Abstract
3,4-Methylendioxymethamphetamine (MDMA) has both stimulatory and hallucinogenic properties which make its psychoactive effects unique and different from those of typical psychostimulant and hallucinogenic agents. The present study investigated the effects of MDMA on extracellular dopamine (DAex) and serotonin (5-HTex) levels in the striatum and prefrontal cortex (PFC) using in vivo microdialysis techniques in mice lacking DA transporters (DAT) and/or 5-HT transporters (SERT). subcutaneous injection of MDMA (3, 10 mg/kg) significantly increased striatal DAex in wild-type mice, SERT knockout mice, and DAT knockout mice, but not in DAT/SERT double-knockout mice. The MDMA-induced increase in striatal DAex in SERT knockout mice was significantly less than in wildtype mice. In the PFC, MDMA dose-dependently increased DAex levels in wildtype, DAT knockout, SERT knockout and DAT/SERT double-knockout mice to a similar extent. In contrast, MDMA markedly increased 5-HTex in wildtype and DAT knockout mice and slightly increased 5-HTex in SERT-KO and DAT/SERT double-knockout mice. The results confirm that MDMA acts at both DAT and SERT and increases DAex and 5-HTex.
Keywords: MDMA, serotonin transporter, dopamine transporter, knockout, microdialysis.
INTRODUCTION
3,4-Methylendioxymethamphetamine (MDMA, street name: ecstasy) exhibits both stimulatory and hallucinogenic properties which make its psychoactive effects unique and different from those of typical hallucinogens or psychostimulants. Under in vitro conditions, MDMA has been shown to increase the release of dopamine (DA), serotonin (5-HT), and norepinephrine (NE) from brain slices and prevent the reuptake of DA, 5-HT, and NE into brain synaptosomes [1-4]. MDMA binds with higher affinity to the 5-HT transporter (SERT) than to the DA transporter (DAT) [5, 6] and produces a greater release of 5-HT than DA [7-9].
In vivo microdialysis studies have revealed that systemic injection of MDMA increases extracellular levels of DA and 5-HT in the striatum and prefrontal cortex (PFC) [7, 10-13]. MDMA induces DA release, at least in the striatum, through several mechanisms. For example, the release of DA elicited by MDMA is hypothesized to involve both transporter- [14, 15] and impulse-dependent processes [8]. Additionally, the increased 5-HT function resulting from MDMA-induced 5-HT release has been suggested to stimulate 5-HT2 receptors, thereby further enhancing DA release [11, 16, 17].
Monoamine transporter knockout (KO) mice provide useful in vivo models to analyze the effects of psychoactive drugs. In SERT-KO mice, Begels et al. (1998) reported a lack of locomotor-stimulating effects of MDMA [18]. MDMA self-administration is also absent in SERT-KO mice [13]. Moreover, the ability of MDMA administration to induce (γ-aminobutyric acid transporter 1 expression in the frontal cortex and midbrain was reduced in SERT-KO mice [19]. In contrast, DAT-KO mice are hyperactive [20, 21] and display perseverative locomotor patterns [22]. MDMA decreases hyperactivity and potentiates the perseverative pattern of locomotor activity in DAT-KO mice [23]. However, the mechanisms underlying these MDMA effects have not been sufficiently elucidated.
To clarify the action of MDMA on the DAT or SERT in the striatum and PFC, we investigated the effects of MDMA on extracellular levels of DA (DAex) and 5-HT (5-HTex) using in vivo microdialysis in mice lacking the DAT and/or SERT.
METHODS
Animals
Wildtype and DAT-KO mouse littermates from crosses of heterozygous/heterozygous DAT-KO mice on a 129/C57 mixed genetic background served as subjects. SERT-KO and DAT/SERT double-KO mouse littermates from crosses of heterozygous DAT/homozygous SERT knockout mice on a 129/C57 mixed genetic background also served as subjects. The experimental procedures and housing conditions were approved by the Institutional Animal Care and Use Committee of Tokyo Institute of Psychiatry, and all animals were cared for and treated humanely in accordance with our institutional animal experimentation guidelines. Naive adult mice were housed in an animal facility maintained at 22 ± 2°C and 55 ± 5% relative humidity under a 12 h light/dark cycle with lights on at 8:00 a.m. and off at 8:00 p.m. Food and water were available ad libitum. In microdialysis experiments, male and female mice from 10 to 24 weeks old were examined.
Surgery and Microdialysis Procedure
Mice were stereotaxically implanted, under sodium pentobarbital anesthesia (50 mg/kg, intraperitoneally), with microdialysis probes in the striatum (anterior +0.6 mm, lateral +1.8 mm, ventral -4.0 mm from bregma) or PFC (anterior +2.0 mm, lateral +0.5 mm, ventral -3.0 mm from bregma), according to the atlas of Franklin and Paxinos [24]. Twenty-four hours after implantation, the dialysis experiments were performed in freely-moving animals. Evaluation of DAex and 5-HTex has been previously described [25]. Basal levels of DAex and 5-HTex were obtained from average concentrations of three consecutive samples when they were stable.
Drugs
Drugs were dissolved in saline and administered subcutaneously (s.c.) in a volume of 10 ml/kg. MDMA (3 and 10 mg/kg) was administered after establishment of stable baseline, and the dialysate was continuously collected for 180 min.
Statistical Analysis
DAex and 5-HTex responses to drugs were expressed as a percentage of basal levels. Areas under the curve (AUC) of DAex and 5-HTex during the 180 min period after drug administration were calculated as the effects of drugs. AUC values of all groups were analyzed using a two-way analysis of variance (ANOVA). Individual post hoc comparisons were performed with Fisher’s protected least significant difference (PLSD) test. In all cases, the PLSD test was applied for multiple comparisons, and values of p < 0.05 were considered statistically significant. Data were analyzed with Statview J5.0 software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baselines of DAex and 5-HTex in the Striatum and PFC
The baselines of DAex and 5-HTex in the striatum and PFC are shown in Table 1. As previously reported [25], baselines of DAex in the striatum were significantly higher in DAT-KO and DAT/SERT-double KO mice than in wildtype mice (one-way ANOVA; F3,66 = 37.708, p < 0.001). Base-lines of DAex in the PFC were not different between wildtype, DAT-KO, SERT-KO, and DAT/SERT double-KO mice (one-way ANOVA; F3,76 = 0.291, p = 0.832). Baselines of 5-HTex were significantly higher in SERT-KO and DAT/SERT double-KO mice than in wildtype mice in both the striatum (one-way ANOVA; F3,66 = 37.716, p < 0.001) and PFC (one-way ANOVA; F3,76 = 47.715, p < 0.001).
Table 1.
The Baselines (fmol/10 min) of DAex and 5-HTex in the Striatum and PFC
| Striatum | PFC | |||||
|---|---|---|---|---|---|---|
| Genotype | n | DAex | 5-HTex | n | DAex | 5-HTex |
| Wildtype | 20 | 43.00 ± 5.15 | 1.24 ± 0.17 | 24 | 1.24 ± 0.18 | 1.87 ± 0.20 |
| DAT-KO | 19 | 486.26 ± 62.00*** | 1.01 ± 0.13 | 21 | 1.16 ± 0.16 | 1.87 ± 0.24 |
| SERT-KO | 16 | 56.18 ± 7.44 | 13.07 ± 1.97*** | 16 | 1.32 ± 0.28 | 15.09 ± 1.73*** |
| DAT/SERT-KO | 15 | 596.18 ± 73.38*** | 15.13 ± 1.91*** | 19 | 1.42 ± 0.22 | 12.21 ± 1.43*** |
Data presented are means ± S.E.M.
P < 0.001 compared to the corresponding wildtype datum.
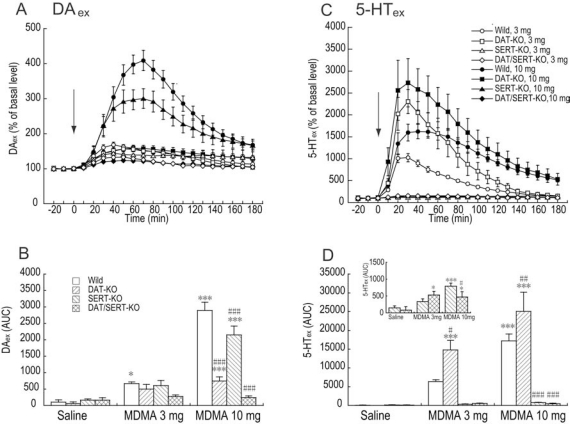
Effects of MDMA on DAex and 5-HTex in the Striatum
MDMA (3 and 10 mg/kg) dose-dependently increased DAex in wildtype and SERT-KO mice, but not in DAT/SERT double-KO mice (Fig. 1A, 1B). Two-way ANOVA (drug × genotype) of the DAex AUC calculated during the 180 min posttreatment period revealed significant effects of drug (F2,58 = 94.751, p < 0.001) and genotype (F3,58 = 26.775, p < 0.001) and a significant drug × genotype interaction (F6,58 = 21.352, p < 0.001). Post hoc comparisons revealed that the effects of MDMA (10 mg/kg) on DAex in SERT-KO mice was significantly less than in wildtype mice (p < 0.001; Fisher’s PLSD post hoc test). However, DAT-KO mice exhibited significant MDMA (10 mg/kg)-induced increases in DAex levels (p < 0.001; Fisher’s PLSD post hoc test), increases that were less than in wildtype mice (p < 0.001; Fisher’s PLSD post hoc test). MDMA (3 and 10 mg/kg) dose-dependently increased 5-HTex in wildtype and DAT-KO mice (Fig. 1C, 1D). Two-way ANOVA (drug × genotype) of 5-HTex revealed significant effects of drug (F2,58 = 23.578, p < 0.001) and genotype (F3,58 = 21.589, p < 0.001) and a significant drug × genotype interaction (F6,58 = 7.769, p < 0.001). The effects of MDMA (3 and 10 mg/kg) on 5-HTex in DAT-KO mice was significantly higher than in wildtype mice (p < 0.05 and p < 0.01, respectively; Fisher’s PLSD post hoc test). When the effects of MDMA were analyzed only in SERT-KO and DAT/SERT double-KO mice, two-way ANOVA (drug × genotype) of 5-HTex revealed a significant effect of drug (F2,25 = 11.858, p < 0.001) but no effect of genotype (F1,25 = 0.492, p = 0.489) and no drug × genotype interaction (F2,25 = 2.773, p = 0.082). The effects of MDMA (10 mg/kg) on 5-HTex in DAT/SERT double-KO mice was significantly less than in SERT-KO mice (p < 0.05; Fisher’s PLSD post hoc test).
Fig. (1).
Effects of MDMA on DAex and 5-HTex in the striatum in wildtype, DAT-KO, SERT-KO, and DAT/SERT double-KO mice. (A, C) Temporal pattern of DAex and 5-HTex before and after injection with saline or MDMA (3 and 10 mg/kg, s.c.). The arrows indicate drug injection time. Each point represents the mean ± SEM of the percentage of DAex or 5-HTex baselines. (B, D) Histogram representing the mean AUC (± SEM) of DAex or 5-HTex during the 180 min period after injection with saline or MDMA (n = 4-8). *p < 0.05, ***p < 0.001, compared with saline group of the same genotype; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with corresponding wildtype data in the same drug treatment (two-way ANOVA followed by Fisher’s PLSD post hoc test).
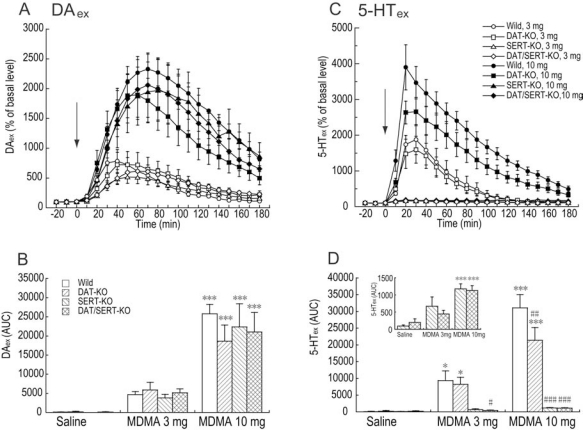
Effects of MDMA on DAex and 5-HTex in the PFC
MDMA (3 and 10 mg/kg) dose-dependently increased DAex in wildtype, DAT-KO, SERT-KO, and DAT/SERT double-KO mice (Fig. 2A, 2B). Two-way ANOVA (drug × genotype) of DAex revealed a significant effect of drug (F2,68 = 53.368, p < 0.001) but no effect of genotype (F3,68 = 0.203, p = 0.894) and no drug × genotype interaction (F6,68 = 0.408, p = 0.871). MDMA (3 and 10 mg/kg) dose-dependently increased 5-HTex in wildtype and DAT-KO mice (Fig. 2C, 2D). Two-way ANOVA (drug × genotype) of 5-HTex revealed significant effects of drug (F2,68 = 32.357, p < 0.001) and genotype (F3,68 = 19.078, p < 0.001) and a significant drug × genotype interaction (F6,68 = 10.596, p < 0.001). The effect of MDMA (10 mg/kg) on 5-HTex in DAT-KO mice was significantly less than in wildtype mice (p < 0.01; Fisher’s PLSD post hoc test). When the effects of MDMA were analyzed only in SERT-KO and DAT/SERT double-KO mice, two-way ANOVA (drug ( genotype) of 5-HTex revealed a significant effect of drug (F2,29 = 28.906, p < 0.001) but no significant effect of genotype (F1,29 = 0.236, p = 0.631) and no drug × genotype interaction (F2,29 = 0.609, p =0.551).
Fig. (2).
Effects of MDMA on DAex and 5-HTex in the PFC in wildtype, DAT-KO, SERT-KO, and DAT/SERT double-KO mice. (A, C) Temporal pattern of DAex or 5-HTex before and after injection with saline or MDMA (3 and 10 mg/kg, s.c.). The arrows indicate drug injection time. Each point represents the mean ± SEM of the percentage of DAex or 5-HTex baselines. (B, D) Histogram representing the mean AUC (± SEM) of DAex or 5-HTex during the 180 min period after injection with saline or MDMA (n = 4-10). *p < 0.05, ***p < 0.001, compared with saline group of the same genotype; #p <0.05, ##p < 0.01, ###p < 0.001, compared with corresponding wildtype data in the same drug treatment (two-way ANOVA followed by Fisher’s PLSD post hoc test).
DISCUSSION
MDMA increased DAex and 5-HTex in the striatum and PFC, consistent with several previous microdialysis studies [7, 10-13]. In DAT/SERT double-KO mice, MDMA did not increase DAex in the striatum, and the increases in 5-HTex were minimal in the striatum and PFC. These results confirm that MDMA acts at both the DAT and SERT.
MDMA increased DAex in wildtype and SERT-KO mice, but not in DAT/SERT double-KO mice. In the absence of the DAT, MDMA-induced changes in DAex were smaller than in wildtype mice. Therefore, the DAT is likely mainly involved in the changes in DAex induced by MDMA. Although DAT-KO mice exhibited significant MDMA-induced increases in DAex levels, these increases were less than in wildtype mice. The increase in DAex produced by MDMA in DAT-KO mice may have two possible explanations. One possibility is that elevated 5-HTex levels produced by MDMA may influence DA release. Microdialysis studies have shown that MDMA, by increasing 5-HTex, indirectly increases DAex via an action at 5-HT2 receptors [7, 8, 17]. Another possibility is that MDMA inhibits DA uptake into 5-HT axon terminals and increases DAex. The SERT is able to transport DA into 5-HT cells [26, 27], and the selective SERT blocker fluoxetine increases DAex in the striatum of DAT-KO mice [25].
Microdialysis studies have shown that NET inhibitors increased DAex in the PFC [28, 29], suggesting that NET can influence DA neurotransmission. Moron et al. (2002) reported that DA uptake in the PFC depends primarily on the NET [30]. This study showed a similar basal extracellular DA concentration in the PFC in DAT-KO and wildtype mice. DAex in the PFC is regulated by the NET. MDMA dose-dependently increased DAex in wildtype, DAT-KO, SERT-KO, and DAT/SERT double-KO mice. Therefore, MDMA may act at the NET and increase DAex levels in the PFC.
MDMA slightly increased 5-HTex in the striatum and PFC in mice lacking the SERT. The selective DAT blocker GBR12909 produced a substantial increase in dialysate 5-HT in SERT-KO mice that was not found in wildtype mice [25]. When the SERT is disrupted in SERT-KO mice, 5-HT is found in DA neurons in the substantia nigra and ventral tegmental area [31]. The DAT appears to play a compensatory role in 5-HT uptake in SERT-KO mice. Therefore, MDMA may act at the DAT and increase 5-HTex levels in the striatum in SERT-KO mice. The NET also appears to be able to play a role in 5-HT uptake [32]. In the PFC, MDMA may increase 5-HTex levels by acting at the NET in SERT-KO mice.
MDMA markedly increased 5-HTex in wildtype and DAT-KO mice. MDMA binds with higher affinity to the SERT than to the DAT [5, 6]. Consistent with in vitro results, MDMA produced greater elevations in 5-HT than DA. Relevant studies have shown that many of the subjective effects of MDMA in human volunteers are reduced after administration of a 5-HT2 receptor antagonist or 5-HT reuptake inhibitors, suggesting that these effects are dependent on SERT-mediated enhancement of serotonergic transmission [33, 34].
In conclusion, the present microdialysis study using DAT- and/or SERT-KO mice demonstrated that MDMA targets monoamine transporters and stimulates predominantly serotonergic transmission.
Acknowledgments
ACKNOWLEDGEMENTS
We acknowledge Mr. Michael Arends for his assistance with editing the manuscript and Ms. Junko Hasegawa for her assistance with genotyping mice. This work was supported by a research grant (17025054) from the MEXT of Japan, by grants from the MHLW of Japan (H17-pharmaco-001, H19-iyaku-023, and 18A-3 and 19A-8 for Nervous and Mental Disorders), by a grant from the Smoking Research Foundation, and by a grant from the Mitsubishi Foundation for Social Welfare Activities.
REFERENCES
- 1.Johnson MP, Conarty PF, Nichols DE. [3H]monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues. Eur. J. Pharmacol. 1991;200:9–16. doi: 10.1016/0014-2999(91)90659-e. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald JL, Reid JJ. Effects of methylenedioxymethamphetamine on the release of monoamines from rat brain slices. Eur. J. Pharmacol. 1990;191:217–220. doi: 10.1016/0014-2999(90)94150-v. [DOI] [PubMed] [Google Scholar]
- 3.Johnson MP, Hoffman AJ, Nichols DE. Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. Eur. J. Pharmacol. 1986;132:269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt CJ, Levin JA, Lovenberg W. In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem. Pharmacol. 1987;36:747–755. doi: 10.1016/0006-2952(87)90729-5. [DOI] [PubMed] [Google Scholar]
- 5.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudelsky GA, Yamamoto BK, Nash JF. Potentiation of 3,4-methylenedioxymethamphetamine-induced dopamine release and serotonin neurotoxicity by 5-HT2 receptor agonists. Eur. J. Pharmacol. 1994;264:325–330. doi: 10.1016/0014-2999(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto BK, Nash JF, Gudelsky GA. Modulation of methylenedioxymethamphetamine-induced striatal dopamine release by the interaction between serotonin and gamma-aminobutyric acid in the substantia nigra. J. Pharmacol. Exp. Ther. 1995;273:1063–1070. [PubMed] [Google Scholar]
- 9.Koch S, Galloway MP. MDMA induced dopamine release in vivo: role of endogenous serotonin. J. Neural Transm. 1997;104:135–146. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- 10.Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol. Biochem. Behav. 2008;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J. Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- 12.Nair SG, Gudelsky GA. Protein kinase C inhibition differentially affects 3,4-methylenedioxymethamphetamine-induced dopamine release in the striatum and prefrontal cortex of the rat. Brain Res. 2004;1013:168–173. doi: 10.1016/j.brainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch KP, Robledo P, Maldonado R. 3,4-methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol. Psychiatry. 2007;62:669–679. doi: 10.1016/j.biopsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Nash JF, Brodkin J. Microdialysis studies on 3,4-methylenedioxy-methamphetamine-induced dopamine release: effect of dopamine uptake inhibitors. J. Pharmacol. Exp. Ther. 1991;259:820–825. [PubMed] [Google Scholar]
- 15.Shankaran M, Yamamoto BK, Gudelsky GA. Mazindol attenuates the 3,4-methylenedioxymethamphetamine-induced formation of hydroxyl radicals and long-term depletion of serotonin in the striatum. J. Neurochem. 1999;72:2516–2522. doi: 10.1046/j.1471-4159.1999.0722516.x. [DOI] [PubMed] [Google Scholar]
- 16.Nash JF. Ketanserin pretreatment attenuates MDMA-induced dopamine release in the striatum as measured by in vivo microdialysis. Life Sci. 1990;47:2401–2408. doi: 10.1016/0024-3205(90)90484-9. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt CJ, Sullivan CK, Fadayel GM. Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J. Neurochem. 1994;62:1382–1389. doi: 10.1046/j.1471-4159.1994.62041382.x. [DOI] [PubMed] [Google Scholar]
- 18.Bengel D, Murphy DL, Andrews AM, Wichems CH, Felt-ner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine ("Ecstasy") in serotonin transporter-deficient mice. Mol. Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 19.Peng W, Simantov R. Altered gene expression in frontal cortex and midbrain of 3,4-methylenedioxymethamphetamine (MDMA) treated mice: differential regulation of GABA transporter subtypes. J. Neurosci. Res. 2003;72:250–258. doi: 10.1002/jnr.10571. [DOI] [PubMed] [Google Scholar]
- 20.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 21.Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc. Natl. Acad. Sci. USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J. Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell SB, Lehmann-Masten VD, Paulus MP, Gainetdinov RR, Caron MG, Geyer MA. MDMA "ecstasy" alters hyperactive and perseverative behaviors in dopamine transporter knockout mice. Psychopharmacology (Berl) 2004;173:310–317. doi: 10.1007/s00213-003-1765-7. [DOI] [PubMed] [Google Scholar]
- 24.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 25.Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt CJ, Lovenberg W. In vitro demonstration of dopamine uptake by neostriatal serotonergic neurons of the rat. Neurosci. Lett. 1985;59:9–14. doi: 10.1016/0304-3940(85)90207-1. [DOI] [PubMed] [Google Scholar]
- 27.Faraj BA, Olkowski ZL, Jackson RT. Active [3H]-dopamine uptake by human lymphocytes: correlates with serotonin transporter activity. Pharmacology. 1994;48:320–327. doi: 10.1159/000139195. [DOI] [PubMed] [Google Scholar]
- 28.Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J. Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J. Neurochem. 1998;71:274–280. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- 30.Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J. Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou FC, Lesch KP, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942:109–119. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]
- 32.Daws LC, Montanez S, Owens WA, Gould GG, Frazer A, Toney GM, Gerhardt GA. Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperometry. J. Neurosci. Methods. 2005;143:49–62. doi: 10.1016/j.jneumeth.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 34.Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA ("Ecstasy") after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]