Abstract
Rationale: We recently implicated a role for CD4+ T cells and demonstrated elevated IL-17A expression in lung ischemia–reperfusion (IR) injury. However, identification of the specific subset of CD4+ T cells and their mechanistic role in IR injury remains unknown.
Objectives: We tested the hypothesis that invariant natural killer T (iNKT) cells mediate lung IR injury via IL-17A signaling.
Methods: Mice underwent lung IR via left hilar ligation. Pulmonary function was measured using an isolated lung system. Lung injury was assessed by measuring edema (wet/dry weight) and vascular permeability (Evans blue dye). Inflammation was assessed by measuring proinflammatory cytokines in lungs, and neutrophil infiltration was measured by immunohistochemistry and myeloperoxidase levels.
Measurements and Main Results: Pulmonary dysfunction (increased airway resistance and pulmonary artery pressure and decreased pulmonary compliance), injury (edema, vascular permeability), and inflammation (elevated IL-17A; IL-6; tumor necrosis factor-α; monocyte chemotactic protein-1; keratinocyte-derived chemokine; regulated upon activation, normal T-cell expressed and secreted; and neutrophil infiltration) after IR were attenuated in IL-17A−/− and Rag-1−/− mice. Anti–IL-17A antibody attenuated lung dysfunction in wild-type mice after IR. Reconstitution of Rag-1−/− mice with wild-type, but not IL-17A−/−, CD4+ T cells restored lung dysfunction, injury, and inflammation after IR. Lung dysfunction, injury, IL-17A expression, and neutrophil infiltration were attenuated in Jα18−/− mice after IR, all of which were restored by reconstitution with wild-type, but not IL-17A−/−, iNKT cells. Flow cytometry and enzyme-linked immunosorbent spot assay confirmed IL-17A production by iNKT cells after IR.
Conclusions: These results demonstrate that CD4+ iNKT cells play a pivotal role in initiating lung injury, inflammation, and neutrophil recruitment after IR via an IL-17A–dependent mechanism.
Keywords: lung transplant, innate immunity, T cells, neutrophils, inflammation
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
CD4+ T cells have been implicated in lung ischemia–reperfusion; however, identification of the specific subset of CD4+ T cells and their role in lung ischemia–reperfusion injury remains to be elucidated.
What This Study Adds to the Field
Acute graft dysfunction, a severe form of ischemia–reperfusion injury, remains a problem in lung transplant patients. This study identifies a mechanistic role for invariant natural killer T-cell–derived IL-17A in the initiation of lung ischemia–reperfusion injury.
Lung transplantation has evolved as the definitive treatment for end-stage pulmonary disease. Despite advances in surgical management, critical care, and immunosuppression, long-term survival has not improved in these patients. In fact, outcomes for lung transplantation are the worst of any solid organ transplant. Lung ischemia–reperfusion (IR) injury, which affects up to 35% of transplant recipients, continues to be a common cause of early morbidity and mortality (1, 2). Lung IR injury also has long-term effects on the allograft, predisposing recipients to a greater risk for the development of bronchiolitis obliterans syndrome (3, 4). To date, no specific therapy exists for the prevention of IR injury, and the development of such a therapy would not only help patients acutely after transplant but could also reduce the incidence of late mortality due to bronchiolitis obliterans syndrome.
IR injury entails oxidative stress–induced injury, inflammation, and innate immune responses (5). A biphasic pattern of injury exists after IR that involves the rapid activation of leukocytes, such as macrophages, followed shortly by activation and infiltration of neutrophils (6, 7). Studies from our laboratory and others have recently implicated an important role for T lymphocytes in lung IR injury (8, 9). CD4+ T cells are known to play a role in initiating innate immune responses via crosstalk with various immune and nonimmune cells (10–12). We previously correlated rapid IL-17A production with infiltrating CD4+ T cells in the lung after IR (8, 13). The IL-17 family of cytokines, including IL-17A, are known to be largely expressed by activated T cells and are involved in host innate immune responses, such as macrophage and neutrophil activation (14, 15). Moreover, recent studies have provided evidence for a role of IL-17–producing CD4+ T cells in organ rejection and IR injury in heart, brain, liver, and intestines (16–19). However, the importance of IL-17–mediated inflammation and the specific cellular sources of IL-17 after lung IR remain to be elucidated.
One subset of CD4+ T cells are type I invariant natural killer T (iNKT) cells, which express the invariant T cell receptor (Vα14Jα18 in mice). The rapid response by iNKT cells to express inflammatory cytokines makes them ideal candidates to participate in the early innate immune response after IR (20, 21). The identification and role of the specific subset of IL-17–producing T cells in lung IR injury is unknown and thus was the focus of the current study. Herein, we tested the hypothesis that IL-17A produced by iNKT cells plays a pivotal role in lung injury and neutrophil infiltration after IR. The results from the present study represent a paradigm shift in the way we understand lung IR injury and neutrophil infiltration as being primarily initiated by CD4+ iNKT cells via an IL-17A–dependent mechanism. Some of the results from this study have been reported previously in the form of an abstract (22).
METHODS
Detailed methods are described in the online supplement.
Animals
This study used 8- to 12-week-old male mice. C57BL/6J wild-type (WT) and Rag-1−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). IL-17A−/− mice (23) and Jα18−/− mice (24) (provided by Dr. M. Taniguchi, RIKEN Research Center for Allergy and Immunology, Chiba University, Chiba, Japan) have been backcrossed onto the C57BL/6J background. For in vivo blocking of IL-17A, WT mice were treated intravenously, 10 minutes before ischemia, with 150 μg of either anti–IL-17 mAb (clone 50104; R&D Systems, Minneapolis, MN) or IgG2A isotype control (clone 20102; R&D Systems). This study conformed to the National Research Council's 8th Edition of the Guide for the Care and Use of Laboratory Animals published by National Academy Press (2011) (25) and was conducted under protocols approved by the University of Virginia's Institutional Animal Care and Use Committee.
Lung IR Model
An in vivo hilar clamp model of IR was used as previously described (8). Mice undergoing IR were subjected to 1 hour left lung ischemia (via left hilar occlusion) followed by 2 hours of reperfusion. Sham animals received the same surgeries as mice undergoing IR but without hilar occlusion.
Pulmonary Function
Pulmonary function was evaluated using an isolated, buffer-perfused lung system (Hugo Sachs Elektronik, March-Huggstetten, Germany) as previously described (8).
Bronchoalveolar Lavage
Left lungs were lavaged with 0.4 ml phosphate-buffered saline, centrifuged, and the bronchoalveolar lavage (BAL) fluid was stored at –80°C.
Cytokine and Myeloperoxidase Measurements
Cytokines in BAL fluid were quantified using a multiplex cytokine panel assay (Bio-Rad Laboratories, Hercules, CA). Myeloperoxidase (MPO) levels were measured in BAL fluid using a mouse MPO ELISA kit (Cell Sciences, Canton, MA).
Lung Wet/Dry Weight
Lungs were weighed and desiccated until a stable dry weight was achieved. Lung wet/dry weight was calculated as an indicator of edema.
Pulmonary Microvascular Permeability
Microvascular permeability was estimated using the Evans blue dye extravasation technique as previously described (13).
Immunohistochemistry and Neutrophil Counting
Immunostaining to identify neutrophils was performed as described previously (8).
Purification and Adoptive Transfer of CD4+ T Cells
CD4+ T cells were isolated from spleens using a magnetic bead–based isolation kit (Miltenyi Biotec, Auburn, CA). Purified CD4+ T cells (2 × 107 cells/animal) were injected into recipient mice via tail vein 7 days before study.
Purification and Adoptive Transfer of iNKT Cells
Splenocytes were incubated with CD1d tetramer-Alexa647 and enriched by positive magnetic bead selection using anti-Alexa647 microbeads (Miltenyi Biotec). The enriched cells were stained with fluorescein isothiocyanate–conjugated anti-CD19 and phycoerythrin-conjugated anti-TCRβ and sorted using a FACSVantage SE Turbo Sorter. Purified iNKT cells (2.5 × 105) were injected into adult male Jα18−/− mice via tail vein 4 days before study.
Flow Cytometry
Flow cytometry was performed as previously published (26) and modified as detailed in the online supplement.
Enzyme-Linked Immunosorbent Spot Assay
A commercially available murine IL-17A enzyme-linked immunosorbent spot (ELISPOT) assay (R&D Systems, Minneapolis, MN) was used. Isolated cells (1 × 105) were stimulated with medium containing 50 ng/ml phorbol 12-myristate-13 acetate (PMA) and 0.5 μg/ml calcium ionomycin. Neutrophils that were not stimulated were also included as controls. Results are presented as the average number of spot-forming cells per total number of cells plated.
Statistics
Values are presented as the mean ± SEM. One-way analysis of variance with post hoc Bonferroni multiple comparisons, Dunnett T3 test, two-way analysis of variance, or Student t test were used as appropriate to compare experimental groups. A P value of less than 0.05 was considered significant.
RESULTS
Pulmonary Dysfunction after IR Is Mediated by IL-17A
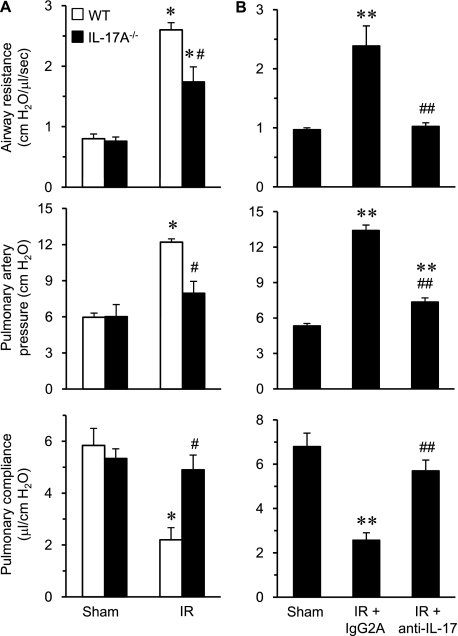
To investigate the importance of IL-17A in lung IR injury, pulmonary function was measured in WT and IL-17A−/− mice after sham or IR surgery (Figure 1A). Significant pulmonary dysfunction occurred after IR in WT mice as indicated by increased airway resistance and pulmonary artery pressure as well as decreased pulmonary compliance. Pulmonary dysfunction after IR was significantly attenuated in IL-17A−/− mice compared with WT. These results suggest that IL-17A is an important mediator of lung dysfunction after IR.
Figure 1.
Pulmonary dysfunction after ischemia–reperfusion (IR) is mediated by IL-17A. (A) Pulmonary function was measured in wild-type (WT) and IL-17A−/− mice after sham or IR surgery (n = 5/group). A significant increase in airway resistance and pulmonary artery pressure as well as a decrease in pulmonary compliance occurred in WT mice after IR compared with sham. Pulmonary dysfunction after IR was significantly attenuated in IL-17A−/− mice versus WT. (B) Pulmonary function was measured in WT mice after sham or IR surgery with or without treatment with anti–IL-17A antibody (n = 5/group). A significant improvement in airway resistance, pulmonary artery pressure, and pulmonary compliance occurred after IR in mice treated with anti–IL-17 antibody versus the isotype control antibody (IgG2A). *P < 0.05 versus corresponding sham; #P < 0.05 versus WT IR, **P < 0.05 versus sham, ##P < 0.05 versus IR+IgG2A.
To confirm the importance of IL-17A in IR injury and to provide a therapeutic relevance to this study, WT mice were treated with either anti–IL-17A antibody or IgG2A isotype control antibody 10 minutes before IR, and pulmonary function was measured. Administration of anti–IL-17A antibody significantly improved airway resistance, pulmonary artery pressure, and pulmonary compliance after IR compared with mice treated with IgG2A isotype control antibody (Figure 1B).
Pulmonary Dysfunction after IR Is Mediated by IL-17A Derived from CD4+ T Cells
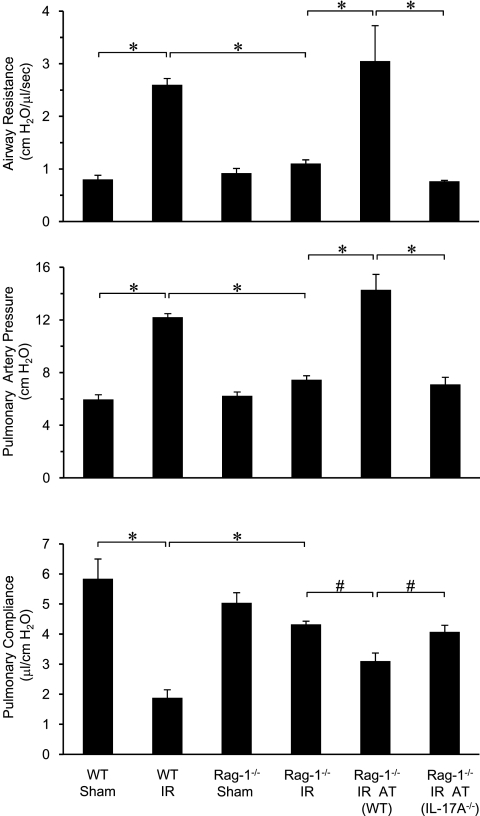
We have previously demonstrated that CD4+ T cells are important contributors to IR injury and could be a major source of IL-17A after IR (8). To further delineate the role of CD4+ T-cell–derived IL-17A in lung IR injury, pulmonary function was measured in WT and Rag-1−/− mice (which lack mature B and T lymphocytes including NKT cells). Compared with WT mice, lung dysfunction after IR in Rag-1−/− mice was significantly attenuated as demonstrated by decreased airway resistance and pulmonary artery pressure as well as increased pulmonary compliance (Figure 2). Sham Rag-1−/− mice were not significantly different from sham WT mice in terms of pulmonary function. To determine if IR-induced pulmonary dysfunction is mediated specifically by CD4+ T-cell–derived IL-17A, Rag-1−/− mice were reconstituted with CD4+ T cells by adoptive transfer. Reconstitution of Rag-1−/− mice with WT CD4+ T cells restored lung dysfunction after IR (Figure 2). However, reconstitution with IL-17A−/− CD4+ T cells failed to restore dysfunction and resulted in pulmonary function comparable to Rag-1−/− mice (Figure 2). These results implicate a key role for CD4+ T-cell–derived IL-17A in pulmonary dysfunction after IR.
Figure 2.
Pulmonary dysfunction after ischemia–reperfusion (IR) is mediated by CD4+ T-cell–derived IL-17A. Pulmonary function was measured after IR in wild-type (WT) and Rag-1−/− mice with or without adoptive transfer (AT) of CD4+ T cells from WT or IL-17A−/− mice as indicated (n = 5/group). IR-induced pulmonary dysfunction (increased airway resistance and pulmonary artery pressure as well as decreased pulmonary compliance) in WT mice was significantly attenuated in Rag-1−/− mice. Adoptive transfer of CD4+ T cells from WT mice, but not IL-17A−/− mice, restored pulmonary dysfunction in Rag-1−/− mice after IR. *P < 0.001, #P < 0.05.
Inflammation after IR Is Mediated by CD4+ T-Cell–produced IL-17A
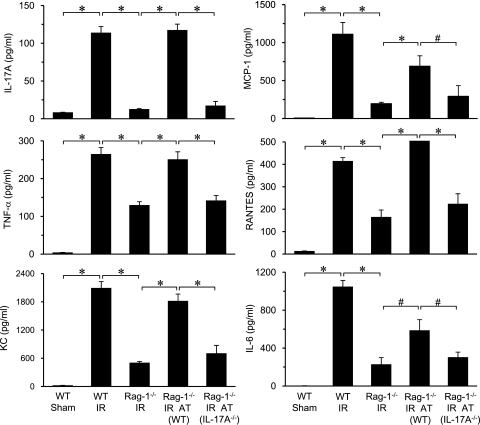
To assess inflammation, the expression of proinflammatory cytokines and chemokines was measured in BAL fluid of WT and Rag-1−/− mice after IR. Significant induction of IL-17A, monocyte chemotactic protein-1 (MCP-1) (CCL2), tumor necrosis factor (TNF)-α, regulated upon activation, normal T-cell expressed and secreted (RANTES) (CCL5), keratinocyte-derived chemokine (KC) (CXCL1), and IL-6 occurred in lungs of WT mice after IR, which was significantly attenuated in Rag-1−/− mice (Figure 3). Cytokine production was similar between sham Rag-1−/− mice and sham WT mice (data not shown). To determine if inflammation is mediated specifically by CD4+ T-cell–derived IL-17A, Rag-1−/− mice were reconstituted with CD4+ T cells by adoptive transfer. Reconstitution of Rag-1−/− mice with WT CD4+ T cells, but not IL-17A−/− CD4+ T cells, restored the induction of all cytokines after IR (Figure 3). Interestingly, expression of IFN-γ was not found to be significantly elevated in WT lungs after IR (data not shown). These results suggest that IL-17A, and not IFN-γ, produced by CD4+ T cells, is a key mediator of lung IR injury.
Figure 3.
Proinflammatory cytokine production after ischemia–reperfusion (IR) is mediated by CD4+ T cell–derived IL-17A. Cytokine levels (IL-17A, monocyte chemotactic protein-1 [MCP-1], tumor necrosis factor [TNF]-α, regulated upon activation, normal T-cell expressed and secreted [RANTES], keratinocyte-derived chemokine [KC], and IL-6) were measured in bronchoalveolar lavage fluid of wild-type (WT) and Rag-1−/− mice with or without adoptive transfer (AT) of CD4+ T cells from WT or IL-17A−/− mice as indicated (n = 5/group). A multifold increase of each cytokine occurred after IR in WT mice compared with sham, which was significantly attenuated in Rag-1−/− mice. Adoptive transfer of CD4+ T cells from WT mice, but not IL-17A−/− mice, restored expression of all cytokines in Rag-1−/− mice after IR. *P < 0.001, #P < 0.05.
Lung Injury after IR Is Mediated by CD4+ T-Cell–produced IL-17A
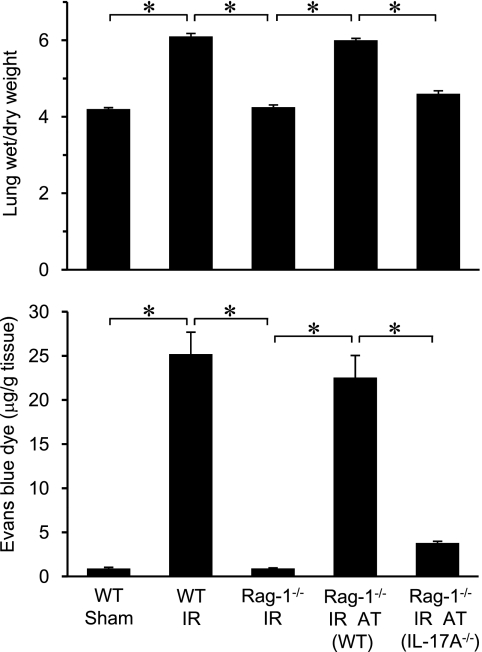
To determine the role of CD4+ T-cell–derived IL-17A in lung injury after IR, pulmonary edema (via wet/dry weight ratio) and vascular permeability (via Evans blue dye extravasation) were measured in WT and Rag-1−/− mice (Figure 4). Wet/dry weight and vascular permeability were similar between sham Rag-1−/− mice and sham WT mice (data not shown). A marked increase in pulmonary edema and microvascular permeability occurred in WT mice after IR, which was completely blocked in Rag-1−/− mice. Adoptive transfer of Rag-1−/− mice with WT CD4+ T cells, but not IL-17A−/− CD4+ T cells, restored pulmonary edema and microvascular permeability after IR (Figure 4). These results suggest that IL-17A from CD4+ T cells is an upstream mediator of vascular and epithelial cell permeability after IR.
Figure 4.
CD4+ T-cell–derived IL-17A mediates lung injury after ischemia–reperfusion (IR). Lung injury was assessed by measuring pulmonary edema (via lung wet/dry weight) and vascular permeability (via Evans blue dye extravasation). Wild-type (WT) and Rag-1−/− mice with or without adoptive transfer (AT) of CD4+ T cells from WT or IL-17A−/− mice were assessed (n = 5/group). Pulmonary edema and vascular permeability were significantly elevated in lungs of WT mice after IR compared with sham, which was blocked in Rag-1−/− mice. Adoptive transfer of CD4+ T cells from WT mice, but not IL-17A−/− mice, restored pulmonary edema and vascular permeability after IR in Rag-1−/− mice. *P < 0.001.
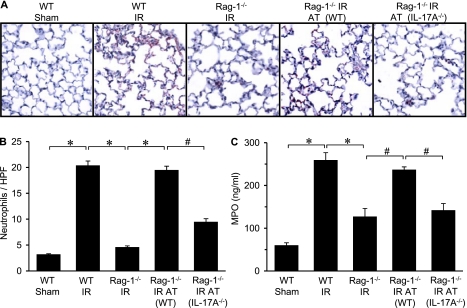
Neutrophil Infiltration and Activation after IR Is Mediated by IL-17A from CD4+ T Cells
IL-17A is known to mediate neutrophil recruitment via the induction of chemokines by IL-17 target cells (15, 27). To determine if CD4+ T-cell–derived IL-17A mediates neutrophil infiltration and activation after IR, neutrophil numbers in lung sections and MPO levels in BAL fluid were measured. Immunohistochemical analysis demonstrated significant neutrophil infiltration in lungs of WT mice after IR, which was prevented in Rag-1−/− mice (Figures 5A and 5B). Neutrophil numbers and MPO levels were similar between sham Rag-1−/− mice and sham WT mice (data not shown). Neutrophil infiltration after IR in Rag-1−/− mice was restored by adoptive transfer of WT CD4+ T cells but not IL-17A−/− CD4+ T cells (Figures 5A and 5B). To evaluate neutrophil activation, BAL fluid was assessed for MPO, a peroxidase enzyme most abundantly present in neutrophil granulocytes and released on activation. MPO levels were significantly elevated after IR in WT mice but were significantly attenuated in Rag-1−/− mice (Figure 5C). MPO levels after IR in Rag-1−/− mice were restored by adoptive transfer of WT CD4+ T cells but not IL-17A−/− CD4+ T cells (Figure 5C). These results support prior studies showing that IL-17A is a potent mediator of neutrophil chemotaxis (15, 27) and demonstrate that CD4+ T-cell–derived IL-17A is a key mediator of neutrophil activation and infiltration in the lung after IR.
Figure 5.
Neutrophil infiltration after ischemia–reperfusion (IR) is mediated by CD4+ T-cell–derived IL-17A. (A) Representative immunohistochemical staining of lung sections. Neutrophils are stained red, sections are counterstained with hematoxylin, and images are at 40× magnification. (B) Quantification of neutrophils per high-power field (HPF) from immunohistochemistry data (n = 5/group). The number of neutrophils/HPF significantly increased after IR in wild-type (WT) mice compared with sham, which was attenuated in Rag-1−/− mice. Adoptive transfer (AT) of Rag-1−/− mice with CD4+ T cells from WT mice, but not IL-17A−/− mice, restored neutrophil infiltration after IR. (C) Neutrophil activation was assessed by measuring myeloperoxidase (MPO) levels in bronchoalveolar lavage fluid (n = 5/group). MPO levels were significantly increased after IR in WT mice and attenuated in Rag-1−/− mice. Adoptive transfer of Rag-1−/− mice with CD4+ T cells from WT mice, but not IL-17A−/− mice, restored MPO levels after IR. *P < 0.001, #P < 0.05.
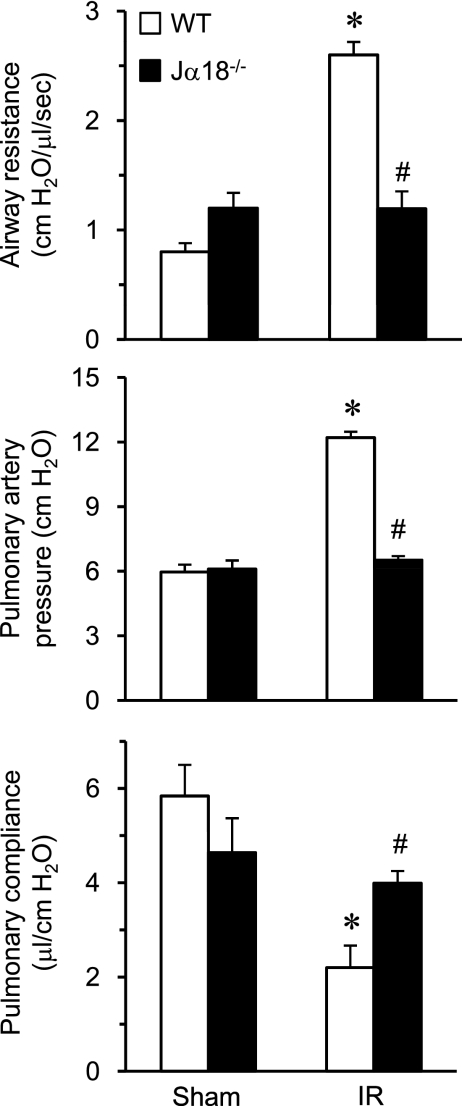
Pulmonary Dysfunction after IR Is Mediated by iNKT Cells
To further define the specific subset of IL-17A–producing CD4+ T cells involved in lung IR injury, we focused on the role of iNKT cells by measuring pulmonary function in Jα18−/− mice, which are deficient in Type 1 iNKT cells. Compared with WT mice, lungs of Jα18−/− mice were significantly protected after IR as shown by reduced airway resistance and pulmonary artery pressure as well as increased pulmonary compliance (Figure 6). Moreover, pulmonary function in Jα18−/− mice after IR was similar to Jα18−/− sham animals. These results demonstrate a key role of iNKT cells in pulmonary dysfunction after IR.
Figure 6.
Pulmonary dysfunction after ischemia–reperfusion (IR) is mediated by invariant natural killer T cells. Pulmonary function was measured in wild-type (WT) and Jα18−/− mice after sham or IR surgery (n = 5/group). A significant increase in airway resistance and pulmonary artery pressure as well as a decrease in pulmonary compliance occurred in WT mice after IR compared with sham. Pulmonary dysfunction after IR was prevented in Jα18−/− mice. *P < 0.001 versus corresponding sham, #P < 0.05 versus WT IR.
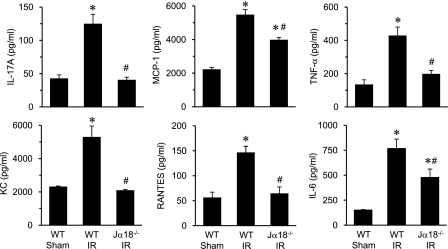
iNKT Cells Mediate Inflammation after IR
To determine if iNKT cells mediate inflammation after IR, the expression of proinflammatory cytokines and chemokines was measured in BAL fluid of WT and Jα18−/− mice. Cytokine levels were similar between sham Jα18−/− mice and WT mice (data not shown). The expression of IL-17A, MCP-1 (CCL2), TNF-α, RANTES (CCL5), KC (CXCL1), and IL-6 were significantly attenuated in Jα18−/− mice after IR compared with WT mice (Figure 7). Interestingly, the expression of IL-17A in Jα18−/− mice after IR was reduced to sham levels. These results demonstrate a pivotal role of iNKT cells in mediating inflammation after IR and suggest that iNKT cells could be primarily responsible for IL-17A production in the lung after IR.
Figure 7.
Proinflammatory cytokine production after ischemia–reperfusion (IR) is mediated by invariant natural killer T cells. Cytokine levels (IL-17A, MCP-1, tumor necrosis factor [TNF]-α, KC, regulated upon activation, normal T-cell expressed and secreted [RANTES], and IL-6) were measured in bronchoalveolar lavage fluid of wild-type (WT) and Jα18−/− mice (n = 5/group). A multifold increase of each cytokine occurred after IR in WT mice compared with sham, which was significantly attenuated in Jα18−/− mice. *P < 0.001 versus WT sham, #P < 0.05 versus WT IR.
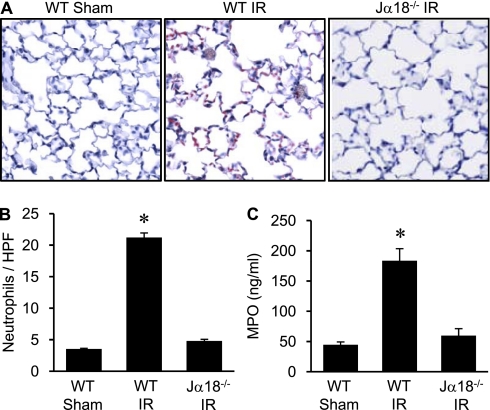
Neutrophil Infiltration and Activation after IR Is Mediated by iNKT Cells
To assess whether iNKT cells contribute to neutrophil activation and infiltration after IR, neutrophil infiltration and MPO levels were quantified. Immunohistochemical analysis demonstrated significant neutrophil infiltration in WT mice after IR which was prevented in Jα18−/− mice (Figures 8A and 8B). Furthermore, MPO levels in BAL fluid were significantly attenuated in Jα18−/− mice compared with WT mice after IR (Figure 8C). Neutrophil numbers and MPO levels in Jα18−/− mice after IR were similar to sham animals. These results demonstrate that iNKT cells are key mediators of neutrophil activation and infiltration after IR.
Figure 8.
Invariant natural killer T cells mediate neutrophil infiltration and activation after ischemia–reperfusion (IR). Neutrophil infiltration and activation were assessed in lungs of Jα18−/− and wild-type (WT) mice (n = 5/group). (A) Representative immunohistochemistry staining of lung sections. Neutrophils are stained red, sections are counterstained with hematoxylin, and images are at 40× magnification. (B) Quantification of neutrophils per high power field (HPF) from immunohistochemistry data. The number of neutrophils/HPF was significantly increased after IR in WT mice compared with sham and was attenuated in Jα18−/− mice. (C) Myeloperoxidase (MPO) levels in bronchoalveolar lavage fluid, as an indicator of neutrophil activation, were significantly increased in WT mice after IR and were attenuated in Jα18−/− mice. *P < 0.001 versus all.
Pulmonary Dysfunction and Inflammation after IR Is Mediated by iNKT-Cell–produced IL-17A
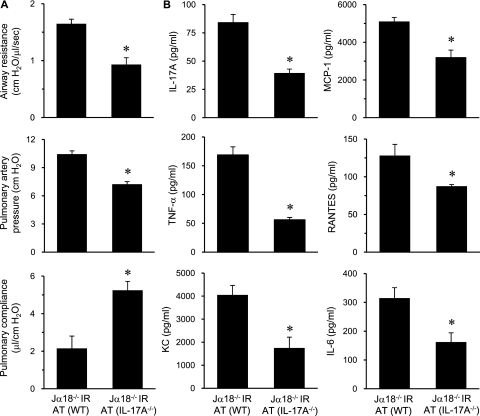
To specifically test if iNKT-cell–derived IL-17A plays an important role in lung IR injury, pulmonary function was assessed in Jα18−/− mice after adoptive transfer of iNKT cells from WT or IL-17A−/− mice. Compared with Jα18−/− mice reconstituted with WT iNKT cells, lung dysfunction in Jα18−/− mice reconstituted with IL-17A−/− iNKT cells was significantly attenuated after IR, as demonstrated by decreased airway resistance and pulmonary artery pressure as well as increased pulmonary compliance (Figure 9A). These results establish a key role for iNKT-cell–derived IL-17A in pulmonary dysfunction after IR. Furthermore, the expression of proinflammatory cytokines and chemokines (IL-17A, TNF-α, KC, MCP-1, RANTES, and IL-6) after IR in lungs of Jα18−/− mice reconstituted with IL-17A−/− iNKT cells was significantly attenuated compared with mice reconstituted with WT iNKT cells (Figure 9B). These results implicate iNKT-cell–derived IL-17A as a critical mediator of inflammation after lung IR.
Figure 9.
Lung dysfunction and inflammation after ischemia–reperfusion (IR) is mediated by IL-17A from invariant natural killer T (iNKT) cells. (A) Pulmonary function and (B) cytokine expression (IL-17A, monocyte chemotactic protein-1 [MCP-1], tumor necrosis factor [TNF]-α, regulated upon activation, normal T-cell expressed and secreted [RANTES], keratinocyte-derived chemokine [KC], and IL-6) in bronchoalveolar lavage fluid were measured after IR in Jα18−/− mice, which underwent adoptive transfer (AT) of iNKT cells purified from either wild-type (WT) or IL-17A−/− mice as indicated (n = 5/group). Lung dysfunction and expression of proinflammatory cytokines after IR were significantly attenuated in Jα18−/− mice reconstituted with IL-17A−/− iNKT cells. *P < 0.05.
iNKT Cells Are a Prominent Source of IL-17A after Lung IR
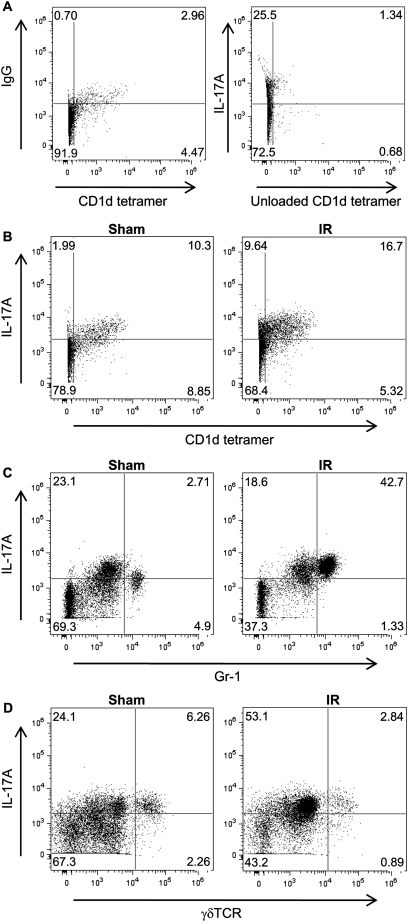
To determine the identity of IL-17A–expressing leukocytes in lungs after IR, cell suspensions of left lungs were evaluated by flow cytometry. To identify iNKT cells, an Alexa647-labeled CD1d tetramer loaded with an analog of α-galactosylceramide (αGalCer) was used because iNKT cells in mice and humans are best identified by binding of fluorochrome-conjugated tetrameric complexes of CD1d loaded with αGalCer (28). The number of IL-17A–expressing iNKT cells (IL-17A+ CD1d tetramer+) and neutrophils (IL-17A+ Gr-1+), was increased after IR compared with sham (Figures 10B and 10C). The number of IL-17A–expressing γδ T cells was not elevated after IR compared with sham (Figure 10D). Quantification of total leukocyte numbers in left lungs by flow cytometry demonstrated a significant increase in iNKT cells (2.1-fold) and neutrophils (10-fold) after IR versus sham (Table 1). After IR, the number of IL-17A+ iNKT cells increased 2.3-fold, and the number of IL-17A+ neutrophils increased 14-fold compared with sham (Table 1). The number of γδTCR+ IL-17A+ γδ T cells was not significantly elevated after IR. The percent of IL-17A+ iNKT cells increased to 81% after IR compared with 75% in sham animals, whereas the percent of IL-17A+ neutrophils increased to 87% after IR compared with 62% in sham animals (Table 1).
Figure 10.
Representative flow cytometric analysis of IL-17A-expressing leukocytes. Wild-type mice underwent either sham or ischemia–reperfusion (IR) surgery, and cell suspensions were prepared from left lungs. CD45+ 7-AAD− B220− TCRβ+ cells were gated and further assessed by flow cytometry as shown. The numbers within each box indicate the percentage of cells gated. (A) IgG isotype and unloaded CD1d tetramer were used as negative controls. (B) The number of IL-17A–expressing invariant natural killer T cells (IL-17+ CD1d tetramer+) was increased after IR compared with sham. (C) A substantial increase in the number of IL-17A–expressing neutrophils (IL-17+ Gr-1+) occurred after IR. (D) The number of IL-17A–expressing γδ T cells (IL-17+ γδTCR+) was not increased after IR compared with sham.
TABLE 1.
FLOW CYTOMETRIC QUANTIFICATION OF IL-17A–EXPRESSING LEUKOCYTES IN LUNGS OF WILD-TYPE MICE AFTER ISCHEMIA–REPERFUSION BY FLOW CYTOMETRY
| CD1d tetramer+ (iNKT cells) | Gr-1+ (neutrophils) | γδ TCR+ (γδ T lymphocytes) | |
|---|---|---|---|
| Total leukocytes* | |||
| Sham (n = 3) | 163,748 ± 19,990 | 74,332 ± 17,235 | 91,452 ± 25,985 |
| IR (n = 4) | 342,502 ± 35,783† | 759,981 ± 139,076† | 102,995 ± 6,076 |
| CD1d tetramer+ IL-17+ |
Gr-1+ IL-17+ |
γδ TCR+ IL-17+ |
|
| IL-17–positive leukocytes* | |||
| Sham (n = 3) | 122,847 ± 25,984 | 45,950 ± 15,428 | 71,476 ± 18,138 |
| IR (n = 4) | 277,848 ± 28,605† | 659,715 ± 122,128† | 76,762 ± 8,375 |
Definition of abbreviation: IR = ischemia–reperfusion.
Total number of cells per left lung are presented.
P < 0.05 versus sham.
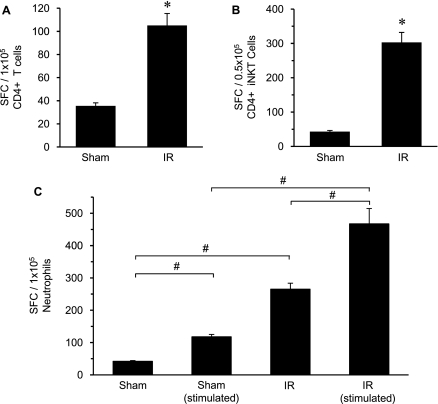
To further delineate an increase in the number of IL-17A–secreting CD4+ iNKT cells in the lung after IR, ELISPOT assay was used. ELISPOT analysis of purified CD4+ T cells and CD4+ CD1d tetramer+ iNKT cells from WT mouse lungs after IR showed a significant, multifold increase in the number of IL-17A–producing cells compared with sham (Figures 11A and 11B). Here, the number of CD4+ T cells and CD4+ iNKT cells expressing IL-17A was increased by threefold and sevenfold, respectively. These results further confirm that infiltrating CD4+ iNKT cells are a prominent source of IL-17A after IR.
Figure 11.
Quantitation of IL-17A–secreting CD4+ T cells, CD4+ invariant natural killer T (iNKT) cells, and neutrophils after ischemia–reperfusion (IR) by enzyme-linked immunosorbent spot (ELISPOT) assay. Wild-type mice (n = 5/group) underwent either sham or IR surgery, and (A) CD4+ T cells or (B) iNKT (CD4+ CD1d tetramer+) cells were isolated from left lungs and stimulated with phorbol 12-myristate-13 acetate (PMA)/ionomycin as described in the Methods. Subsequent analysis by ELISPOT showed a significant increase in the number of IL-17A–producing CD4+ T cells and CD4+ iNKT cells compared with sham. (C) Ly-6G+ neutrophils were also isolated and evaluated by ELISPOT after either stimulation with PMA/ionomycin or after no stimulation. The number of IL-17A–producing cells was significantly higher in stimulated and unstimulated neutrophils after IR compared with corresponding shams. The number of IL-17A–producing cells was significantly greater after IR in stimulated versus unstimulated neutrophils. The average number of spot forming cells (SFC) per number of cells plated is shown. *P < 0.05 versus corresponding sham, #P < 0.05.
ELISPOT assay was also used to assess IL-17A production by neutrophils isolated from WT mouse lungs after sham or IR surgery. Because abundant expression of IL-17A receptor (IL-17RA) on neutrophils can bind and potentially release IL-17A (29), Ly-6G+ neutrophils stimulated by PMA/ionomycin were compared with unstimulated neutrophils. The stimulated sham neutrophils had significantly higher numbers of IL-17A–producing cells versus unstimulated sham (Figure 11C). A sixfold increase in IL-17A–producing cells occurred in unstimulated neutrophils after IR versus unstimulated sham, whereas a fourfold increase in IL-17A–producing cells occurred in stimulated neutrophils after IR versus stimulated sham (Figure 11C). Importantly, a further, significant increase (1.7-fold) in IL-17A–producing cells occurred in stimulated neutrophils versus unstimulated neutrophils after IR (Figure 11C), suggesting that neutrophils may produce IL-17A.
DISCUSSION
The present study demonstrates for the first time that rapid production of IL-17A from infiltrating CD4+ iNKT cells plays a pivotal role in initiating lung IR injury and neutrophil infiltration. We have shown that both IL-17A−/− and Rag-1−/− mice are protected from lung IR injury. Although reconstitution of Rag-1−/− mice with WT CD4+ T cells restored lung IR injury, reconstitution with IL-17A−/− CD4+ T cells failed to do so. These results demonstrate that production of IL-17A specifically from CD4+ T cells plays a pivotal role in the initiation of lung IR injury. Furthermore, Jα18−/− mice, which lack iNKT cells, were also protected from lung IR injury, thereby implicating a crucial role for iNKT cells. The fact that reconstitution of Jα18−/− mice with IL-17A−/− iNKT cells failed to restore IR injury, whereas reconstitution with WT iNKT cells restored injury, demonstrates that IL-17A specifically from iNKT cells is a key mediator of lung IR injury. The specific identification of IL-17A–producing iNKT cells as being a CD4+ T cell subpopulation was confirmed by flow cytometry and ELISPOT assay. Taken together, these data support our hypothesis that CD4+ iNKT cells mediate lung injury, dysfunction, and inflammation after IR via the rapid production and secretion of IL-17A.
It should be noted that although Jα18−/− mice reconstituted with WT iNKT cells had significantly greater lung dysfunction and injury compared with mice reconstituted with IL-17A−/− iNKT cells (Figure 9), some parameters of injury (e.g., airway resistance and IL-17A levels) were not fully restored to levels comparable to WT mice after IR. This difference is likely related to the number of iNKT cells (2.5 × 105 cells) adoptively transferred, a relatively low number in comparison to that typically used in other studies described in the literature. It is likely that reconstitution with higher numbers of WT iNKT cells would have resulted in full restoration of injury. Because restoration of lung injury in Jα18−/− mice reconstituted with WT iNKT cells was not complete, we cannot exclude the possibility that CD4+ T cells other than iNKT cells contribute to IR injury. However, this seems unlikely because Jα18−/− mice, which are deficient only in iNKT cells, are completely protected against IR injury to the extent of sham animals. Nevertheless, the principal conclusion is that Jα18−/− mice reconstituted with IL-17−/− iNKT cells are protected versus mice reconstituted with WT iNKT cells, demonstrating specifically that iNKT cells contribute significantly to lung IR injury via IL-17A production.
Additionally, we noted an apparently high number of IL-17A–producing iNKT cells in lungs of sham animals as shown by flow cytometry (Figure 10B). This is likely due to the elevated background staining for IL-17A as shown by the IgG isotype control. This level of IL-17A background expression in the sham animals was not apparent by ELISPOT assay (Figure 11), suggesting that the high level of IL-17A background expression is limited to the flow cytometry technique. However, this should not significantly affect the relative change in numbers of IL-17A+ iNKT cells between sham and IR animals. We have also observed, by flow cytometry, that there is no difference in the number of IL-17A–expressing cells between sham and unoperated animals (data not shown), demonstrating that the surgical procedure alone does not elevate IL-17A expression.
Previous studies from our group demonstrated that CD3+ T cells rapidly infiltrate the lung after IR, but only depletion of CD4+ T cells, not CD8+ T cells, is protective (8). We also observed that depletion of CD4+ T cells attenuates neutrophil infiltration after IR, whereas depletion of neutrophils does not affect T cell infiltration. In the present study, we show that IL-17A mediates neutrophil recruitment after IR. Neutrophil recruitment was prevented in Rag-1−/− mice after IR and was restored by reconstitution with WT CD4+ T cells but not IL-17A−/− CD4+ T cells. In addition, neutrophil infiltration was attenuated in Jα18−/− mice after IR, but reconstitution with IL-17A−/− iNKT cells failed to restore neutrophil infiltration. These results demonstrate a role for CD4+ iNKT-cell–derived IL-17A in neutrophil recruitment after IR. IL-17A has been shown to up-regulate cell activation via the induction of various proinflammatory cytokines by IL-17A target cells, including IL-6, KC, IL-1β, GM-CSF, CXCL1, CXCL10, and TNF-α (14, 30). Thus, neutrophil recruitment after lung IR is likely mediated by IL-17A–dependent induction of potent chemokines (e.g., KC, TNF-α, and MIP-1α) from IL-17A target cells, chemokines that we have previously shown to be up-regulated after lung IR (13). Because MCP-1 and IL-6 levels were only partially restored after IR in Jα18−/− mice and in Rag-1−/− mice reconstituted with WT CD4+ T cells, there may be an additional mechanism, independent of T cells, that mediates pulmonary MCP-1 and IL-6 production after IR. In vitro data from our laboratory (unpublished observations) and earlier studies (31, 32) suggest that IL-17A modulates neutrophil recruitment and activation by stimulating chemotaxis via TNF-α and KC production by macrophages and epithelial cells, respectively. Collectively, these data suggest a key role of CD4+ iNKT-cell–derived IL-17A in neutrophil activation and recruitment after lung IR.
The role of immune cells in the inflammatory response after IR is complex; however, recent studies have provided evidence for T cell involvement in early innate immune responses after IR. IL-17–producing iNKT cells have also been shown to be frequent in the lung and to induce neutrophil recruitment into airways in response to αGalCer (33). Our data implicate iNKT cells as early responders to IR in the lung. As such, iNKT cells rapidly transition from a resting to an activated state after IR, whereby infiltrating as well as resident iNKT cells secrete IL-17A among other cytokines/chemokines. These findings represent a paradigm shift in our understanding of the role of T cells in lung IR injury.
Human iNKT cell populations have been shown to be composed of CD4+, CD8+, or CD4− CD8− cells, among which CD4+ iNKT cells account for approximately 50% of total iNKT cells (34). A recent study also described the existence of functionally distinct CD4+ and CD4− iNKT subsets in mice (35). Although it is possible that both CD4+ and CD4− iNKT cells produce IL-17A after IR, taken together, our results demonstrate that CD4+ iNKT cells compose the critical cell population that initiates lung IR injury via an IL-17–dependent mechanism. NKT cells have been shown to play an important role in regulating innate and adaptive immunity via production of a variety of cytokines, including IFN-γ, and increasing evidence suggests that different NKT cell subsets may exert distinct functions (36). It is interesting to note that IFN-γ expression was not significantly elevated after IR in our model, which is an important difference from what has been described for kidney (37) or liver (21) models of IR in which CD4+ T-cell–derived IFN-γ plays an important role. Thus it appears that T-cell–mediated lung IR injury is largely an IL-17A–dependent mechanism, and that IFN-γ is not a prominent player. It is possible, however, that reperfusion times longer than the 2 hours used in the present study could reveal a later role for IFN-γ as well as other cytokines in lung IR injury.
The flow cytometry and ELISPOT data in the present study demonstrate a significant increase in the number of IL-17A–producing CD4+ iNKT cells after IR. It is possible that the increase (recruitment) in the number of IL-17A–producing iNKT cells, and not the induction of IL-17A per se, is the main stimulus for IL-17–mediated IR injury. However, we believe that iNKT cells do significantly produce IL-17A in response to IR, which is supported by preliminary data demonstrating that production of IL-17A is significantly induced by primary CD4+ T cells when exposed to acute hypoxia/reoxygenation (38).
Although the present study identifies iNKT cells as a source of IL-17A after lung IR, a contribution from other T cell populations, such as CD8+ T cells, Th17 cells, or γδ T cells, remains plausible (27, 32, 33, 39, 40). Flow cytometry data in the present study suggest that γδ T cells are not significant contributors of IL-17A after IR. The acute time frame of IL-17A production in our model of lung IR (i.e., 2-h reperfusion) is not consistent with a role for Th17 cells, which are not normally present in the lung and which require differentiation from naive CD4+ T cells via TGF-β and IL-6 as well as the transcription factor RORγt (41). Moreover, we have previously shown that depletion of CD8+ T cells does not affect lung injury or IL-17A production after IR (8), suggesting that CD8+ T cells are not a source of IL-17A and not required for lung IR injury. The identification of iNKT cells as a critical source of IL-17A in the present study and its pronounced effect in mediating inflammation after IR thus signifies a paradigm shift in the current view of the role of T lymphocytes in lung IR injury.
Several studies have provided evidence that neutrophils can express IL-17A either at the protein level using immunohistochemistry or flow cytometry (19, 26) or at the mRNA level using real-time PCR (42, 43). However, it has been shown that the IL-17A receptor (IL-17RA) is ubiquitously expressed, including neutrophils, and that abundant expression of IL-17RA could allow neutrophils to avidly bind IL-17A secreted by other cells (29, 44, 45). The current study demonstrates, via flow cytometry and ELISPOT assay, that neutrophils express IL-17A after IR. This expression could result from production of IL-17A by neutrophils or from IL-17A bound to IL-17RA on neutrophils. The flow cytometry and ELISPOT methods cannot discriminate between bound versus produced IL-17. However, the fact that PMA/ionomycin-stimulated neutrophils had significantly more spot-forming cells versus unstimulated neutrophils after IR via ELISPOT assay suggests that neutrophils could be producing IL-17A after IR. Definitive proof for this will require evaluation of IL-17A gene expression in neutrophils sorted by fluorescence-activated cell sorter or the use of IL-17A reporter mice.
In conclusion, we have presented novel and compelling evidence that IL-17A produced by CD4+ iNKT cells mediates neutrophil infiltration and activation resulting in lung injury and dysfunction after IR. The identification of the pivotal role of iNKT-cell–derived IL-17A after IR advances our understanding of the molecular and cellular mechanisms underlying the initiation of IR injury after lung transplantation. Further studies will help determine if specific inhibition of iNKT cell-produced IL-17A via pharmacologic means can provide an effective therapeutic strategy to prevent IR injury and primary graft dysfunction after lung transplantation.
Supplementary Material
Acknowledgments
The authors thank Dr. Jeffrey Lysiak for assistance with the Bioplex instrument and Bioplex Bead Array system and Drs. Jie Sun, Thomas Braciale, and Joanne Lannigan for their assistance with flow cytometry. They also thank the National Institutes of Health tetramer core facility for providing the CD1d tetramer.
Supported by National Institutes of Health grants R01HL077301 (V.E.L.), T32HL007849 (I.L.K.), R01DK062324 and R01DK056223 (M.D.O.), and an American Heart Association Scientist Development grant (L.L.).
This article has online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201007-1173OC on February 11, 2011
Author Disclosure: A.K.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.L. received institutional grant support from the American Heart Association. C.L.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.L.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.D.O. received grant support from Genzyme and was a consultant for AstraZeneca. He owns a patent through and receives royalties from PGxHealth/Adenosine Therapeutics LLC. V.E.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ailawadi G, Lau CL, Smith PW, Swenson BR, Hennessy SA, Kuhn CJ, Fedoruk LM, Kozower BD, Kron IL, Jones DR. Does reperfusion injury still cause significant mortality after lung transplantation? J Thorac Cardiovasc Surg 2009;137:688–694. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Taylor DO, Kucheryavaya AY, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report-2009. J Heart Lung Transplant 2009;28:1031–1049. [DOI] [PubMed] [Google Scholar]
- 3.Granton J. Update of early respiratory failure in the lung transplant recipient. Curr Opin Crit Care 2006;12:19–24. [DOI] [PubMed] [Google Scholar]
- 4.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, Robbins MK, Kron IL. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg 2002;73:1041–1047. [DOI] [PubMed] [Google Scholar]
- 5.Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 2005;79:505–514. [DOI] [PubMed] [Google Scholar]
- 6.Eppinger MJ, Jones ML, Deeb GM, Bolling SF, Ward PA. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J Surg Res 1995;58:713–718. [DOI] [PubMed] [Google Scholar]
- 7.Fiser SM, Tribble CG, Long SM, Kaza AK, Cope JT, Laubach VE, Kern JA, Kron IL. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg 2001;121:1069–1075. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2009;137:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Perrot M, Young K, Imai Y, Liu M, Waddell TK, Fischer S, Zhang L, Keshavjee S. Recipient T cells mediate reperfusion injury after lung transplantation in the rat. J Immunol 2003;171:4995–5002. [DOI] [PubMed] [Google Scholar]
- 10.Debbabi H, Ghosh S, Kamath AB, Alt J, Demello DE, Dunsmore S, Behar SM. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L274–L279. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest 2004;114:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation 2008;86:710–718. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Sharma AK, Marshall M, Kron IL, Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol 2009;40:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 1998;160:3513–3521. [PubMed] [Google Scholar]
- 15.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C–X-C chemokine release in the airways. J Immunol 1999;162:2347–2352. [PubMed] [Google Scholar]
- 16.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med 2009;15:946–950. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 2005;289:G969–G976. [DOI] [PubMed] [Google Scholar]
- 18.Edgerton C, Crispin JC, Moratz CM, Bettelli E, Oukka M, Simovic M, Zacharia A, Egan R, Chen J, Dalle Lucca JJ, et al. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol 2009;130:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min SI, Ha J, Park CG, Won JK, Park YJ, Min SK, Kim SJ. Sequential evolution of IL-17 responses in the early period of allograft rejection. Exp Mol Med 2009;41:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 2007;178:5899–5911. [DOI] [PubMed] [Google Scholar]
- 21.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 2006;203:2639–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma AK, Zhao Y, LaPar D, Lau C, Kron I, Laubach V. Pivotal role of NKT cells and IL-17 In pulmonary ischemia-reperfusion injury [abstract]. Am J Respir Crit Care Med 2010;181:A1099. [Google Scholar]
- 23.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002;17:375–387. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 1997;278:1623–1626. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals, 8th ed. Washington, DC: National Academies Press; 2011.
- 26.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 2010;120:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821–852. [DOI] [PubMed] [Google Scholar]
- 28.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med 2000;191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001;194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol 2007;19:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004;21:467–476. [DOI] [PubMed] [Google Scholar]
- 32.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation 2008;31:167–179. [DOI] [PubMed] [Google Scholar]
- 33.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med 2007;204:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, Rugeles MT, Atkinson MA, Landay AL, Wilson SB. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 2007;122:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med 2005;202:1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009;86:513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol 2006;176:3108–3114. [DOI] [PubMed] [Google Scholar]
- 38.Sharma AK, Hajzus V, Kazemi A, Kron IL, Laubach VE. NADPH oxidase-dependent activation of CD4+ T cells modulates macrophage and alveolar epithelial cell activation during lung ischemia-reperfusion injury. Am J Respir Crit Care Med 2010;181:A1095. [Google Scholar]
- 39.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J 2005;25:159–172. [DOI] [PubMed] [Google Scholar]
- 40.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 2006;177:4662–4669. [DOI] [PubMed] [Google Scholar]
- 41.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity 2008;28:445–453. [DOI] [PubMed] [Google Scholar]
- 42.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol 2003;170:2106–2112. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino A, Nagao T, Nagi-Miura N, Ohno N, Yasuhara M, Yamamoto K, Nakayama T, Suzuki K. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J Autoimmun 2008;31:79–89. [DOI] [PubMed] [Google Scholar]
- 44.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 1995;3:811–821. [DOI] [PubMed] [Google Scholar]
- 45.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine 1997;9:794–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.