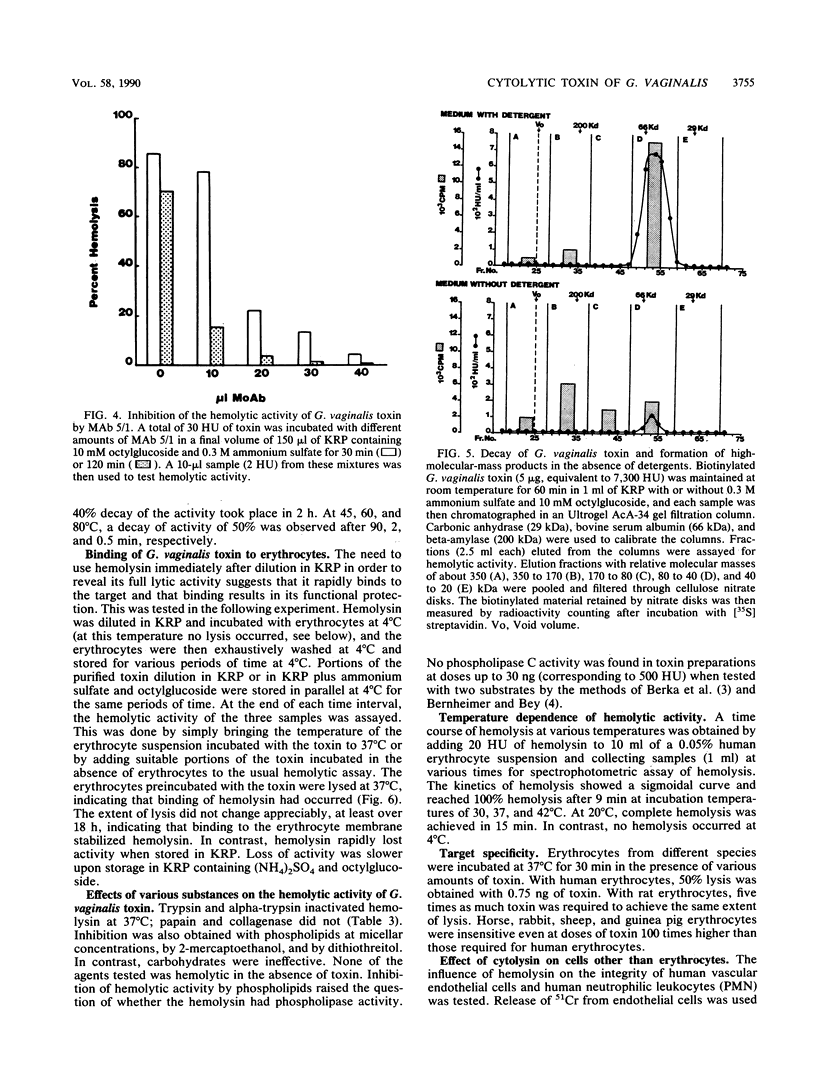
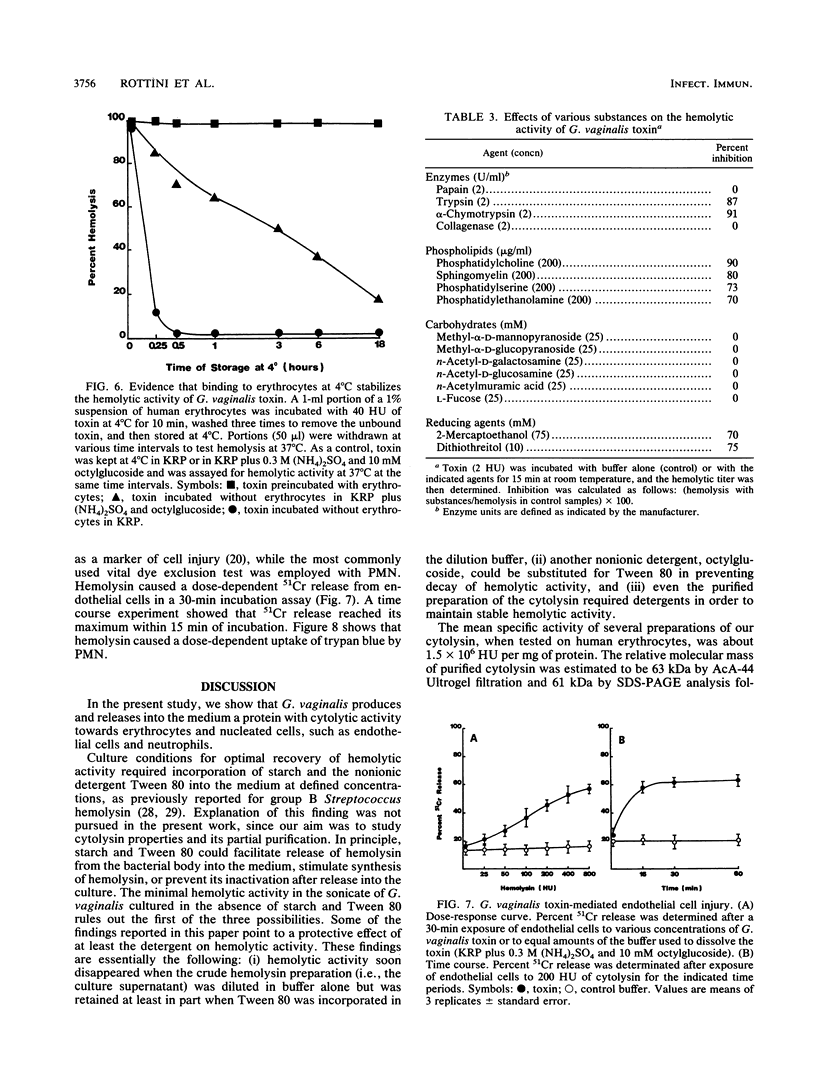
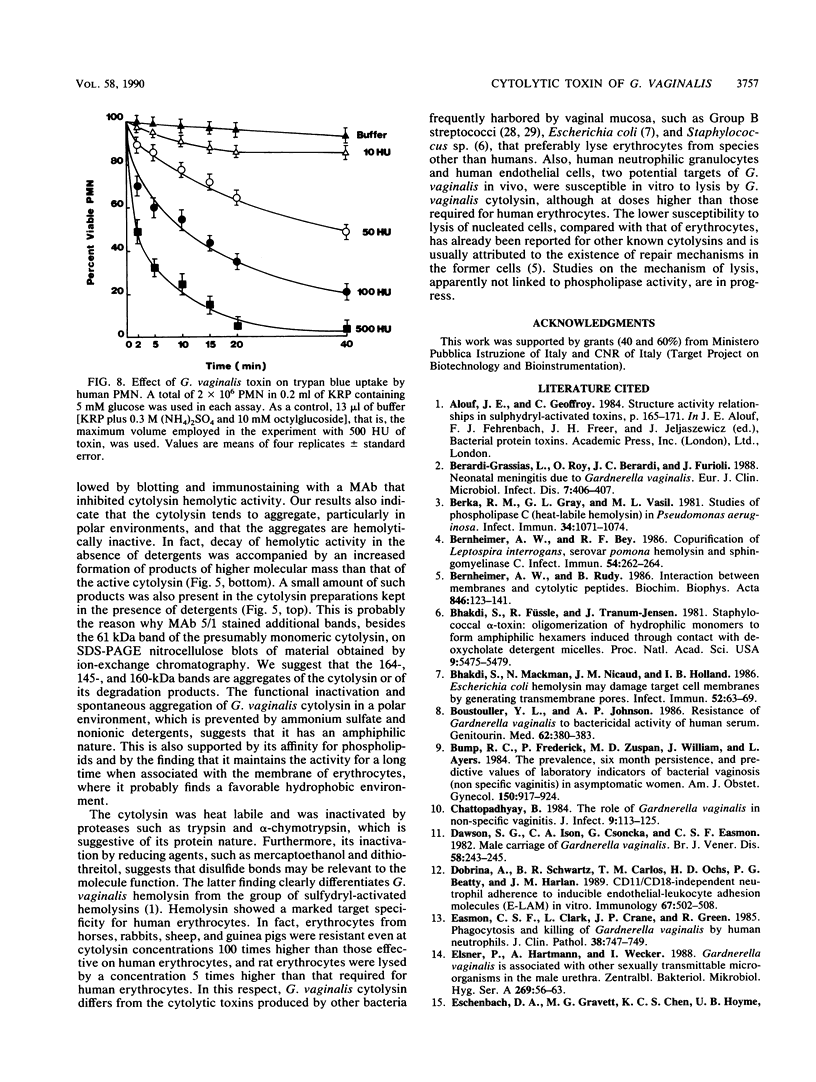
Abstract
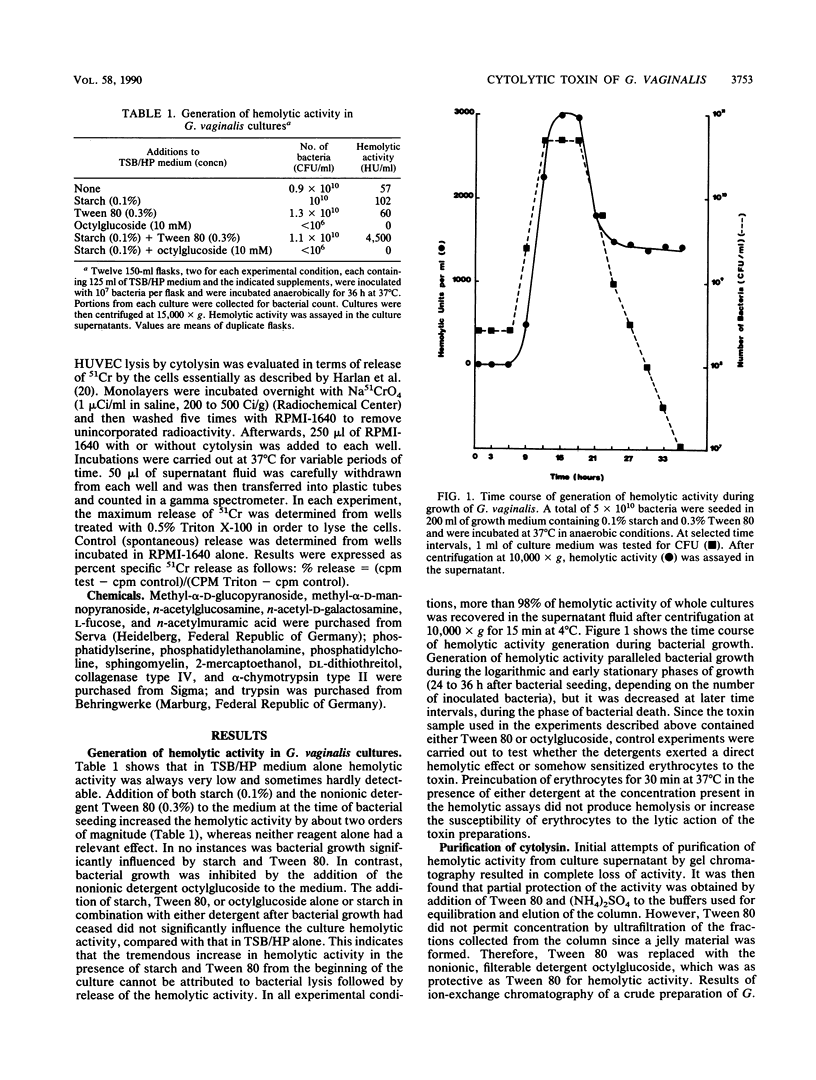
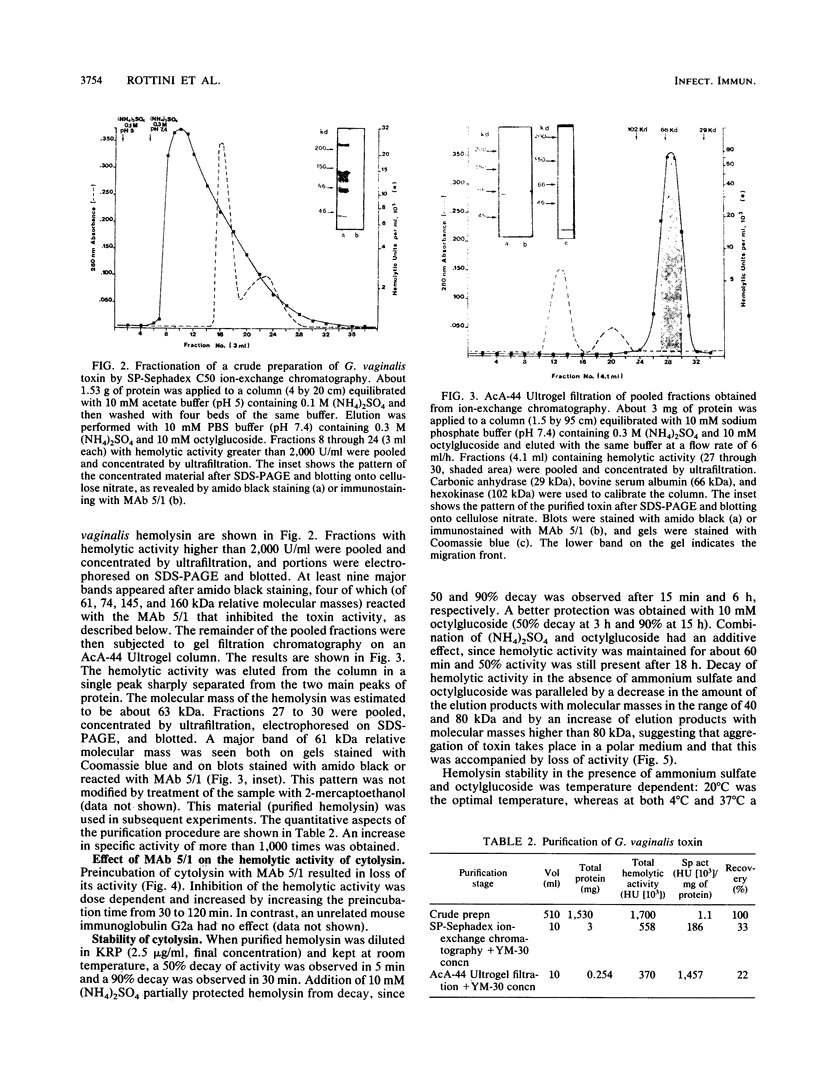
Generation and release into the culture medium of a cytolytic toxin by Gardnerella vaginalis has been demonstrated. Addition of starch and of the nonionic detergent Tween 80 to the culture medium was essential to recover cytolytic activity. A protein with an apparent molecular mass of 61 to 63 kDa was purified from the culture supernatants showing lytic activity towards erythrocytes and nucleated cells, such as human endothelial cells and human neutrophils. The protein had marked selectivity for human erythrocytes, while erythrocytes from other species were not lysed or were lysed at much higher concentrations of the protein than those needed for human erythrocytes. The cytolytic activity was remarkably unstable in polar media, but was stabilized by nonionic detergents, by binding, or by insertion into the target cell membrane, suggesting its amphiphilic nature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berardi-Grassias L., Roy O., Berardi J. C., Furioli J. Neonatal meningitis due to Gardnerella vaginalis. Eur J Clin Microbiol Infect Dis. 1988 Jun;7(3):406–407. doi: 10.1007/BF01962348. [DOI] [PubMed] [Google Scholar]
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Bey R. F. Copurification of Leptospira interrogans serovar pomona hemolysin and sphingomyelinase C. Infect Immun. 1986 Oct;54(1):262–264. doi: 10.1128/iai.54.1.262-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustouller Y. L., Johnson A. P. Resistance of Gardnerella vaginalis to bactericidal activity of human serum. Genitourin Med. 1986 Dec;62(6):380–383. doi: 10.1136/sti.62.6.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bump R. C., Zuspan F. P., Buesching W. J., 3rd, Ayers L. W., Stephens T. J. The prevalence, six-month persistence, and predictive values of laboratory indicators of bacterial vaginosis (nonspecific vaginitis) in asymptomatic women. Am J Obstet Gynecol. 1984 Dec 15;150(8):917–924. doi: 10.1016/0002-9378(84)90381-8. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay B. The role of Gardnerella vaginalis in 'non-specific' vaginitis. J Infect. 1984 Sep;9(2):113–125. doi: 10.1016/s0163-4453(84)90883-1. [DOI] [PubMed] [Google Scholar]
- Dawson S. G., Ison C. A., Csonka G., Easmon C. S. Male carriage of Gardnerella vaginalis. Br J Vener Dis. 1982 Aug;58(4):243–245. doi: 10.1136/sti.58.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrina A., Schwartz B. R., Carlos T. M., Ochs H. D., Beatty P. G., Harlan J. M. CD11/CD18-independent neutrophil adherence to inducible endothelial-leucocyte adhesion molecules (E-LAM) in vitro. Immunology. 1989 Aug;67(4):502–508. [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Clark L., Crane J. P., Green R. Phagocytosis and killing of Gardnerella vaginalis by human neutrophils. J Clin Pathol. 1985 Jul;38(7):747–749. doi: 10.1136/jcp.38.7.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner P., Hartmann A. A., Wecker I. Gardnerella vaginalis is associated with other sexually transmittable microorganisms in the male urethra. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jul;269(1):56–63. doi: 10.1016/s0176-6724(88)80084-1. [DOI] [PubMed] [Google Scholar]
- Eschenbach D. A., Gravett M. G., Chen K. C., Hoyme U. B., Holmes K. K. Bacterial vaginosis during pregnancy. An association with prematurity and postpartum complications. Scand J Urol Nephrol Suppl. 1984;86:213–222. [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gardner H. L. Pathogenicity of Gardnerella vaginalis (Haemophilus vaginalis). Scand J Infect Dis Suppl. 1983;40:37–40. [PubMed] [Google Scholar]
- Gibbs R. S., Weiner M. H., Walmer K., St Clair P. J. Microbiologic and serologic studies of Gardnerella vaginalis in intra-amniotic infection. Obstet Gynecol. 1987 Aug;70(2):187–190. [PubMed] [Google Scholar]
- Greenwood J. R., Pickett M. J. Salient features of Haemophilus vaginalis. J Clin Microbiol. 1979 Feb;9(2):200–204. doi: 10.1128/jcm.9.2.200-204.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier S. L., Martius J., Krohn M., Kiviat N., Holmes K. K., Eschenbach D. A. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988 Oct 13;319(15):972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Davies H. A. Demonstration by electron microscopy of pili on Gardnerella vaginalis. Br J Vener Dis. 1984 Dec;60(6):396–397. doi: 10.1136/sti.60.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson S., Thomason J., Sturino K., Zabransky R., Williams J. Gardnerella vaginalis in the urinary tract: incidence and significance in a hospital population. Obstet Gynecol. 1988 Feb;71(2):245–250. [PubMed] [Google Scholar]
- Kristiansen F. V., Oster S., Frost L., Boustouller Y., Korsager B., Møller B. R. Isolation of Gardnerella vaginalis in pure culture from the uterine cavity of patients with irregular bleedings. Br J Obstet Gynaecol. 1987 Oct;94(10):979–984. doi: 10.1111/j.1471-0528.1987.tb02273.x. [DOI] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz B. A., Duncan J. L. Lysis of erythrocytes by a hemolysin produced by a group B Streptococcus sp. Infect Immun. 1981 Dec;34(3):787–794. doi: 10.1128/iai.34.3.787-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz B. A., Duncan J. L. Properties of a hemolysin produced by group B streptococci. Infect Immun. 1980 Dec;30(3):805–813. doi: 10.1128/iai.30.3.805-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piot P., Van Dyck E., Totten P. A., Holmes K. K. Identification of Gardnerella (Haemophilus) vaginalis. J Clin Microbiol. 1982 Jan;15(1):19–24. doi: 10.1128/jcm.15.1.19-24.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Schneider J., Kaye D., Levison M. E. Adherence of bacteria to vaginal epithelial cells at various times in the menstrual cycle. Infect Immun. 1981 Apr;32(1):194–197. doi: 10.1128/iai.32.1.194-197.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A. W. Gardnerella vaginalis in infections of the urinary tract. J Infect. 1989 Jan;18(1):45–49. doi: 10.1016/s0163-4453(89)93642-6. [DOI] [PubMed] [Google Scholar]
- Sturm A. W., de Leeuw J. H., de Pree N. T. Post-operative wound infection with Gardnerella vaginalis. J Infect. 1983 Nov;7(3):264–266. doi: 10.1016/s0163-4453(83)97223-7. [DOI] [PubMed] [Google Scholar]
- Tedesco F., Rottini G., Patriarca P. Moculating effect of the late-acting components of the complement system on the bactericidal activity of human polymorphonuclear leukocytes on E. coli 0111:B4. J Immunol. 1981 Nov;127(5):1910–1915. [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updyke T. V., Nicolson G. L. Immunoaffinity isolation of membrane antigens with biotinylated monoclonal antibodies and immobilized streptavidin matrices. J Immunol Methods. 1984 Oct 12;73(1):83–95. doi: 10.1016/0022-1759(84)90034-6. [DOI] [PubMed] [Google Scholar]