Abstract
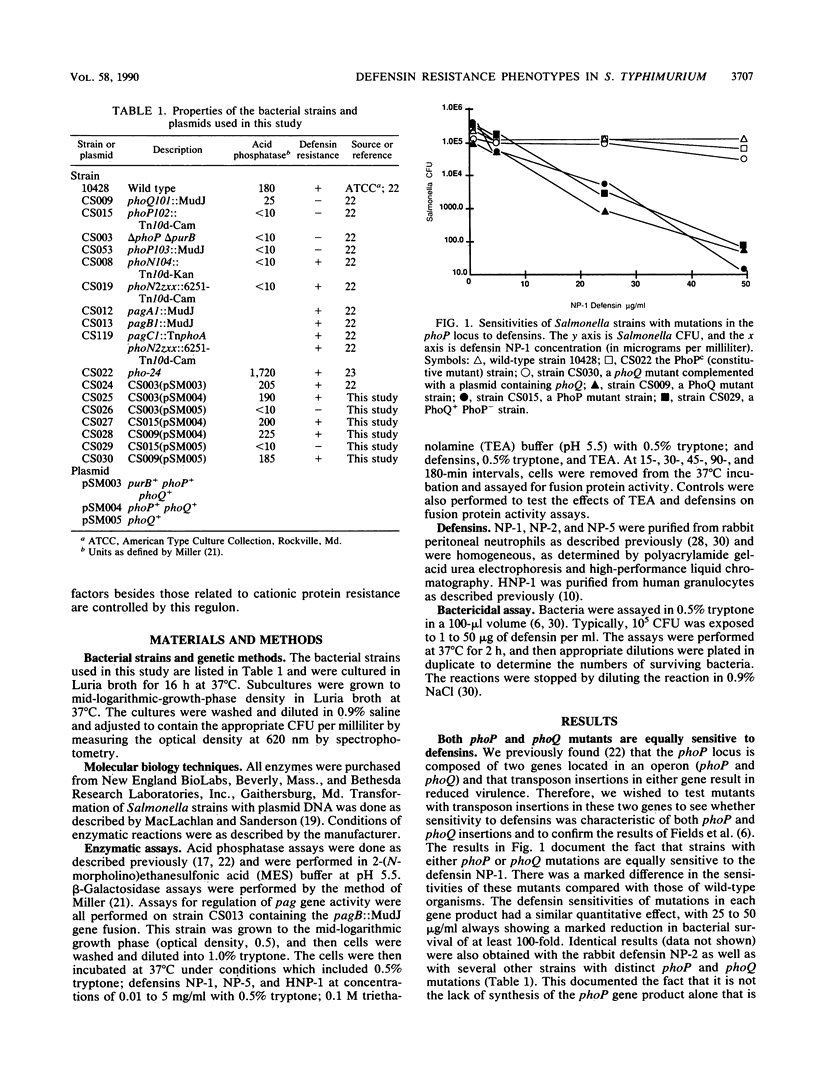
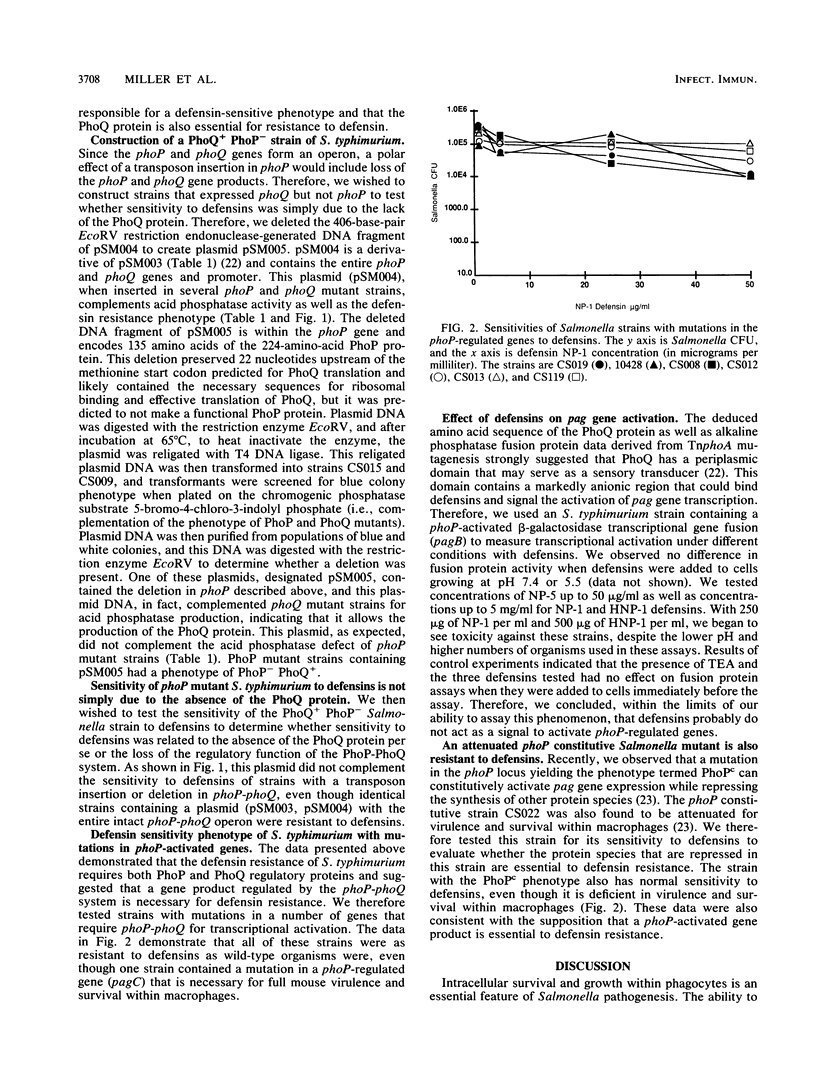
The defensin sensitivities of Salmonella typhimurium strains with mutations in the phoP/phoQ two-component virulence regulon were tested by using purified defensins NP-1 and NP-2. Strains with mutations in either gene of the regulatory pair (phoP [transcriptional activator] or phoQ [membrane sensor kinase]) had increased sensitivities to defensin. The predicted periplasmic domain of the PhoQ protein contained a markedly anionic domain that could interact with cationic proteins and that could be responsible for resistance to defensin. Because insertion mutations in phoP are polar on phoQ, we constructed strains that expressed the PhoQ protein in the absence of PhoP to test whether resistance to defensin requires only the phoQ gene product. We found that resistance to defensin requires the function of both components of this regulatory system, because strains expressing PhoQ without PhoP were still markedly sensitive to defensins. This implied that a pag (phoP-activated gene) product is responsible for defensin resistance. We also tested for the ability of defensins NP-1, NP-5, and HNP-1 to activate pag expression and found that these peptides have no effect. Defensin resistance is not the only virulence characteristic controlled by the PhoP-PhoQ regulon because mutations in pagC, as well as ones in the phoP locus that resulted in constitutive pag activation (phenotype PhoPc), had no effect on defensin resistance, even though they rendered the organism avirulent and deficient in survival within macrophages. The virulence defect conferred by mutations in the phoP-phoQ two-component regulatory system is not completely explained by alterations in resistance to cationic proteins and involves the control of other proteins necessary for S. typhimurium survival within macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989 Jun;6(6):433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Chiao E., Lipps C. J., Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Aricò B., Rappuoli R. Genetics of pertussis toxin. Mol Microbiol. 1989 Jan;3(1):119–124. doi: 10.1111/j.1365-2958.1989.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. 2. N Engl J Med. 1970 Oct 1;283(14):739–746. doi: 10.1056/NEJM197010012831406. [DOI] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. N Engl J Med. 1970 Sep 24;283(13):686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R. M., Ames B. N. Regulation of nonspecific acid phosphatase in Salmonella: phoN and phoP genes. J Bacteriol. 1979 Apr;138(1):155–161. doi: 10.1128/jb.138.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Selsted M. E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983 Oct;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Mekalanos J. J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990 May;172(5):2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. M., Mekalanos J. J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988 Nov;56(11):2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Harwig S. S., Lehrer R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985 Apr 25;260(8):4579–4584. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984 Sep;45(3):655–659. doi: 10.1128/iai.45.3.655-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984 Sep;45(3):655–659. doi: 10.1128/iai.45.3.655-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Lehrer R. I., Rest R. F. Human neutrophil antimicrobial activity. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S450–S456. doi: 10.1093/cid/10.supplement_2.s450. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


