1 receptor antagonist or vehicle was administered locally, and the amount of scarring was quantified using histology.
Supplementary Material
Figure 4. Epidermal injury results in a profibrotic response in the uninjured dermis of skin.
Quantitative reverse transcriptase PCR was performed on RNA extracted from the unwounded connective tissue that lay beneath the injured cutaneous and mucosal epithelium (N=8 per time point). Remarkably, an epithelial-restricted injury resulted in significant profibrotic changes in the underlying mesenchymal tissue of skin, but not mucosa. The cutaneous dermis demonstrated significant upregulation of the profibrotic growth factor TGF-β (A), the principal extracellular matrix component, collagen 1 (B), and the matrix metalloproteinase inhibitor TIMP1 (C). These changes were not observed in the mucosal lamina propria. * = p<0.05, ** = p<0.01, *** = p<0.001.
Acknowledgements
Funding was provided by NIH P20 GM078426-01.
Abbreviations
- IL
Interleukin
- IL-1RA
Interleukin 1 receptor antagonist
- iNOS
Inducible nitric oxide synthase
- MMP
Matrix metalloproteinase
- NZW
New Zealand White rabbit
- Q-RT-PCR
Quantitative reverse transcriptase polymerase chain reaction
- RNA
Ribonucleic acid
- SEI
Scar elevation index
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
Footnotes
Conflict of Interest
The authors state no conflict of interest
References
- Abdollahi-Roodsaz S, Joosten LA, Koenders MI, van den Brand BT, van de Loo FA, van den Berg WB. Local interleukin-1-driven joint pathology is dependent on toll-like receptor 4 activation. Am J Pathol. 2009;175:2004–2013. doi: 10.2353/ajpath.2009.090262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Basson MD. In vitro evidence for matrix regulation of intestinal epithelial biology during mucosal healing. Life Sci. 2001;69:3005–3018. doi: 10.1016/s0024-3205(01)01408-4. [DOI] [PubMed] [Google Scholar]
- Blyszczuk P, Kania G, Dieterle T, Marty RR, Valaperti A, Berthonneche C, Pedrazzini T, Berger CT, Dirnhofer S, Matter CM, Penninger JM, Luscher TF, Eriksson U. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res. 2009;105:912–920. doi: 10.1161/CIRCRESAHA.109.199802. [DOI] [PubMed] [Google Scholar]
- Bussi M, Valente G, Curato MP, Carlevato MT, Cortesina G. Is transposed skin transformed in major head and neck mucosal reconstruction? Acta Otolaryngol. 1995;115:348–351. doi: 10.3109/00016489509139327. [DOI] [PubMed] [Google Scholar]
- Chang CC, Kuo YF, Chiu HC, Lee JL, Wong TW, Jee SH. Hydration, not silicone, modulates the effects of keratinocytes on fibroblasts. J Surg Res. 1995;59:705–711. doi: 10.1006/jsre.1995.1227. [DOI] [PubMed] [Google Scholar]
- Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics. 2010;11:471. doi: 10.1186/1471-2164-11-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Tsou R, Wallace K, Gibran N, Isik F. Early gene expression profile of human skin to injury using high-density cDNA microarrays. Wound Repair Regen. 2001;9:360–370. doi: 10.1046/j.1524-475x.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Duncan MR, Berman B. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factor-alpha and beta. J Invest Dermatol. 1989;92:699–706. doi: 10.1111/1523-1747.ep12696891. [DOI] [PubMed] [Google Scholar]
- Eslami A, Gallant-Behm CL, Hart DA, Wiebe C, Honardoust D, Gardner H, Hakkinen L, Larjava HS. Expression of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming wound healing. J Histochem Cytochem. 2009;57:543–557. doi: 10.1369/jhc.2009.952572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant-Behm CL, Mustoe TA. Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen. 2010;18:235–244. doi: 10.1111/j.1524-475x.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1324–1331. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch JD, Li Q, Ashraf F, O’Brien TP, Stark WJ, Liu SH. Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol. 1999;91:34–40. doi: 10.1006/clim.1998.4681. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Ghavami S. S100A8/A9: a mediator of severe asthma pathogenesis and morbidity? Can J Physiol Pharmacol. 2009;87:743–755. doi: 10.1139/Y09-054. [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Jones LK, O’Sullivan KM, Semple T, Kuligowski MP, Fukami K, Ma FY, Nikolic-Paterson DJ, Holdsworth SR, Kitching AR. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant. 2009;24:3024–3032. doi: 10.1093/ndt/gfp214. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, McCarthy SA, Watkins SC, Wright TM. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J Rheumatol. 2004;31:1946–1954. [PubMed] [Google Scholar]
- Kawaguchi Y, Nishimagi E, Tochimoto A, Kawamoto M, Katsumata Y, Soejima M, Kanno T, Kamatani N, Hara M. Intracellular IL-1alpha-binding proteins contribute to biological functions of endogenous IL-1alpha in systemic sclerosis fibroblasts. Proc Natl Acad Sci U S A. 2006;103:14501–14506. doi: 10.1073/pnas.0603545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeters O, Schierle C, Tandara A, Mustoe TA. The use of a semiocclusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair Regen. 2008;16:568–575. doi: 10.1111/j.1524-475X.2008.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Raftery MJ, Goyette J, Hsu K, Geczy CL. Oxidative modifications of S100 proteins: functional regulation by redox. J Leukoc Biol. 2009;86:577–587. doi: 10.1189/jlb.1008608. [DOI] [PubMed] [Google Scholar]
- Maas-Szabowski N, Fusenig NE. Interleukin-1-induced growth factor expression in postmitotic and resting fibroblasts. J Invest Dermatol. 1996;107:849–855. doi: 10.1111/1523-1747.ep12331158. [DOI] [PubMed] [Google Scholar]
- Mak K, Manji A, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, Hakkinen L. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009;56:168–180. doi: 10.1016/j.jdermsci.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Marionnet C, Bernerd F, Dumas A, Verrecchia F, Mollier K, Compan D, Bernard B, Lahfa M, Leclaire J, Medaisko C, Mehul B, Seité S, Mauviel A, Dubertret L. Modulation of gene expression induced in human epidermis by environmental stress in vivo. J Invest Dermatol. 2003;121:1447–1458. doi: 10.1111/j.1523-1747.2003.12629.x. [DOI] [PubMed] [Google Scholar]
- Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg. 2008;32:82–92. doi: 10.1007/s00266-007-9030-9. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy KD, De La Garza M, Roy NK, Mustoe TA. Homeostasis of the epidermal barrier layer: a theory of how occlusion reduces hypertrophic scarring. Wound Repair Regen. 2009;17:700–708. doi: 10.1111/j.1524-475X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- Sandulache VC, Chafin JB, Li-Korotky HS, Otteson TD, Dohar JE, Hebda PA. Elucidating the role of interleukin 1beta and prostaglandin E2 in upper airway mucosal wound healing. Arch Otolaryngol Head Neck Surg. 2007;133:365–374. doi: 10.1001/archotol.133.4.365. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Sone K. Hydration and occlusion treatment for hypertrophic scars and keloids. Br J Plast Surg. 1992;45:599–603. doi: 10.1016/0007-1226(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Schierle C, O’Shaughnessy K, Ding XZ, Galiano RD, Mustoe TA. Silicone gel occlusion suppresses epidermal activation in a murine cutaneous wound model. Wound Repair Regen. 2007;15:A23. [Google Scholar]
- Schreuder H, Tardif C, Trump-Kallmeyer S, Soffientini A, Sarubbi E, Akeson A, Bowlin T, Yanofsky S, Barrett RW. A new cytokine-receptor binding mode revealed by the crystal structure of the IL-1 receptor with an antagonist. Nature. 1997;386:194–200. doi: 10.1038/386194a0. [DOI] [PubMed] [Google Scholar]
- Sextius P, Marionnet C, Bon FX, de La Chapelle AL, Tacheau C, Lahfa M, Mauviel A, Bernard BA, Leclaire J, Bernerd F, Dubertret L. Large scale study of epidermal recovery after stratum corneum removal: dynamics of genomic response. Exp Dermatol. 2010;19:259–268. doi: 10.1111/j.1600-0625.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- Spies M, Dasu MR, Svrakic N, Nesic O, Barrow RE, Perez-Polo JR, Herndon DN. Gene expression analysis in burn wounds of rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R918–930. doi: 10.1152/ajpregu.00170.2002. [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res. 2005;84:309–314. doi: 10.1177/154405910508400403. [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- Tandara AA, Kloeters O, Mogford JE, Mustoe TA. Hydrated keratinocytes reduce collagen synthesis by fibroblasts via paracrine mechanisms. Wound Repair Regen. 2007;15:497–504. doi: 10.1111/j.1524-475X.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg. 2008;61:1219–2125. doi: 10.1016/j.bjps.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, Reichner JS, Albina JE. Disruption of interleukin-1 signaling improves the quality of wound healing. Am J Pathol. 2009;174:2129–2136. doi: 10.2353/ajpath.2009.080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg Clin North Am. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- Varga J. Scleroderma and Smads: dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 2002;46:1703–1713. doi: 10.1002/art.10413. [DOI] [PubMed] [Google Scholar]
- Wong JW, Gallant-Behm C, Wiebe C, Mak K, Hart DA, Larjava H, Hakkinen L. Wound healing in oral mucosa results in reduced scar formation as compared with skin: evidence from the red Duroc pig model and humans. Wound Repair Regen. 2009;17:717–729. doi: 10.1111/j.1524-475X.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69:986–994. [PubMed] [Google Scholar]
- Yano S, Banno T, Walsh R, Blumenberg M. Transcriptional responses of human epidermal keratinocytes to cytokine interleukin-1. J Cell Physiol. 2008;214:1–13. doi: 10.1002/jcp.21300. [DOI] [PubMed] [Google Scholar]
- Zelles T, Purushotham KR, Macauley SP, Oxford GE, Humphreys-Beher MG. Saliva and growth factors: the fountain of youth resides in us all. J Dent Res. 1995;74:1826–1832. doi: 10.1177/00220345950740120301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.