Abstract
Rationale: It has been nearly 20 years since sarcoidosis mortality was examined at the population level in the United States.
Objectives: To examine mortality rates and underlying causes of death among United States decedents with sarcoidosis from 1988–2007.
Methods: We used data from the National Center for Health Statistics to (1) calculate age-adjusted sarcoidosis-associated mortality rates; (2) examine how those rates differ by age, sex, and race and ethnicity; and (3) determine underlying causes of death among sarcoidosis decedents.
Measurements and Main Results: From 1988–2007, there were 46,450,489 deaths in the United States and 23,679 decedents with sarcoidosis mentioned on their death certificates. Over this time, the age-adjusted, sarcoidosis-related mortality rate increased 50.5% in women and 30.1% in men. The greatest absolute increase in death rates was among non-Hispanic black females. Regardless of sex or race, mortality rates climbed most in decedents 55 years or older. The most common cause of death was sarcoidosis itself. Younger sarcoidosis decedents with pulmonary fibrosis were more likely to be black than white, and younger sarcoidosis decedents were more likely than similarly aged decedents in the general population to have a cardiac cause contribute to death.
Conclusions: From 1988–2007, sarcoidosis-related mortality rates increased significantly, particularly in non-Hispanic black females aged 55 years or older. The underlying cause of death in most patients with sarcoidosis was the disease itself. Among young sarcoidosis decedents, those with pulmonary fibrosis or a cardiac cause contributing to death were more likely to be black than white.
Keywords: sarcoidosis, mortality, epidemiology
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
It has been over 15 years since investigators examined mortality in sarcoidosis. In this study, we determined mortality rates and underlying causes of death among decedents with sarcoidosis from 1988–2007.
What This Study Adds to the Field
We found that mortality rates increased from 1988–2007, particularly in black females. Younger age and black race are risk factors for having pulmonary fibrosis or a cardiac cause contribute to death.
Sarcoidosis is a multisystem, granulomatous disease of unknown cause (1) that most commonly affects young adults, particularly females (2). Sarcoidosis affects the thorax (lungs or mediastinal lymph nodes) in more than 90% of patients (3). Worldwide, the highest annual incidence of sarcoidosis (5–40 cases per 100,000 population) has consistently been observed in northern European countries (4). In the United States, the adjusted annual incidence of sarcoidosis is over three times greater in blacks than whites (35.5 cases vs. 10.9 cases per 100,000 population) (4). In the Black Women's Health Study, a study that enrolled a cohort of 59,000 black United States women, investigators calculated an annual incidence rate of 71 per 100,000 (5). Black Americans with sarcoidosis are more likely than other racial groups in the United States to suffer extrathoracic organ (e.g., skin or eye) involvement and a chronic disease course (3, 6).
For most patients, sarcoidosis is a benign disorder; many never develop clinically significant disease, and spontaneous remission is not infrequent (6, 7). However, for a significant minority of patients, sarcoidosis is a chronic, debilitating, and even life-threatening condition: investigators have reported mortality rates up to 6% (8–10), with deaths attributed to respiratory, neurologic, or cardiovascular system involvement (9, 11, 12). Among patients with sarcoidosis awaiting lung transplantation, the median survival is less than 2 years (13).
Few studies have examined mortality rates in sarcoidosis at the national level. In Japan, nationwide surveys found the age-adjusted mortality rate to be 0.2 per 1 million population in 1972 and 0.1 per 1 million population in 1984 (14). In the United States, age-adjusted (to the 1990 United States population) mortality rates (among decedents who died from sarcoidosis or one of its complications [e.g., pneumonia]) increased steadily from 1979–1991: for men, from 1.3–1.6 deaths per million men; and for women, from 1.9–2.5 deaths per million women (15).
United States data collected after 1991 have not been systematically analyzed. We examined National Center for Health Statistics (NCHS) data files that were compiled from all deaths in the United States from 1988–2007. From these and United States census data, for decedents with sarcoidosis, we calculated age-adjusted mortality rates; examined differences in mortality rates by age, sex, and race and ethnicity; determined the underlying causes of death; and assessed the geographic variability of mortality rates among the 50 United States.
METHODS
We used methods described in detail elsewhere (16, 17). The online supplement provides a full description of methods. Briefly, we used data files, compiled and manipulated annually by the NCHS, that were derived from all United States death certificates from 1988–2007. The NCHS applies computer algorithms to the death certificate data to produce a standardized “record axis.” The record axis includes up to 20 associated causes of death, including the underlying cause of death (UCD). We included in this study files from any decedent with “sarcoidosis” in the record axis. Details of the UCD determination and state-level analyses are outlined in the online supplement.
We used July 1st intercensal population estimates (from 1988–1999) and July 1st postcensal population estimates (from 2000–2007) from the United States Census Bureau (18) to determine denominators for corresponding yearly mortality rates. Direct standardization was the method used to standardize mortality rates to the 2000 United States Census population.
For certain analyses focused on pulmonary hypertension (PH) and cardiac dysrhythmia and sudden cardiac death, we dichotomized the data set into two International Classification of Disease (ICD) eras: the ICD-9 era from 1988–1998 and the ICD-10 era from 1999–2007. This allowed us to examine the influence of coding changes (from ICD-9 to ICD-10) along with evolutions in therapy for PH and sudden cardiac death (e.g., implantable cardiac defibrillators) that have become commonplace in the ICD-10 era.
We used chi-square tests, and the Mantel-Haenszel statistic where appropriate, to compare proportions between groups, t tests to compare continuous variables between groups, and Poisson multivariable regression analysis to test for changes in mortality rates over time. Mortality rates were calculated using Microsoft Office Excel 2003 SP2 (Microsoft, Redmond, WA). All other data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC). We were not required to obtain Institutional Review Board approval for this study, because all data contained in the database files have been de-identified.
RESULTS
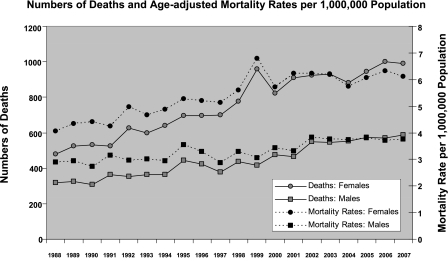
From 1988–2007, there were 46,450,489 deaths in the United States, and 23,679 Multiple Cause of Death (MCOD) records contained a diagnostic code for sarcoidosis (Table 1). Over the study period, for males and females, the numbers of sarcoidosis-related deaths and age-adjusted, sarcoidosis-related mortality rates increased (Figure 1). From 1988–2007, the average age- and sex-adjusted sarcoidosis-related mortality rate was 4.32 per 1,000,000 population. The age-adjusted, sarcoidosis-related mortality rate increased 50.5% among females (from 4.06 per 1,000,000 in 1988 to 6.11 per 1,000,000 women in 2007) and 30.1% among males (from 2.89 per 1,000,000 in 1988 to 3.76 per 1,000,000 men in 2007). For all-comers with sarcoidosis, over the study period, the average yearly increase in mortality rate was 3%.
TABLE 1.
PERCENTAGE OF UNITED STATES DECEDENTS WITH SARCOIDOSIS STRATIFIED ON AGE
| Total United States Decedents |
Decedents with Sarcoidosis |
% |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age in Years | Female | Male | Total | Female | Male | Total | Female | Male | Total |
| <14 | 38,4215 | 509,260 | 89,3475 | 7 | 7 | 14 | 0.0016 | 0.0016 | 0.0016 |
| 15–24 | 17,2408 | 507,700 | 680,108 | 49 | 74 | 123 | 0.0284 | 0.0146 | 0.0181 |
| 25–34 | 28,4785 | 704,166 | 988,951 | 570 | 639 | 1,209 | 0.2002 | 0.0907 | 0.1223 |
| 35–44 | 608,447 | 117,0428 | 1,778,875 | 2,101 | 1,774 | 3,875 | 0.3453 | 0.1516 | 0.2178 |
| 45–54 | 1,122,151 | 1,889,669 | 3,011,820 | 3,282 | 2,122 | 5,404 | 0.2925 | 0.1123 | 0.1794 |
| 55–64 | 1,997,909 | 3,038,099 | 5,036,008 | 3,211 | 1,739 | 4,950 | 0.1607 | 0.0572 | 0.0983 |
| 65–74 | 3,888,161 | 5,106,897 | 8,995,058 | 2,911 | 1,389 | 4,300 | 0.0749 | 0.0273 | 0.0480 |
| 75–84 | 6,728,086 | 6,442,435 | 13,170,521 | 2,108 | 908 | 3,016 | 0.0313 | 0.0141 | 0.0229 |
| 85+ | 7,950,410 | 3,936,634 | 1,188,7044 | 617 | 171 | 788 | 0.0078 | 0.0043 | 0.0066 |
| Totals | 23,138,683 | 2,331,1806 | 46,450,489 | 14,856 | 8,823 | 23,679 | 0.0642 | 0.0379 | 0.0510 |
Figure 1.
Numbers of deaths and age-adjusted mortality rates per 1,000,000 population.
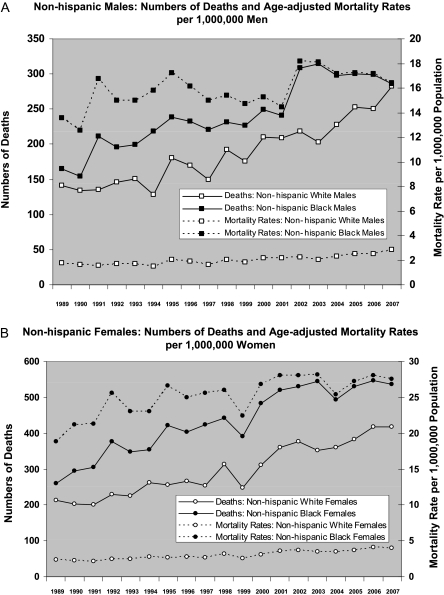
For any given year, mortality rates were uniformly greater (nearly 10-fold for both males and females) for non-Hispanic blacks than non-Hispanic whites (Figures 2A and 2B). However, the relative increase from 1989–2007 in age-adjusted, sarcoidosis-related mortality rates was greater for non-Hispanic whites than for non-Hispanic blacks (for females, the relative increase was 74% in whites vs. 46% in blacks; for males, the relative increase was 65% in whites vs. 21% in blacks). In non-Hispanic blacks, age-adjusted sarcoidsosis-related mortality rates seemed to plateau in 2001 (females) and 2002 (males); and from 2002 through 2007, these rates remained stable in females and even declined slightly in males.
Figure 2.
(A) Non-Hispanic males. Numbers of deaths and age-adjusted mortality rates per 1,000,000 men. (B) Non-Hispanic females. Numbers of deaths and age-adjusted mortality rates per 1,000,000 women.
Among Hispanics, over the entire study, the average age- and sex-adjusted sarcoidosis-related mortality rate was 0.82 per 1,000,000 population. The age-adjusted, sarcoidosis-related mortality rates from 1989–2007 increased from 0.85–1.45 per million for females and from 0.43–0.64 per million for males; however, from 2003–2007, rates seemed to be declining in males and generally rising in females (see Figure E1 in the online supplement).
From 1989–2007, the greatest absolute increase in sarcoidosis-related deaths was among non-Hispanic black females; their rate increased by nearly 10 deaths per million (from 18.85–27.58 deaths per million). For black males, the rate only increased by about three deaths per million, and for white men or women, the rates increased by at most one death per million.
When we stratified the data by age, we made the following observations (see Table E1 and Figure E2; Table 2): (1) among non-Hispanic black females, sarcoidosis-related mortality rates declined from 1989–2007 for those 25–44 years of age (P < 0.0001) but more than doubled for those aged 55–64 years (P < 0.0001) and for those aged 65–74 years (P < 0.0001); (2) for non-Hispanic black males, the pattern was similar. Rates declined among those 25–34 years of age and increased in those 45–84 years (P < 0.0001). The increase in rates was greatest among those aged 65–74 years (for this age stratum, rates increased from 13.4 deaths per million in 1989 to 57.7 deaths per million in 2007); and (3) among non-Hispanic whites, mortality rates did not decline in any age group from 1988–2007. On balance, sarcoidosis-related mortality rates were highest and rose the greatest amounts in the three oldest age strata. Considering all female decedents from 1988–2007, the age strata with the highest proportions of sarcoidosis-related deaths were 35- to 44-year-olds (0.34%) and 45- to 54-year-olds (0.29%). For males, it was the same: 35- to 44-year-olds (0.15%) and 45- to 54-year-olds (0.11%) (Table 1).
TABLE 2.
RESULTS OF POISSON REGRESSION ANALYSES PERFORMED TO DETERMINE WHETHER TRENDS IN MORTALITY OVER TIME WERE SIGNIFICANT
| Sex | Race | Age in Years | Estimate* | P |
|---|---|---|---|---|
| Female | Black | 25–34 | −0.0561 | <0.0001 |
| Female | Black | 35–44 | −0.0191 | 0.003 |
| Female | Black | 45–54 | 0.007 | 0.2 |
| Female | Black | 55–64 | 0.0427 | <0.0001 |
| Female | Black | 65–74 | 0.0482 | <0.0001 |
| Female | Black | 75–84 | 0.0577 | <0.0001 |
| Female | White | 25–34 | −0.0040 | 0.9 |
| Female | White | 35–44 | 0.0311 | 0.03 |
| Female | White | 45–54 | 0.0263 | 0.001 |
| Female | White | 55–64 | 0.0270 | <0.0001 |
| Female | White | 65–74 | 0.0261 | <0.0001 |
| Female | White | 75–84 | 0.0400 | <0.0001 |
| Male | Black | 25–34 | −0.0301 | <0.0001 |
| Male | Black | 35–44 | −0.0071 | 0.2 |
| Male | Black | 45–54 | 0.0106 | 0.04 |
| Male | Black | 55–64 | 0.0319 | <0.0001 |
| Male | Black | 65–74 | 0.0504 | <0.0001 |
| Male | Black | 75–84 | 0.0508 | <0.0001 |
| Male | White | 25–34 | 0.0547 | 0.001 |
| Male | White | 35–44 | 0.0237 | 0.002 |
| Male | White | 45–54 | 0.0200 | 0.009 |
| Male | White | 55–64 | 0.0396 | <0.0001 |
| Male | White | 65–74 | 0.0197 | 0.0003 |
| Male | White | 75–84 | 0.0330 | <0.0001 |
Estimate for year of study; negative values correspond to decreasing rates over time, and positive values correspond to increasing rates over time.
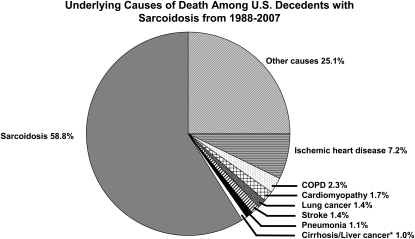
From 1988–2007, the average age of death among decedents with sarcoidosis was 57.2 ± 1.8 years, compared with 71.4 ± 1.1 years for the nonsarcoidosis background population (P < 0.0001 for comparison). Black decedents with sarcoidosis were significantly younger (51.8 ± 2.3 yr vs. 65.2 ± 1 yr; P < 0.0001) than white decedents with sarcoidosis. In decedents with sarcoidosis, the disease itself was the UCD in 13,923 (58.8%) (Figure 3). Among decedents in whom sarcoidosis was the UCD, pulmonary fibrosis (PF) was mentioned as a contributing cause in 9.1 ± 1.4% (n = 1,267); 22.9% were 45–54 years old, 22.2% were 55–64 years old, 21.1% were 65–74 years old, and 14.7% were 75–85 years old. Overall, these 1,267 decedents were significantly more likely to be black than white (P = 0.001). Among them, those in younger age strata were significantly more likely to be black than white; however, those in older age strata were significantly more likely to be white than black (Table 3). Among decedents 25–84 years old, in whom sarcoidosis was the UCD, a cardiac etiology (coded as ischemia or myocardial infarction, sudden cardiac death or arrhythmia, congestive heart failure, or cardiomyopathy) contributed to death in 3,473 (24.9%). Regardless of race or sex, among decedents in the 25- to 34-year-old age strata, those with sarcoidosis as the UCD were more likely than those in the background population to have a cardiac etiology contribute to death; the same was true for white females or males in the 35- to 44-year-old age strata. However, for most other age strata (among decedents of either black or white race and either sex) the presence of sarcoidosis was protective from having a cardiac etiology contribute to death (Table 4).
Figure 3.
Ultimate underlying causes of death among United States decedents with sarcoidosis from 1988–2007. COPD = chronic obstructive pulmonary disease.
TABLE 3.
STRATIFICATION, BY RACE AND AGE, OF DECEDENTS WITH SARCOIDOSIS FOR WHOM SARCOIDOSIS WAS THE UNDERLYING CAUSE OF DEATH AND PULMONARY FIBROSIS CONTRIBUTED SIGNIFICANTLY TO DEATH
| Age Group | Blacks | Whites | P |
|---|---|---|---|
| 25–34 yr | 36 | 3 | <0.0001* |
| 35–44 yr | 134 | 16 | |
| 45–54 yr | 231 | 58 | |
| 55–64 yr | 179 | 100 | |
| 65–74 yr | 105 | 163 | |
| 75–84 yr | 38 | 145 | |
| ≥ 85 yr | 9 | 40 |
For chi-square for general association between races and for the Mantel-Haenszel chi-square statistic for trends in numbers of decedents in increasing age groups.
TABLE 4.
RISK OF CARDIAC DEATH FOR UNITED STATES DECEDENTS FROM 1988–2007 WITH SARCOIDOSIS AS UNDERLYING CAUSE OF DEATH RELATIVE TO BACKGROUND POPULATION
| Blacks |
Whites |
|||
|---|---|---|---|---|
| Age in Years | Female | Male | Female | Male |
| 25–34 | 1.43 (1.22–2.00)* | 2.14 (1.81–2.52)† | 2.25 (1.32–3.82)‡ | 2.31 (1.61–3.31)† |
| 35–44 | 1.02 (0.93–1.13) | 1.08 (0.97–1.20) | 1.61 (1.25–2.08)‡ | 1.26 (1.03–1.54)§ |
| 45–54 | 0.87 (0.81–0.95)* | 0.81 (0.73–0.90)† | 1.19 (1.01–1.41)§ | 0.76 (0.64–0.91)* |
| 55–64 | 0.72 (0.65–0.79)† | 0.75 (0.66–0.86)† | 0.72 (0.62–0.83)† | 0.63 (0.53–0.74)† |
| 65–74 | 0.63 (0.56–0.72)† | 0.74 (0.61–0.90)* | 0.71 (0.63–0.79)† | 0.66 (0.57–0.77)† |
| 75–84 | 0.78 (0.65–0.93)‡ | 0.73 (0.52–1.03)§ | 0.68 (0.61–0.77)† | 0.61 (0.51–0.73)† |
Cardiac death includes codes for ischemia or myocardial infarction, sudden cardiac death or arrhythmia, congestive heart failure, or cardiomyopathy.
P ≤ 0.001.
P < 0.0001.
P ≤ 0.005.
P < 0.05.
In the ICD-9 era, among the 10,882 decedents with sarcoidosis, PH was the UCD in 0.48% (n = 52), and PH was mentioned on the death certificate (either as the UCD or as present and contributing to death) in 2.81% (n = 306). Among these 306 records that contained both sarcoidosis and PH, sarcoidosis itself was coded as the UCD in 81% (n = 248); no other entity (except PH) was coded as the UCD in more than 2% of people.
In the ICD-10 era, among the 12,797 decedents with sarcoidosis, PH was the UCD in 0.95% (n = 122), and PH was mentioned on the death certificate (either as the UCD or as present and contributing to death) in 7.05% (n = 902). Among these 902 records that contained both sarcoidosis and PH, sarcoidosis itself was coded as the UCD in 80% (n = 722); no other entity (except PH) was coded as the UCD in more than 1% of people. Among all-comers with sarcoidosis, in the ICD-9 era, cardiac dysrhythmia and sudden cardiac death were the UCD 1.52%, and that fell to 0.66% in the ICD-10 era.
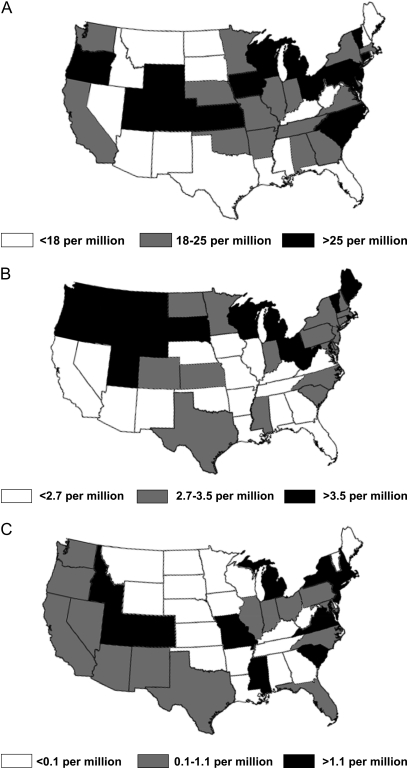
For non-Hispanic blacks, non-Hispanic whites, and Hispanics, we identified significant variability in mortality rates among the 50 United States (Figures 4A–4C).
Figure 4.
(A) State-level age-adjusted mortality rates among United States non-Hispanic black decedents with sarcoidosis from 2000–2007. (B) State-level age-adjusted mortality rates among United States non-Hispanic white decedents with sarcoidosis from 2000–2007. (C) State-level age-adjusted mortality rates among United States Hispanic decedents with sarcoidosis from 2000–2007.
DISCUSSION
This study yields the first systematic assessment of sarcoidosis-related mortality in the United States since 1991, and to our knowledge, the first-ever estimates for sarcoidosis-related mortality among Hispanics. Over the study period, the average sarcoidosis-related mortality rate increased 3% per year. This acceleration is the same as that for idiopathic pulmonary fibrosis (IPF) over the same time period, although in any given year, the mortality rate for IPF is about 10-fold greater than the rate for sarcoidosis (IPF data not shown).
We examined sex-, race/ethnicity-, and age-stratified mortality rates in decedents with sarcoidosis and found the highest rates in females, non-Hispanic blacks, and decedents in older age strata. Considering all decedents from 1988–2007, sarcoidosis-related mortality rose in both females and males, but on a steeper trajectory in females than males. Age-adjusted, sarcoidosis-related mortality rates in non-Hispanic whites rose slightly but steadily over the study period in both females and males. In non-Hispanic blacks, rates were consistently higher than non-Hispanic whites and rose more sharply until 2001 or 2002; from then until 2007, rates have been stable for non-Hispanic black females overall and have declined slightly for non-Hispanic black males overall.
Regardless of sex or race, sarcoidosis-related mortality rates climbed most steeply over the study period in older decedents, those between 55 and 74 years of age. Rates actually declined over time in the two youngest age strata of black females; whether this is caused by improved therapies that prolong survival is unknown.
There are only a few other studies in which investigators systematically examined population-level data on sarcoidosis-related mortality. By using MCOD mortality data from 1979 through 1991, Gideon and Mannino (15) identified 9,014 decedents on whose death certificate sarcoidosis was mentioned. Although the time frame of our study was twice as long, and we standardized to the year 2000 United States population (Gideon and Mannino [15] standardized to the 1990 United States population, thus precluding direct comparisons of results between studies), the general patterns of results are similar between studies. Like us, they found that age-adjusted, sarcoidosis-related mortality rates were greater in females than males; that rates were consistently higher in blacks than whites but rose steadily over time in both races; that particularly in females, rates were highest in decedents in older age strata; and that the highest percentage of total sarcoidosis-related deaths occurred in decedents aged 35–44 years. To our knowledge, in the only other population-level, epidemiologic study of sarcoidosis, Yamaguchi and colleagues (14) calculated age-adjusted mortality rates of 0.2 and 0.1 per million population. Gribbin and colleagues (19) used a computerized longitudinal primary care database to identify 1,019 subjects with sarcoidosis, 78 (7.6%) of whom died over an average follow-up period of 5.4 years. Their study was not designed to accomplish the same goals as our study.
We also examined the UCD (the disease or injury that initiated the train of events leading directly to death [20, 21]) among decedents for whom death records mentioned sarcoidosis. For the 23,679 decedents with sarcoidosis mentioned on their death certificate, sarcoidosis was the UCD in nearly 14,000 (58.8%). Because of the confines of the data set, we were not able to determine the actual cause of (or dysfunctional organ system primarily responsible for) death for these decedents. Historical data suggest that patients with sarcoidosis-related mortality have died from neurologic, cardiovascular, or most commonly respiratory system involvement of sarcoidosis. When we looked at the subset of decedents for whom sarcoidosis was the UCD, we found that PF was mentioned as a contributing factor in about 9%, and a cardiac etiology was mentioned in about 25%, suggesting that at a minimum 9% and 25%, respectively, of patients with sarcoidosis severe enough to warrant coding on the death certificate have associated PF or a cardiac condition (possibly sarcoidosis-related) contributing to death. In a recent publication, Crawshaw and colleagues (22) noted an association between sarcoidosis and congestive heart failure among patients admitted to UK National Health Service hospitals over a 35-year period.
From the ICD-9 to the ICD-10 era, we identified a substantial increase in PH (coded either as the ultimate UCD or somewhere in the chain of events leading to death) among decedents with sarcoidosis. In contrast, from one ICD era to the next, we found a decrease in cardiac dysrhythmia and sudden cardiac death among decedents with sarcoidosis. Whether these trends over time are caused by increased disease recognition in the case of PH and the development and increased use of implantable cardiac defibrillators in the case of the latter is speculative and unable to be answered by using this data set.
Reflecting historical data revealing that blacks are more likely to develop severe disease at younger ages than other racial and ethnic groups, we found that young sarcoidosis decedents (those <55 yr of age) with PF were significantly more likely to be black than white. We also found that black and white sarcoidosis decedents in the 25–34 year old age strata were more likely than similarly aged decedents in the background population to have a cardiac etiology contribute to death. There is no way to decipher whether or not these were sarcoidosis-related, but we believe it is a safe assumption. For decedents in older age strata, those with sarcoidosis were less likely than similarly aged decedents in the background population to have a cardiac etiology contribute to death. If older decedents with sarcoidosis are less likely to die from a cardiac cause, then from what are they dying?
We suspect the true number of patients dying from sarcoidosis-related PF is actually much greater than the 9% we identified, but that death certifiers may simply write “sarcoidosis” on death certificates without mentioning PF. A clue that PF is a key driver of sarcoidosis-related deaths lies in the mortality rates we observed among older decedents. For blacks or whites, men or women, the bulk of the increase in mortality rates over the study period could be attributed to decedents 55 years and older, and for whites, to decedents 75 years and older, in particular. It has been well established that many forms of PF, particularly idiopathic, are more prevalent in older people, and mortality rates for PF, particularly among the elderly, are rising steeply (16, 23). A theory gaining traction is that aging plays a key role in the pathogenesis of lung fibrosis (24, 25). Given our observations, we speculate that lung fibrosis is also steering the trends we observed in decedents with sarcoidosis. The hypothesis linking age and PF asserts that, in a susceptible individual (susceptibility being governed by some combination of genetics and age) lung insult or injury is likely to result in a fibrotic response, in contrast to the nonsusceptible individual, in whom the insult or injury would either not cause disease at all or would resolve with normal healing. This would hold regardless of the etiology for the insult or injury (e.g., inhalational, granulomatous, caused by an autoimmune process). Whatever the case, accumulating data suggest that research is needed to discern the cause of the trends in mortality among older decedents with sarcoidosis; to determine whether current approaches to therapy are less effective for older patients with sarcoidosis; and given the apparent increased risk of dying from sarcoidosis among older patients, to decipher whether more aggressive treatment strategies are needed in that group.
In a cohort of 59,000 black women (83% of whom were <50 yr old) in the United States, Cozier and colleagues (5) found no differences in geographic region of residence between 435 incident and 685 prevalent cases of sarcoidosis. For each group, the fewest number of cases came from Western states. We found significant geographic variability in mortality rates among the 50 United States, similar to Gideon and Mannino (15). Whether this variability within and among racial and ethnic groups is caused by actual differences in geographic distribution of disease (raising the question of whether environmental exposures influence disease development), caused by regional variation in methods to identify disease, or some other cause is uncertain but merits further exploration.
In this study, we faced hurdles common in epidemiologic studies. For example, it is possible that the generally rising mortality rates we observed might simply reflect an increase in the clinical recognition of sarcoidosis. During the 1990s, computed tomography (CT) scans became commonplace in the evaluation of patients with respiratory conditions. Nodal enlargement and interstitial abnormalities that might not have been obvious on chest radiography could now be easily identified on chest CT scans and prompt diagnostic evaluation. This may be particularly relevant for older patients: over the past decade or so, evidence showing that sarcoidosis occurs in patients greater than 50 years old is mounting, a trend likely contributed to by the ubiquitous use of chest CT scans. Thus, for example, an older patient with progressive, fibrotic, parenchymal lung disease who might have been classified as having IPF in 1990 could be diagnosed more accurately with sarcoidosis in 2005. However, we do not think increased case-finding entirely explains the rising rates for sarcoidosis, because the mortality rates for IPF and rheumatoid arthritis–related PF also increased significantly over the time frame of this study (15, 16). We would have expected the rates for IPF to flatten or decline if large numbers of decedents were being pulled from the IPF group to be categorized as having sarcoidosis.
It is possible that the incidence of sarcoidosis is increasing: more people are dying a sarcoidosis-related death simply because there are increasing numbers of people developing sarcoidosis. If, however the incidence of sarcoidosis is not increasing, as suggested by Gribbin and colleagues (19), then perhaps there has been a shift in disease phenotype: the sarcoidosis that patients develop now is just more severe than in years past. The flat to downward trends in mortality in the younger age strata, including the strata with patients at greatest risk for developing sarcoidosis, argues against this possibility, at least in the young, but leaves open (and reinforces) the possibility that sarcoidosis is becoming more severe in people in older age strata.
The nature of the database forced us to rely on death certifiers to identify cases correctly and then accurately code these conditions on the death certificate. Unfortunately, we had no way to assess the fidelity of the diagnosis in this dataset. Clearly, sarcoidosis will not appear on the death certificates of many or perhaps most patients who develop sarcoidosis at some point in their lives: spontaneous remission or clinically insignificant disease is common. Thus, mortality rates here should not be considered surrogates for the incidence of sarcoidosis. We believe they represent floor values; actual rates may be higher, but they are extremely unlikely to be lower. We hypothesize that for sarcoidosis to be mentioned on an individual's death certificate, the condition must be chronic or severe; that nearly 60% of decedents with sarcoidosis mentioned in their death record had sarcoidosis as their UCD supports this. Finally, like many other epidemiologic studies, this one should be viewed only as hypothesis-generating, sparking ideas for prospective research.
Conclusions
Overall, United States sarcoidosis-related mortality rates increased from 1988–2007. Rates were consistently higher in females than males and higher in blacks than whites. Over the study period, rates rose steadily but mildly in Hispanics and non-Hispanic whites and more steeply in non-Hispanic blacks; however, rates seem to have plateaued since the early 2000s in blacks. This was largely caused by modestly declining rates among decedents 25–34 years. Regardless of race or sex, mortality rates rose most sharply, and seem to be continuing to rise, among decedents 55 years of age and older. Younger sarcoidosis decedents with pulmonary fibrosis were more likely to be black than white, and younger decedents of either sex were more likely than similarly aged decedents in the background population to have a cardiac etiology contribute to death. It is hoped that our observations will fuel more research, particularly on the topic of the effects of age on clinical phenotype, disease course, and therapy of patients with sarcoidosis.
Supplementary Material
Supported in part by a Career Development Award from the NIH (K23 HL092227) (to J.J.S.).
Conception and design: J.S., A.L.O., T.J.H., E.R.F.-P., J.S., D.S., K.K.B. Analysis and interpretation: J.S., A.L.O., T.J.H., E.R.F.-P., J.S., D.S., K.K.B. Drafting the manuscript for important intellectual content: J.S., A.L.O., T.J.H., E.R.F.-P., J.S., D.S., K.K.B.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201010-1679OC on February 17, 2011
Author Disclosure: J.J.S. received consultancy fees from Actelion, Bayer, Genentech, and Gilead. A.L.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.J.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.R.F.-P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.K.B. received lecture fees from Biogen; consultancy fees from Genzyme, Actelion, MondoBiotech, Phillips, Pacific Therapeutics, Celgene, Elan, Stromedix, Fibrogen, and Amgen; advisory board fees from Novartis, Boehringer-Ingelheim, Centocor, and Gilead; and sponsored grants from Genzyme, Actelion, Novartis, Gilead, and Amgen.
References
- 1.Boeck C. Multiple benign sarcoid of the skin. J Cutan Genito-Urin Dis 1899;17:543–550. [Google Scholar]
- 2.Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997;145:234–241. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885–1889. [DOI] [PubMed] [Google Scholar]
- 4.Pietinalho A, Hiraga Y, Hosoda Y, Lofroos AB, Yamaguchi M, Selroos O. The frequency of sarcoidosis in Finland and Hokkaido, Japan. A comparative epidemiological study. Sarcoidosis 1995;12:61–67. [PubMed] [Google Scholar]
- 5.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the black women's health study. Chest 2011;139:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153–2165. [DOI] [PubMed] [Google Scholar]
- 7.Rybicki BA, Maliarik MJ, Major M, Popovich J Jr, Iannuzzi MC. Epidemiology, demographics, and genetics of sarcoidosis. Semin Respir Infect 1998;13:166–173. [PubMed] [Google Scholar]
- 8.Chappell AG, Cheung WY, Hutchings HA. Sarcoidosis: a long-term follow up study. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:167–173. [PubMed] [Google Scholar]
- 9.Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983;52:525–533. [PubMed] [Google Scholar]
- 10.Hillerdal G, Nou E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis 1984;130:29–32. [DOI] [PubMed] [Google Scholar]
- 11.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med 1995;119:167–172. [PubMed] [Google Scholar]
- 12.Takada K, Ina Y, Noda M, Sato T, Yamamoto M, Morishita M. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clin Epidemiol 1993;46:359–366. [DOI] [PubMed] [Google Scholar]
- 13.Arcasoy SM, Christie JD, Pochettino A, Rosengard BR, Blumenthal NP, Bavaria JE, Kotloff RM. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest 2001;120:873–880. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi M, Hosoda Y, Sasaki R, Aoki K. Epidemiological study on sarcoidosis in Japan. Recent trends in incidence and prevalence rates and changes in epidemiological features. Sarcoidosis 1989;6:138–146. [PubMed] [Google Scholar]
- 15.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med 1996;100:423–427. [DOI] [PubMed] [Google Scholar]
- 16.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007;176:277–284. [DOI] [PubMed] [Google Scholar]
- 17.Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, Murphy J, Cohen M, Raghu G, Brown KK. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Census Bureau. Population estimates. 2009. (accessed 2010 September 10). Available from: http://www.census.gov.
- 19.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax 2006;61:980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. ICD-10: International statistical classification of diseases and related health problems. Tenth revision. Geneva: World Health Organization; 2003. [PubMed]
- 21.Redelings M, Sorvillo F, Simon P. A comparison of underlying cause and multiple causes of death. Epidemiology 2006;17:100–103. [DOI] [PubMed] [Google Scholar]
- 22.Crawshaw AP, Wotton CJ, Yeates DG, Goldacre MJ, Ho LP. Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax 2011;66:447. [DOI] [PubMed] [Google Scholar]
- 23.Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, Gross BH, Myers J, Travis WD, Colby TV, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2010;181:832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collard HR. The age of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2010;181:771–772. [DOI] [PubMed] [Google Scholar]
- 25.Thannickal VJ. Aging, antagonistic pleiotropy and fibrotic disease. Int J Biochem Cell Biol 2010;42:1398–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.