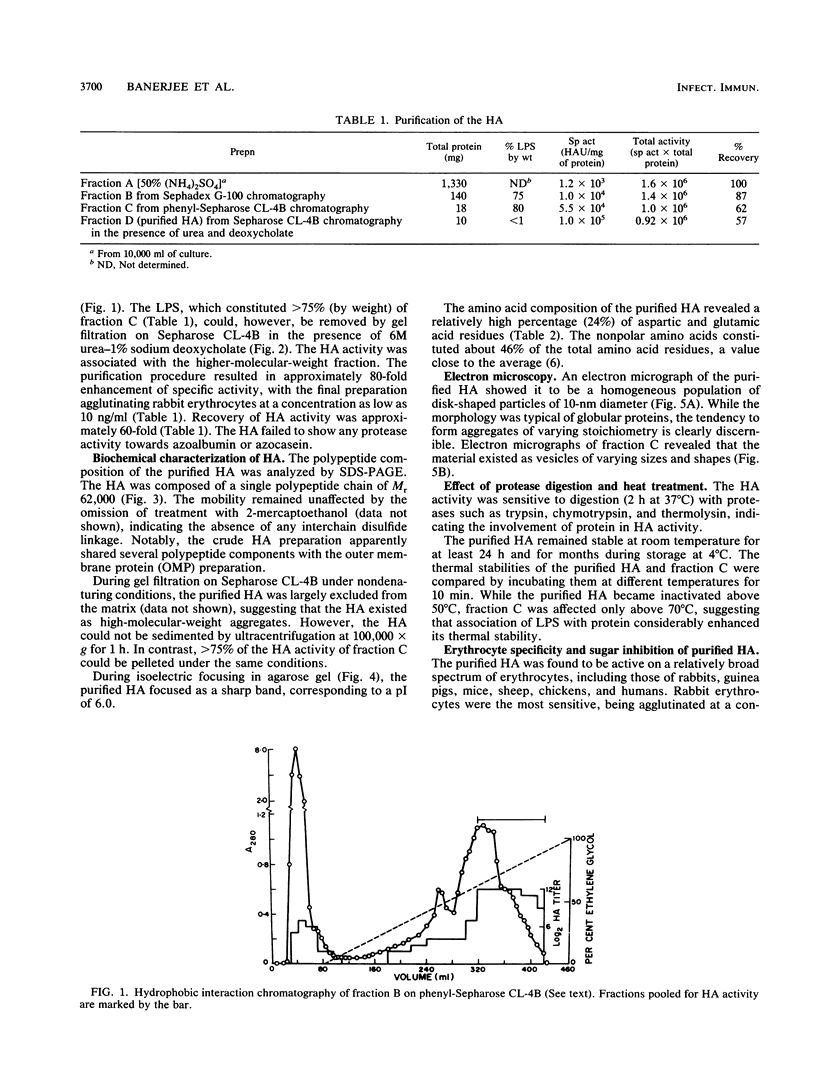
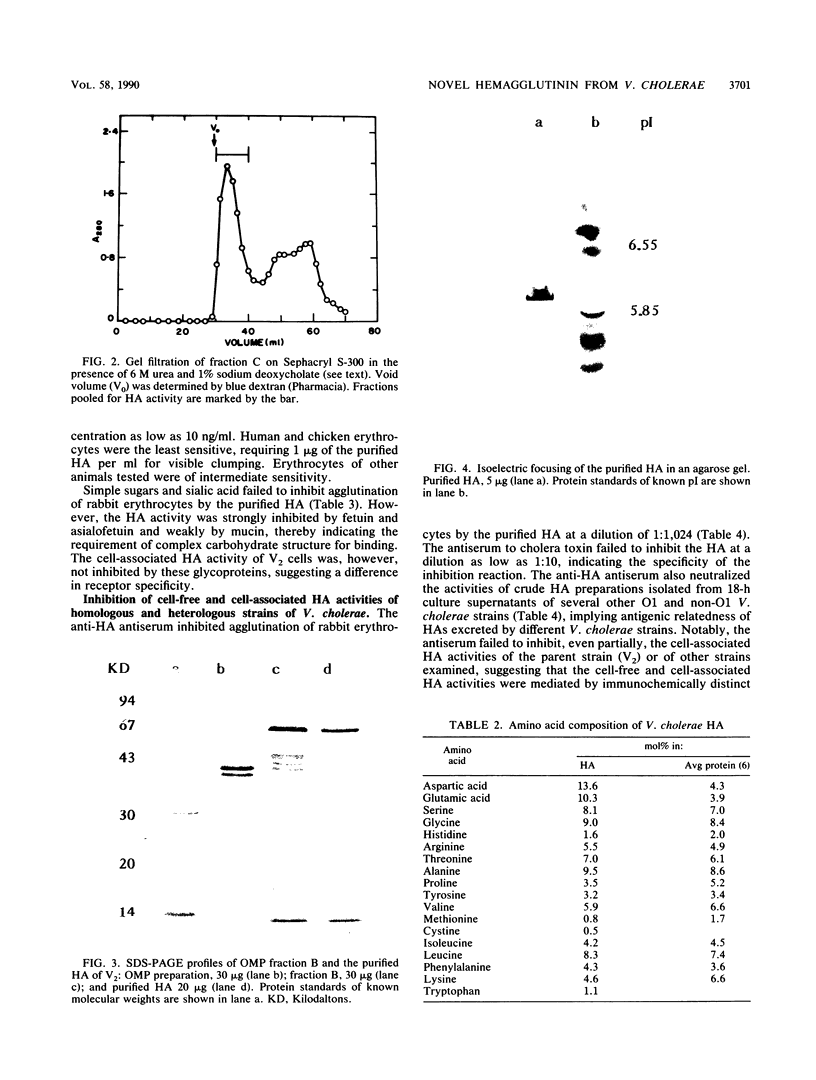
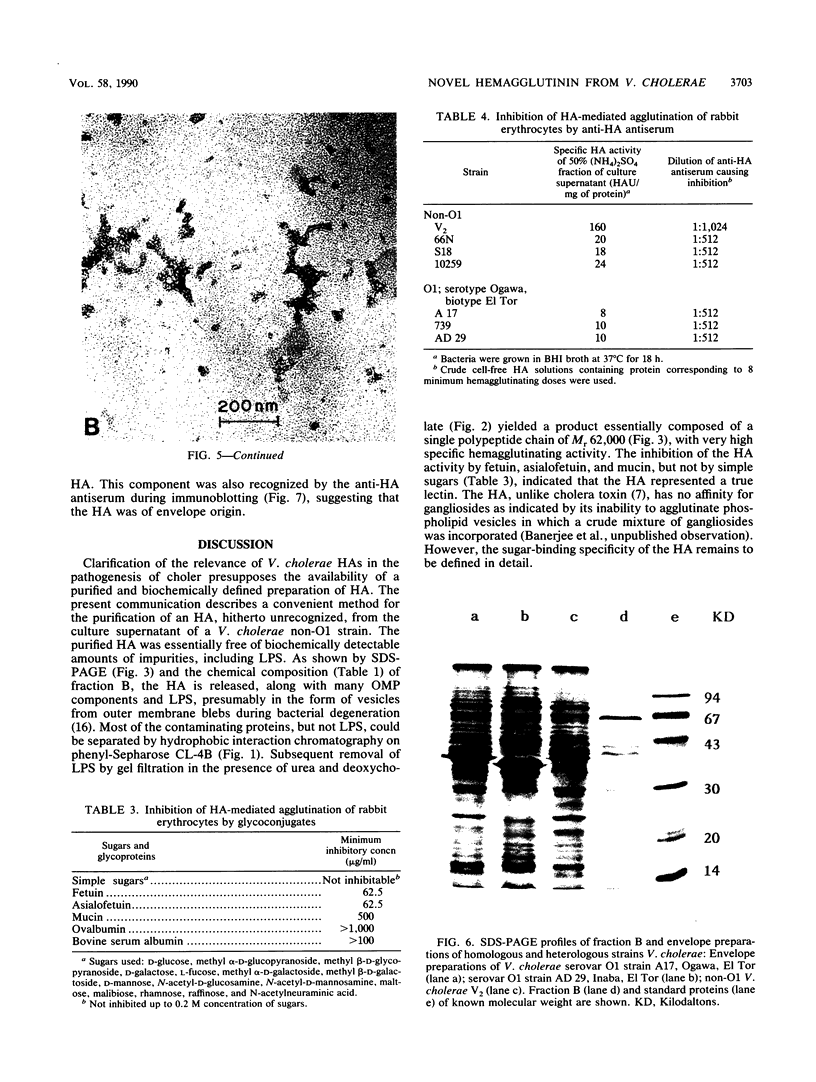
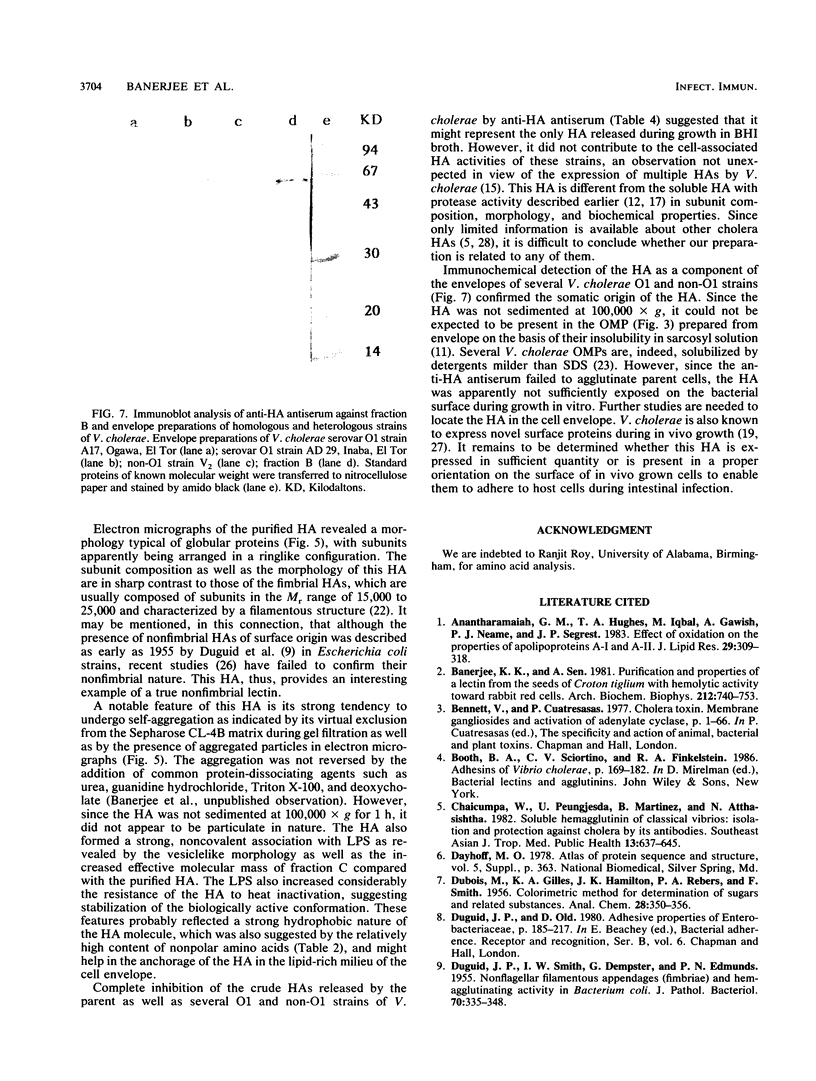
Abstract
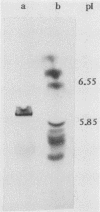
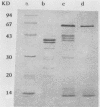
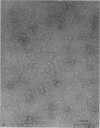
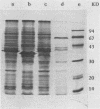
A lectin with strong hemagglutinating activity toward erythrocytes of several animal species was isolated from an 18-h culture supernatant of a diarrheagenic strain, V2, of non-O1 Vibrio cholerae. The hemagglutinin (HA) was purified free of lipopolysaccharide by salt fractionation followed by gel filtration, hydrophobic interaction chromatography, and, finally, gel filtration in the presence of urea and deoxycholate. The purification procedure resulted in an HA preparation with 80-fold enhancement of specific activity. The HA consisted of noncovalently bound subunits of Mr 62,000 and behaved essentially as a single component with pI 6.0. Nonpolar and acidic amino acids contributed 46 and 24%, respectively, to the total amino acid residues. Electron micrographs of the HA showed it to consist of large, nonstoichiometric aggregates' of disklike molecules of 10-nm diameter. Inhibition of the HA by the glycoproteins fetuin, asialofetuin, and mucin, but not by ovalbumin and simple sugars, suggested the specific requirement of complex carbohydrates for binding. Rabbit antisera to the purified HA inhibited the hemagglutinating activities of the crude cell-free HA preparations, but not cell-associated HA activities of the parent (V2) or of other O1 and non-O1 V. cholerae strains. This suggested that the released and cell-associated HA activities were mediated by antigenically distinct components. Immunoblotting experiments showed that the antisera recognized a polypeptide component of Mr 62,000 in the cell envelope preparations of the parent and several other V. cholerae O1 and non-O1 strains. These data suggested that the HA was a nonfimbrial lectin of somatic origin with no protease activity and was apparently distinct from V. cholerae HAs described so far.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anantharamaiah G. M., Hughes T. A., Iqbal M., Gawish A., Neame P. J., Medley M. F., Segrest J. P. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988 Mar;29(3):309–318. [PubMed] [Google Scholar]
- Banerjee K. K., Sen A. Purification and properties of a lectin from the seeds of Croton tiglium with hemolytic activity toward rabbit red cells. Arch Biochem Biophys. 1981 Dec;212(2):740–753. doi: 10.1016/0003-9861(81)90418-5. [DOI] [PubMed] [Google Scholar]
- Chaicumpa W., Peungjesda U., Martinez B., Atthasishtha N. Soluble haemagglutinin of classical vibrios: isolation and protection against cholera by its antibodies. Southeast Asian J Trop Med Public Health. 1982 Dec;13(4):637–645. [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Datta-Roy K., Banerjee K., De S. P., Ghose A. C. Comparative study of expression of hemagglutinins, hemolysins, and enterotoxins by clinical and environmental isolates of non-O1 Vibrio cholerae in relation to their enteropathogenicity. Appl Environ Microbiol. 1986 Oct;52(4):875–879. doi: 10.1128/aem.52.4.875-879.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOSH A. K., GANGULY R., SHRIVASTAVA D. L. STUDIES IN IMMUNOCHEMISTRY OF VIBRIO CHOLERAE. VI. HAEMAGGLUTINATION. Indian J Med Res. 1965 Jan;53:1–7. [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Honda T., Lertpocasombat K., Hata A., Miwatani T., Finkelstein R. A. Purification and characterization of a protease produced by Vibrio cholerae non-O1 and comparison with a protease of V. cholerae O1. Infect Immun. 1989 Sep;57(9):2799–2803. doi: 10.1128/iai.57.9.2799-2803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson G., Svennerholm A. M., Holmgren J. Vibrio cholerae expresses cell surface antigens during intestinal infection which are not expressed during in vitro culture. Infect Immun. 1989 Jun;57(6):1809–1815. doi: 10.1128/iai.57.6.1809-1815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANKFORD C. E. Factors of virulence of Vibrio cholerae. Ann N Y Acad Sci. 1960 Nov 21;88:1203–1212. doi: 10.1111/j.1749-6632.1960.tb20111.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Lectins as molecules and as tools. Annu Rev Biochem. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Orskov I., Birch-Andersen A., Duguid J. P., Stenderup J., Orskov F. An adhesive protein capsule of Escherichia coli. Infect Immun. 1985 Jan;47(1):191–200. doi: 10.1128/iai.47.1.191-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Strömberg G. J., Holmgren J. Purification of Vibrio cholerae soluble hemagglutinin and development of enzyme-linked immunosorbent assays for antigen and antibody quantitations. Infect Immun. 1983 Jul;41(1):237–243. doi: 10.1128/iai.41.1.237-243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]