Abstract
Punica granatum is commonly used in Korea as a traditional medicine for the treatment of pathogenic bacteria. In this study, we investigated the in vitro and in vivo antimicrobial activity of P. granatum peel EtOH extract (PGPE) against 16 strains of Salmonella. The minimal inhibitory concentrations of PGPE were in the range of 62.5–1000 x03BCg mL−1. In addition, the in vivo antibacterial activity of the PGPE extract was examined in a S. typhimurium infection mouse model. Mice were initially infected with S. typhimurium and then with PGPE. The extract was found to have significant effects on mortality and the numbers of viable S. typhimurium recovered from feces. Although clinical signs and histological damage were rarely observed in the treated mice, the untreated controls showed signs of lethargy and histological damage in the liver and spleen. Taken together, the results of this study indicate that PGPE has the potential to provide an effective treatment for salmonellosis.
1. Introduction
Salmonella enterica, which are Gram-negative bacterial pathogens capable of infecting humans and animals, cause significant morbidity and mortality worldwide [1]. S. enterica serovar typhimurium is a clinically important intracellular bacterial pathogen that causes food poisoning and gastroenteritis in millions of people worldwide each year [2]. The Centers for Disease Control (CDC) estimates that there are nearly 1.4 million food-borne Salmonella infections annually in the USA [3]. This bacterium infects the intestinal tract and causes systemic infection of various organs such as the liver and spleen [4].
Fluoroquinolones and tetracyclines are the antibiotics most commonly used to treat Salmonella, and until recently most strains were susceptible to these drugs. However, a high incidence of Salmonella strains resistant to commonly prescribed antibiotics has recently been reported in Korea and other countries [5, 6], and the increased appearance of antibiotic resistant strains of Salmonella further exacerbates this problem [7]. One major concern to public health has been the global dissemination of S. typhimurium Definitive Type 104, which is commonly resistant to five or more antimicrobial agents [8–11]. The rise in antibiotic-resistant pathogens has led to the development of new therapeutic agents that are effective against these bacteria. Recently, there has been considerable interest in the use of plant materials as an alternative method to control pathogenic microorganisms [12], and many compounds of plant products have been shown to be specifically targeted against resistant pathogenic bacteria [13].
Punica granatum, which belongs to the family of Punicaceae, is commonly known as pomegranate, grenade, granats and punica apple [14]. Punica granatum has been used extensively as a traditional medicine in many countries [15] for the treatment of dysentery, diarrhea, helminthiasis, acidosis, hemorrhage and respiratory pathologies [16, 17]. In addition, P. granatum is reported to have antioxidant [18, 19], anti-atherosclerotic [20, 21], antibacterial [22, 23] and antiviral [24] properties. The constituents of P. granatum include gallocatechins, delphinidin, cyanidin, gallic acid, ellagic acid, pelargonidin and sitosterol, which are very well known for their therapeutic properties [25].
Punica granatum peel is used to treat infections found in human sexual organs as well as mastitis, acne, folliculitis, pile, allergic dermatitis, tympanitis, scalds, diarrhea, dysentery and as an antioxidant [26]. In addition, it is reported that the extracts of P. granatum have antimicrobial activity against Salmonella [23]. However, to date, no studies regarding the antimicrobial activity of P. granatum peels have been conducted. Therefore, the goal of this study is to evaluate the antimicrobial activity of the EtOH extract of P. granatum peel using various in vitro and in vivo models.
2. Methods
2.1. Bacterial Strains and Culture Medium
Salmonella typhi (ATCC 19943), S. dublin (ATCC 39184), S. derby (ATCC 6960), S. choleraesuis (ATCC 7001) and S. gallinarum (ATCC 9184) were used in this study (Table 1). In addition, this study included local isolates of S. enteritidis, S. typhimurium, S. gallinarum and S. paratyphi A, which were provided by the National Veterinary Research and Quarantine Service, Republic of Korea. Bacterial strains were suspended in Mueller Hinton broth (MHB, Difco, USA) and then incubated at 37°C for 20 h. Mueller Hinton agar (MHA, Difco) was used for the agar diffusion method and minimal inhibitory concentration (MIC). Salmonella typhimurium (JOL 389) was used for in vivo assays in mice.
Table 1.
List of Salmonella strains used in this study.
| Strain | Serotypes | Origin | Resistant antibiotics |
|---|---|---|---|
| JOL 380 | S. typhi ATCC 19943 | Human | — |
| JOL 381 | S. paratyphi A | Human | — |
| JOL 386 | S. enteritidis | Chicken | — |
| JOL 387 | S. typhimurium | Cattle | — |
| JOL 388 | S. typhimurium | Cattle | — |
| JOL 389 | S. typhimurium | Pig | AM, C, G, S, TIC |
| JOL 407 | S. enteritidis | Chicken | — |
| JOL 408 | S. typhimurium | Pig | — |
| JOL 409 | S. dublin ATCC 39184 | Cattle | — |
| JOL 410 | S. derby ATCC 6960 | Pig | — |
| JOL 411 | S. choleraesuis ATCC 7001 | Pig | AM, SXT |
| JOL 419 | S. gallinarum | Chicken | CF, G, SXT |
| JOL 420 | S. gallinarum | Chicken | CF, CIP, NA |
| JOL 421 | S. gallinarum | Chicken | G, NA, S |
| JOL 422 | S. gallinarum | Chicken | — |
| JOL 423 | S. gallinarum ATCC 9184 | Chicken | AM, AMC, C, G, S |
AM, ampicillin; AMC, amoxicillin/clavulanic acid; C, chloramphenicol; CF, cephalothin; G, sulfisoxazole; NA, nalidixic acid; S, streptomycin; SXT, trimethoprim/sulfamethoxazole; TIC, ticarcillin.
2.2. Extraction of Plant Material
Punica granatum peel was purchased from an Oriental drug store, Daehak Hanyakkuk (Iksan, Korea), and then authenticated by Dr D.Y. Kwon. A voucher specimen (no. 06-022) was deposited in the Laboratory of Herbalogy, College of Pharmacy, Wonkwang University, Iksan, Korea. Next, the P. granatum peel was air-dried in the dark at room temperature and then ground into a powder using a mechanical grinder. Approximately 500 g of the powdered materials were then boiled in 1500 mLof EtOH for 3 h. The solvent was then removed under reduced pressure in a rotary evaporator (N-1000S, EYELA, Japan) and dissolved in water or 50% dimethyl sulfoxide (DMSO, Sigma, USA) prior to use.
2.3. High-Performance Liquid Chromatography Analysis
The High-performance liquid chromatography (HPLC) system consisted of a Shimadzu LC-6A model (Shimadzu, Tokyo, Japan), with a column of ODS-C18 (4.6 × 250 mm, 5 μm) and a detection of SPD- 6AV with a sensitivity of 0.04 AUFS and a wavelength of 254 nm. Elution was carried out at a flow rate of 0.8 mL/min under a linear gradient of acetonitrile (solvent A) and H2O with 1% formic acid (solvent B) from 5% A to 100% A in 50 min. The P. granatum peel EtOH extract (PGPE) was dissolved in a mixture of methanol and water (6 : 4 v v−1), and 20 μlL was injected into the HPLC. The presence of gallic acid and ellagic acid was confirmed by the same retention time of their standards (Sigma Chemical Co, St Louis, USA) (Figure 1). The obtained chromatogram is shown in Figure 2 [27].
Figure 1.
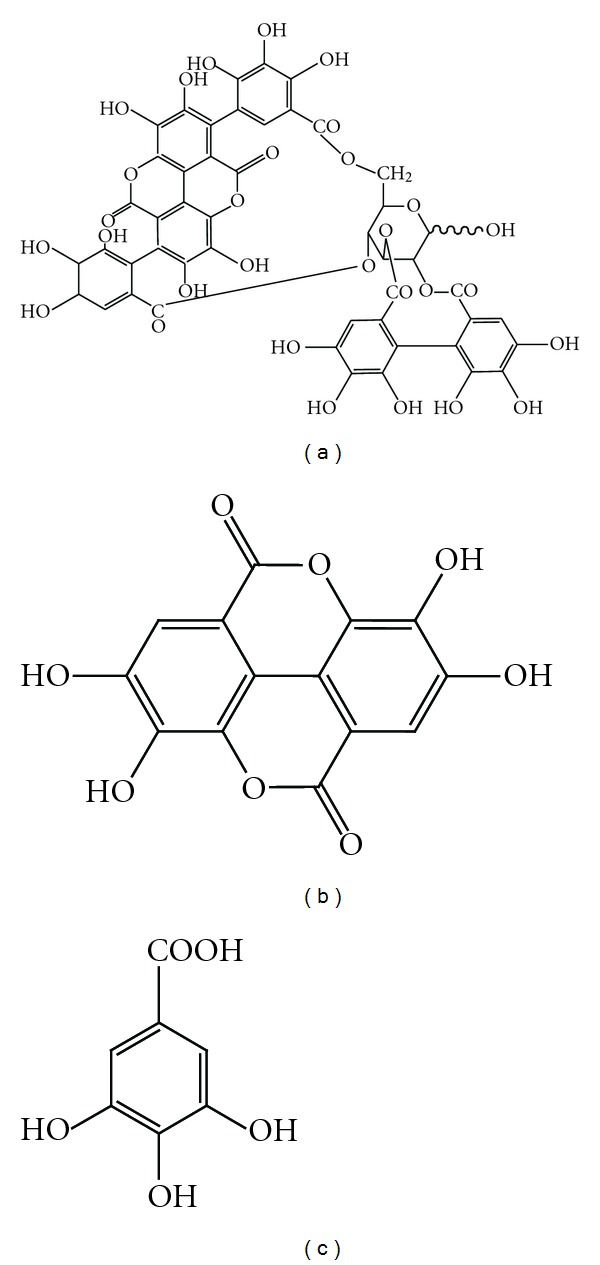
The chemical structure of punicalagin (a), ellagic acid (b) and gallic acid (c).
Figure 2.
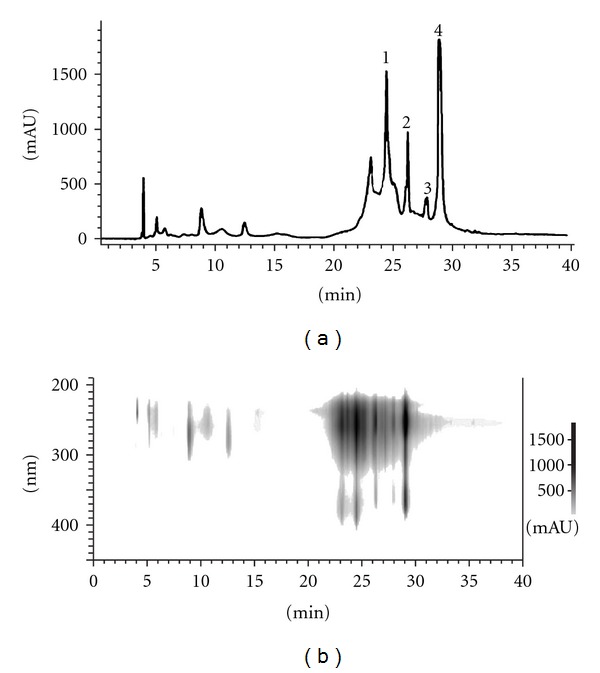
HPLC analysis of PGPE (a) and 3D HPLC analysis (b). (1) and (2) punicalagin isomers; (3) gallic acid; (4) ellagic acid.
2.4. Antimicrobial Resistance Testing
The resistance of the various Salmonella strains to different antimicrobial agents was determined using the disk-agar method standardized by the Clinical and Laboratory Standards Institute [28]. The quality control strain used was Enterococcus faecalis ATCC 29212.
2.5. Disc Diffusion Method
The antibacterial activities of the isolates on the different extracts were tested using the disk-agar method described by the Clinical and Laboratory Standards Institute Standards and by using a modified agar-well diffusion method [28, 29]. Briefly, sterile paper discs (6 mm; Toyo Roshi Kaihsa, Japan) were loaded with 20 μL of PGPE (varying concentrations: 100, 200 and 500 μg) dissolved in 50% DMSO and then left to dry for 18 h at 37°C in a sterile room. The bacterial suspensions were then diluted to a turbidity of approximately 0.5 McFarland (∼1.5 × 108 CFU mL−1), and then further diluted to obtain the final inoculum. Next, the MHA was poured into Petri dishes and inoculated with 100 μlL of the suspension containing 1 × 105 CFU mL−1 of bacteria. Ampicillin (Sigma Chemical Co) was used as the positive control and discs treated with 50% DMSO were used as the negative control. The plates were then placed in an incubator (Vision Co, Seoul, Korea) at 37°C for 24 h, after which the diameter of the zone of inhibition around each of the discs was measured and recorded. Each experiment was performed in triplicate.
2.6. Determination of MICs
The MIC values were determined for microorganisms that were found to be sensitive to PGPE during the disc diffusion assay. To accomplish this, the microorganism inocula were prepared from 12-h broth cultures and the suspensions were then adjusted to a turbidity of 0.5 McFarland. Susceptibility tests were then conducted using the standard broth micro dilution method in accordance with the CLSI guidelines [30] in MHB with an inoculum of ∼5 × 104 CFU mL−1. The MHB was then supplemented with serial dilutions of P. granatum peel of EtOH extracts ranging from 3.9 to 2000 μg mL−1 and ampicillin concentration was ranging from 0.03 to 250 μg mL−1. The lowest concentration of PGPE capable of inhibiting visible growth after 24 h of incubation at 37°C was then recorded as the MIC [30].
2.7. Animals
Mice were obtained from Da Mool Science (Deajeon, Korea). All mice experiments in this study were approved by the Wonkwang University Animal Ethics Committee in accordance with the guidelines of the Korean Council on Animal Care. Fifteen male Balb/c mice (15–17 g) aged between 5 and 6 weeks were used for all in vivo experiments. They were kept in a temperature-controlled room under a 12 h light 12 h dark cycle. Animals had free access to commercial solid food (SCF Co. Ltd, Korea) and water ad libitum, and were acclimatized for at least 1 week prior to beginning the experiments.
2.8. In Vivo Assay Using Mice
Mice were divided into the following groups: control (CON), Salmonella-infected (SI) and Salmonella-infected + PGPE (SIPG). Each treatment group contained five mice. Throughout the experiment, mice were provided with water that contained streptomycin (5 mg mL−1) in order to reduce the level of facultative anaerobic bacteria that normally colonize the mouse intestine [31]. The inhibition of the growth of test organisms in mice was then determined by monitoring S. typhimurium in the feces of the mice. Briefly, S. typhimurium (JOL 389) was grown overnight in Luria–Bertani broth (Difco), centrifuged, washed in phosphate-buffered saline (PBS) and then diluted into 20% sucrose to achieve a final concentration of 1 × 105 CFU. The SI and SIPG groups exclusively were then inoculated using gavage needle orally with approximately 105 CFU of S. typhimurium in a 0.1 mL volume. One hour after infection, animals in the SIPG group were orally administered 5 mg (using gavage needle) of the PGPE daily, whereas CON and SI animals were not. Fecal samples were then collected 0, 1, 2, 3, 4, 5 and 6 days after the bacterial suspensions were administered and the numbers of the bacteria per gram of feces were determined. Aliquots (100 μl) of fecal suspensions were serially diluted in PBS and then plated on duplicate Salmonella-Shigella agar plates (Difco), which were subsequently incubated overnight at 37°C. Typical colonies were then counted on plates that contained between 30 and 300 colonies [32], after which confirmation of S. typhimurium was performed by a PCR assay using a previously described method [33]. At Day 4 post-infection, the mice were sacrificed, and tissue specimens of the kidney, liver, intestine and spleen organs were transferred to 10% buffered neutral formalin for histopathologic examinations and then processed using standard procedures. Sections of paraffin-embedded tissues were then stained with hematoxylin and eosin.
3. Results
3.1. Determination of Antibacterial Activity by the Disc Diffusion Method
The antimicrobial efficacy of PGPE against the 16 Salmonella strains was evaluated by the disc diffusion method via determination of the surrounding zones of inhibition, as well as by evaluating the MIC using the agar dilution method. Table 2 shows the antimicrobial activity of P. granatum peel extract as determined by the disc diffusion method. The mean values of the zones of inhibition produced against the tested bacteria ranged from 13.3 to 18.6 mm, with the growth of each of the tested strains being inhibited at 500 μg per disc and the zone of inhibition increasing in a dose-dependant manner.
Table 2.
Antimicrobial activity (as inhibition zone diameters) of PGPE and ampicillin (APCL) against 16 strains of Salmonella.
| Strain | Serotypes | Diameter of clear zone (mm) | |||
|---|---|---|---|---|---|
| PGPE | APCLa | ||||
| 100 μg | 200 μg | 500 μg | 10 μg | ||
| JOL 380 | S. typhi ATCC 19943 | 13.3 ± 1.1 | 16.3 ± 1.5 | 17.3 ± 1.1 | 34.2 ± 1.0 |
| JOL 381 | S. paratyphi A | 14.3 ± 0.5 | 15.6 ± 1.1 | 18.6 ± 1.1 | 28.5 ± 1.1 |
| JOL 386 | S. enteritidis | 9.0 ± 1.0 | 12.6 ± 1.5 | 14.3 ± 1.1 | 31.1 ± 0.3 |
| JOL 387 | S. typhimurium | 9.6 ± 0.5 | 11.6 ± 0.5 | 14.6 ± 0.5 | 27.5 ± 1.0 |
| JOL 388 | S. typhimurium | 11.0 ± 1.0 | 14.0 ± 1.0 | 15.0 ± 1.0 | 26.0 ± 1.0 |
| JOL 389 | S. typhimurium | 9.0 ± 1.0 | 12.0 ± 1.0 | 12.6 ± 0.5 | ND |
| JOL 407 | S. enteritidis | 9.3 ± 1.1 | 12.3 ± 0.5 | 14.6 ± 0.5 | 26.7 ± 1.2 |
| JOL 408 | S. typhimurium | 8.6 ± 1.1 | 10.6 ± 0.5 | 14.0 ± 2.0 | 30.2 ± 0.5 |
| JOL 409 | S. dublin ATCC 39184 | 8.0 ± 0.0 | 10.0 ± 0.0 | 13.3 ± 0.5 | 27.0 ± 1.1 |
| JOL 410 | S. derby ATCC 6960 | 10.0 ± 0.0 | 12.0 ± 0.0 | 14.3 ± 0.5 | 25.6 ± 1.1 |
| JOL 411 | S. choleraesuis ATCC 7001 | 11.6 ± 0.5 | 15.6 ± 1.0 | 16.0 ± 0.0 | 11.2 ± 1.0 |
| JOL 419 | S. gallinarum | 12.3 ± 0.5 | 16.0 ± 1.0 | 16.6 ± 1.1 | 27.3 ± 1.5 |
| JOL 420 | S. gallinarum | 16.0 ± 0.0 | 16.0 ± 0.7 | 17.6 ± 0.5 | 25.8 ± 1.1 |
| JOL 421 | S. gallinarum | 11.3 ± 0.5 | 15.0 ± 0.0 | 16.3 ± 0.5 | 25.2 ± 0.3 |
| JOL 422 | S. gallinarum | 12.0 ± 0.0 | 14.0 ± 1.7 | 16.0 ± 1.0 | 28.5 ± 0.5 |
| JOL 423 | S. gallinarum ATCC 9184 | 7.6 ± 0.5 | 10.0 ± 0.0 | 13.3 ± 1.1 | ND |
Data shown represent the mean ± SE of three experiments that consisted of three replicates. ND, No activity detected.
aPositive control.
3.2. Determination of MICs
The MICs of the PGPE against the 16 strains of Salmonella are shown in Table 3. The MICs determined using the broth dilution method confirmed the results obtained using the disc diffusion method. PGPE showed antimicrobial activity against each of the tested strains, and these values ranged from 62.5 to 1000 μg mL−1. The in vivo experiment was therefore conducted with the EtOH extract.
Table 3.
Antimicrobial activity of PGPE and ampicillin (APCL) against 16 strains of Salmonella.
| Strain | Serotypes | MIC (μg mL−1) | |
|---|---|---|---|
| PGPE | APCLa | ||
| JOL 380 | S. typhi ATCC 19943 | 250 | 0.97 |
| JOL 381 | S. paratyphi A | 62.5 | 1.95 |
| JOL 386 | S. enteritidis | 1000 | 1.95 |
| JOL 387 | S. typhimurium | 1000 | 1.95 |
| JOL 388 | S. typhimurium | 500 | 0.97 |
| JOL 389 | S. typhimurium | 250 | >250 |
| JOL 407 | S. enteritidis | 250 | 1.95 |
| JOL 408 | S. typhimurium | 500 | 1.95 |
| JOL 409 | S. dublin ATCC 39184 | 500 | 0.97 |
| JOL 410 | S. derby ATCC 6960 | 500 | 1.95 |
| JOL 411 | S. choleraesuis ATCC 7001 | 62.5 | >250 |
| JOL 419 | S. gallinarum | 62.5 | 1.95 |
| JOL 420 | S. gallinarum | 62.5 | 1.95 |
| JOL 421 | S. gallinarum | 125 | 1.95 |
| JOL 422 | S. gallinarum | 250 | 1.95 |
| JOL 423 | S. gallinarum ATCC 9184 | 1000 | >250 |
aPositive control.
3.3. Antibacterial Efficacy of PGPE in Mice
The in vivo antibacterial activity of PGPE was examined using a mouse S. typhimurium infection model. Briefly, mice were infected with 1 × 105 CFU of S. typhimurium (SI). One-hour later, the mice were orally administered PGPE (SIPG). As shown in Table 4, treatment with the extract of P. granatum peel was found to have marked effects on mortality and on the number of viable S. typhimurium recovered from feces. At Day 1 post-infection, ten mice in the SI and SIPG group shed viable S. typhimurium in feces, with the feces of mice in the SI group being found to contain bacteria at a concentration of 3 × 103 to 4 × 105 CFU g−1 and feces of mice in the SIPG group being found to contain bacteria at a concentration of 2 × 102 to 2 × 103 CFU g−1. In addition, at Day 6 post-injection, none of the mice in the SIPG group had died, whereas all five mice in the SI group had succumbed as illustrated in Figure 3.
Table 4.
Effects of treatment with PGPE on fecal shedding of S. typhimurium (CFU g−1) by mice.
| Group | Day of post-feeding | ||||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| SI-1 | 0 | 3 × 103 | 2 × 103 | 1 × 104 | 2 × 104 | 1 × 104 | Death |
| SI-2 | 0 | 2 × 104 | 5 × 104 | 7 × 105 | Death | Death | Death |
| SI-3 | 0 | 3 × 104 | 1 × 105 | 2 × 105 | Death | Death | Death |
| SI-4 | 0 | 7 × 104 | 3 × 104 | 2 × 104 | 1 × 106 | Death | Death |
| SI-5 | 0 | 4 × 105 | 2 × 105 | 1 × 105 | 3 × 106 | Death | Death |
| SIPG-1 | 0 | 1 × 103 | 1 × 103 | 1 × 103 | 6 × 103 | 2 × 103 | 0 |
| SIPG-2 | 0 | 2 × 103 | 1 × 103 | 2 × 103 | 3 × 103 | 2 × 103 | 2 × 103 |
| SIPG-3 | 0 | 2 × 102 | 2 × 103 | 8 × 103 | 3 × 103 | 3 × 102 | 2 × 102 |
| SIPG-4 | 0 | 1 × 103 | 3 × 102 | 2 × 102 | 1 × 102 | 3 × 102 | 2 × 102 |
| SIPG-5 | 0 | 4 × 102 | 8 × 102 | 1 × 103 | 2 × 102 | 3 × 102 | 1 × 102 |
Figure 3.

Importance of PGPE against S. typhimurium infection.
3.4. Organ Histopathologic Changes
Salmonella typhimurium-infected mice that did not receive the PGPE were lethargic and showed signs of histological damage in the liver and spleen. In addition, the central and portal veins of the liver showed congestion with focal necrotic emboli-like materials (Figure 4). There were multiple small necrotizing nodular lesions in the liver parenchyma with Kuffer cell hyperplasia and inflammatory cell infiltrate. The spleen showed extensive hemorrhagic necrosis in the red pulp with multiple apoptotic bodies in the white pulp (Figure 4). No specific abnormal findings were observed in the kidney or the small intestine. Conversely, clinical signs and histological damage were rarely observed in S. typhimurium-infected mice fed with the PGPE.
Figure 4.
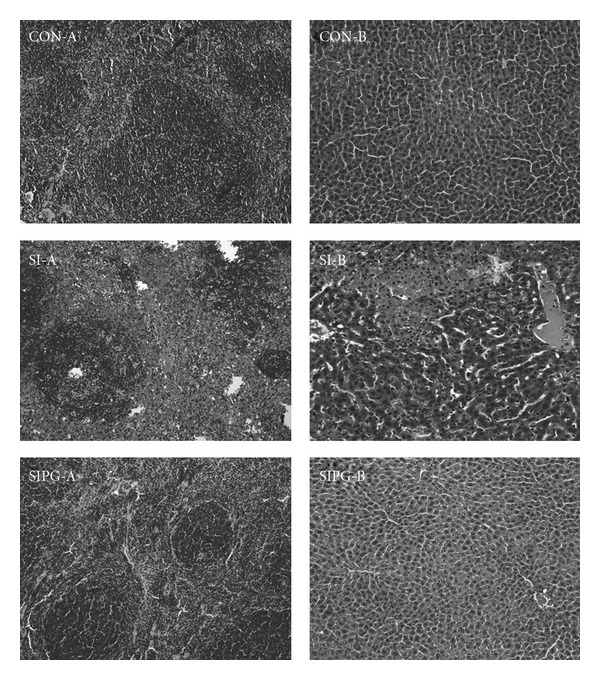
Histopathological changes in organs in CON, SI and SIPG. (a) spleen (×200) and (b) liver (×200).
4. Discussion
Recently, a number of antibiotics have lost their effectiveness due to the development of resistant strains of bacteria, which has primarily occurred through the expression of resistance genes [34, 35]. In addition to inducing resistance, antibiotics are sometimes associated with opposing effects such as hypersensitivity, immune-suppression and allergic reactions [36]. Therefore, there is a need to develop alternative antimicrobial drugs for the treatment of infectious diseases [37, 38].
In the present study, the PGPE exhibited antibacterial activity against all 16 strains of eight different Salmonella serotypes tested. In addition, the results of the MIC assays also confirmed its antibacterial effects against all tested salmonella strains. The PGPE also exhibited antibacterial activities against Salmonella strains JOL 389, JOL 411, JOL 419, JOL 420, JOL 421 and JOL 423, all of which have been shown to be resistant to two to five antibiotics (Table 1). The in vivo antibacterial assay also revealed that the extract effectively inhibited the growth of S. typhimurium and significantly reduced mouse mortality (Table 4). Furthermore, clinical signs of infection and histological damage were rarely observed in test mice, whereas untreated SI mice showed severe clinical signs and histological damage in the tested organs. This is the first study describing the antibacterial activity of P. granatum peel extract against Salmonella. Based on these promising in vitro and in vivo assay findings, we believe that P. granatum peel extract is likely to become a novel antimicrobial treatment for salmonellosis. It has been reported that P. granatum peel extracts have shown antibacterial activity against Escherichia coli O157 and methicillin-resistant Staphylococcus aureus bacteria [14, 39]. This antibacterial activity may be indicative of the presence of several metabolic toxins or broad-spectrum antibiotics. Several metabolites from herb species, including alkaloids, tannins and sterols, have previously been associated with antimicrobial activity [40].
In order to investigate components from the PGPE, the HPLC analysis was performed as shown in Figures 2(a) and 2(b). This HPLC analysis among some other minor constituents mainly shows some major phenolic compounds [26]; gallic acid and ellagic acids in addition to punicalagin as a major ellagitannin. The retention time shows it to be gallic acid and ellagic acid [41, 42] and also the presence of punicalagin isomers (Figure 1) could be deduced to be one of the major components from the results of literature reported previously [27]. Gallic acid was reported to have antibacterial activity against some intestinal bacteria [43], ellagic acid has anti-microbial activity [44] and punicalagin was reported to show anti-food-borne pathogens [45]. The site and the number of hydroxyl groups on the phenol components may increase the toxicity against the microorganisms [46]. However, it has been reported that gallic acid, ellagic acid and punicalagin have weak antibacterial activity against Salmonella.
The antibacterial activity of P. granatum peel extract might be related to the action of its antibiotic compounds or to the presence of metabolic toxins. This suggests that these components may also provide antibacterial activity against Salmonella and provide a plausible explanation for the higher antibacterial activity of the EtOH extract. On the other hand, the unknown minor components present have not been elucidated in terms of their activity. Further studies then need to be done. In the future, thorough investigation is needed to better ascertain the antibacterial effect of this herb extract.
Funding
Grant No. RTI 05-03-02 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE) in Republic of Korea.
References
- 1.Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cellular Microbiology. 2007;9(11):2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 2.Grassl GA, Valdez Y, Bergstrom KSB, Vallance BA, Finlay BB. Chronic enteric Salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134(3):768–780. doi: 10.1053/j.gastro.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 3.Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999;5(5):607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunology and Cell Biology. 2007;85(2):112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 5.Choi S-H, Woo JH, Lee JE, et al. Increasing incidence of quinolone resistance in human non-typhoid Salmonella enterica isolates in Korea and mechanisms involved in quinolone resistance. Journal of Antimicrobial Chemotherapy. 2005;56(6):1111–1114. doi: 10.1093/jac/dki369. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson JE, Gay K, Barrett TJ, Medalla F, Chiller TM, Angulo FJ. Increase in nalidixic acid resistance among non-typhi Salmonella enterica isolates in the United States from 1996 to 2003. Antimicrobial Agents and Chemotherapy. 2007;51(1):195–197. doi: 10.1128/AAC.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. The Lancet. 2005;366(9487):749–762. doi: 10.1016/S0140-6736(05)67181-4. [DOI] [PubMed] [Google Scholar]
- 8.Gebreyes WA, Thakur S, Davies PR, Funk JA, Altier C. Trends in antimicrobial resistance, phage types and integrons among Salmonella serotypes from pigs, 1997–2000. Journal of Antimicrobial Chemotherapy. 2004;53:997–1003. doi: 10.1093/jac/dkh247. [DOI] [PubMed] [Google Scholar]
- 9.Perron GG, Bell G, Quessy S. Parallel evolution of multidrug-resistance in Salmonella enterica isolated from swine. FEMS Microbiology Letters. 2008;281(1):17–22. doi: 10.1111/j.1574-6968.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 10.Poppe C, Ziebell K, Martin L, Allen K. Diversity in antimicrobial resistance and other characteristics among Salmonella typhimurium DT104 isolates. Microbial Drug Resistance. 2002;8:107–122. doi: 10.1089/107662902760190653. [DOI] [PubMed] [Google Scholar]
- 11.Threlfall EJ, Ward LR, Frost JA, Willshaw GA. Spread of resistance from food animals to man–the UK experience. Acta Veterinaria Scandinavica. Supplementum. 2000;93:63–68. [PubMed] [Google Scholar]
- 12.Aqil F, Khan MS, Owais M, Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of beta-lactamase producing methicillin resistant Staphylococcus aureus . Journal of Basic Microbiology. 2005;45:106–114. doi: 10.1002/jobm.200410355. [DOI] [PubMed] [Google Scholar]
- 13.Nostro A, Cellini L, Di Bartolomeo S, et al. Effects of combining extracts (from propolis or Zingiber officinale) with clarithromycin on Helicobacter pylori . Phytotherapy Research. 2006;20(3):187–190. doi: 10.1002/ptr.1830. [DOI] [PubMed] [Google Scholar]
- 14.Voravuthikunchai SP, Sririrak T, Limsuwan S, Supawita T, Iida T, Honda T. Inhibitory effects of active compounds from Punica granatum pericarp on verocytotoxin production by enterohemorrhagic Escherichia coli O157:H7. Journal of Health Science. 2005;51(5):590–596. [Google Scholar]
- 15.Singh RP, Chidambara MKN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. Journal of Agricultural and Food Chemistry. 2002;50(1):81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 16.Ricci D, Giamperi L, Bucchini A, Fraternale D. Antioxidant activity of Punica granatum fruits. Fitoterapia. 2006;77(4):310–312. doi: 10.1016/j.fitote.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Lamar A, Fonseca G, Fuentes JL, et al. Assessment of the genotoxic risk of Punica granatum L. (Punicaceae) whole fruit extracts. Journal of Ethnopharmacology. 2007;115(3):416–422. doi: 10.1016/j.jep.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Related A, LinksHeber D, Seeram NP, et al. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. Journal of Agricultural and Food Chemistry. 2007;55:10050–10054. doi: 10.1021/jf071689v. [DOI] [PubMed] [Google Scholar]
- 19.Parmar HS, Kar A. Medicinal values of fruit peels from Citrus sinensis, Punica granatum, and Musa paradisiaca with respect to alterations in tissue lipid peroxidation and serum concentration of glucose, insulin, and thyroid hormones. Journal of Medicinal Food. 2008;11(2):376–381. doi: 10.1089/jmf.2006.010. [DOI] [PubMed] [Google Scholar]
- 20.Aviram M, Rosenblat M, Gaitini D, et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clinical Nutrition. 2004;23:423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Parmar HS, Kar A. Protective role of Citrus sinensis, Musa paradisiaca, and Punica granatum peels against diet-induced atherosclerosis and thyroid dysfunctions in rats. Nutrition Research. 2007;27(11):710–718. [Google Scholar]
- 22.Braga LC, Shupp JW, Cummings C, et al. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. Journal of Ethnopharmacology. 2005;96(1-2):335–339. doi: 10.1016/j.jep.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Naz S, Siddiqi R, Ahmad S, Rasool SA, Sayeed SA. Antibacterial activity directed isolation of compounds from Punica granatum . Journal of Food Science. 2007;72(9):M341–M345. doi: 10.1111/j.1750-3841.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Zhan B, Yao X, Gao Y, Shong J. Antiviral activity of tannin from the pericarp of Punica granatum L. against genital Herpes virus in vitro. Zhongguo Zhong yao za zhi. 1995;20(9):556–576. [PubMed] [Google Scholar]
- 25.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. Journal of Ethnopharmacology. 2007;109(2):177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Singh RP, Chidambara MKN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. Journal of Agricultural and Food Chemistry. 2002;50(1):81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 27.Kwak HM, Jeong HH, Sohng BH, et al. Quantitative analysis of antioxdants in Korea pomegranate Husk (Granati pericarpium) cultivated in different site. Journal of the Korean Society for Applied Biological Chemistry. 2005;48:431–434. [Google Scholar]
- 28. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standards. CLSI document M2-A7. Wayne, Pa, USA, 2001.
- 29.Okunji CO, Okeke CN, Gugnani HC, Iwu MM. An antifungal spirostanol saponin from fruit pulp of Dracaena mannii . International Journal of Crude Drug Research. 1990;28(3):193–199. [Google Scholar]
- 30. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standards. CLSI document M7-A5. Wayne, Pa, USA, 2000.
- 31.Myhal ML, Laux DC, Cohen PS. Relative colonizing abilities of human fecal and K-12 stains of Escherichia coli in the large intestines of streptomycin treated mice. European Journal of Clinical Microbiology. 1982;1:186–192. doi: 10.1007/BF02019621. [DOI] [PubMed] [Google Scholar]
- 32.Lee M-H, Kwon HA, Kwon D-Y, et al. Antibacterial activity of medicinal herb extracts against Salmonella . International Journal of Food Microbiology. 2006;111(3):270–275. doi: 10.1016/j.ijfoodmicro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez J, Sota M, Vivanco AB, et al. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. Journal of Clinical Microbiology. 2004;42(4):1734–1738. doi: 10.1128/JCM.42.4.1734-1738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264(5157):375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 35.Service RF. Antibiotics that resist resistance. Science. 1995;270(5237):724–727. doi: 10.1126/science.270.5237.724. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. Journal of Ethnopharmacology. 1998;62(2):183–193. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 37.Berahou A, Auhmani A, Fdil N, Benharref A, Jana M, Gadhi CA. Antibacterial activity of Quercus ilex bark’s extracts. Journal of Ethnopharmacology. 2007;112(3):426–429. doi: 10.1016/j.jep.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Salomão K, Pereira PRS, Campos LC, et al. Brazilian propolis: correlation between chemical composition and antimicrobial activity. Evidence-Based Complementary and Alternative Medicine. 2008;5(3):317–324. doi: 10.1093/ecam/nem058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado TDB, Leal ICR, Amaral ACF, Dos Santos KRN, Da Silva MG, Kuster RM. Antimicrobial ellagitannin of Punica granatum fruits. Journal of the Brazilian Chemical Society. 2002;13(5):606–610. [Google Scholar]
- 40.Leven MD, Vanden BDA, Marten T, Vilientmick A, Lomweas EC. Screening of higher plants for biological activity. Planta Medica. 1979;36:311–312. doi: 10.1055/s-0028-1097277. [DOI] [PubMed] [Google Scholar]
- 41.Lu J, Wei Y, Yuan Q. Preparative separation of punicalagin from pomegranate husk by high-speed countercurrent chromatography. Journal of Chromatography B. 2007;857(1):175–179. doi: 10.1016/j.jchromb.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 42.Aviram M, Volkova N, Coleman R, et al. Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: studies in vivo in atherosclerotic apolipoprotein e-deficient (E0) mice and in vitro in cultured macrophages and lipoproteins. Journal of Agricultural and Food Chemistry. 2008;56:1148–1157. doi: 10.1021/jf071811q. [DOI] [PubMed] [Google Scholar]
- 43.Ahn Y-J, Lee C-O, Kweon J-H, Ahn J-W, Park J-H. Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria. Journal of Applied Microbiology. 1998;84(3):439–443. doi: 10.1046/j.1365-2672.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- 44.Thiem B, Goślińska O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoterapia. 2004;75(1):93–95. doi: 10.1016/j.fitote.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Taguri T, Tanaka T, Kouno I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biological and Pharmaceutical Bulletin. 2004;27(12):1965–1969. doi: 10.1248/bpb.27.1965. [DOI] [PubMed] [Google Scholar]
- 46.Cowan MM. Plant products as antimicrobial agents. Clinical Microbiology Reviews. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]


