Abstract
Infection with Helicobacter pylori is strongly associated with gastric cancer and gastric adenocarcinoma. WHO classified H. pylori as a group 1 carcinogen in 1994. Impatiens balsamina L. has been used as indigenous medicine in Asia for the treatment of rheumatism, fractures and fingernail inflammation. In this study, we isolated anti-H. pylori compounds from this plant and investigated their anti- and bactericidal activity. Compounds of 2-methoxy-1,4-naphthoquinone (MeONQ) and stigmasta-7,22-diene-3β-ol (spinasterol) were isolated from the pods and roots/stems/leaves of I. balsamina L., respectively. The minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) for MeONQ were in the ranges of 0.156–0.625 and 0.313–0.625 μg mL−1, respectively, and in the ranges of 20–80 μg mL−1 both of MICs and MBCs for spinasterol against antibiotic (clarithromycin, metronidazole and levofloxacin) resistant H. pylori. Notably, the activity of MeONQ was equivalent to that of amoxicillin (AMX). The bactericidal H. pylori action of MeONQ was dose-dependent. Furthermore, the activity of MeONQ was not influenced by the environmental pH values (4–8) and demonstrated good thermal (121°C for 15 min) stability. MeONQ abounds in the I. balsamina L. pod at the level of 4.39% (w/w db). In conclusion, MeONQ exhibits strong potential to be developed as a candidate agent for the eradication of H. pylori infection.
1. Introduction
Infection with Helicobacter pylori is strongly associated with gastric cancer and gastric adenocarcinoma [1]. WHO classified H. pylori as a group 1 carcinogen in 1994. Antibiotics combined with proton-pump inhibitors, H2-blockers and bismuth salts are the suggested standard treatment modalities to eradicate H. pylori [2]. However, H. pylori resistance to antibiotics is thought to be the most important factor in eradication failure. Resistance rates are the highest for metronidazole (MTZ) (19.9–39.2%), followed by clarithromycin (CLR) (1.7–27.7%), and the lowest for amoxicillin (AMX), tetracycline and trovafloxacin (0–4.7%) [3, 4].
Impatiens balsamina L. (Balsaminaceae) is an annual herb which originated in Asia. The whole plant has been used as indigenous medicine in Taiwan for the treatment of rheumatism, swelling and fingernail inflammation. Modern pharmacological studies have reported this plant demonstrating antifungal, antibacterial, antitumor, antipruritic and antianaphylactic activities [5, 6]. The active compounds isolated from this plant include peptides (Ib-AMP1-4) from seeds, quinones[1, 4-naphthoquinone, lawsone, 2-methoxy-1,4-naphthoquinone (MeONQ), balsaquinone, impatienol, naphthalene-1,4-dione] from petals, pericarp and aerial parts, and flavonoids (kaempferol, quercetin, rutin, astragalin, nicotiflorin, naringenin and their derivatives) from petals and leaves [5–8].
MeONQ (Figure 1) was isolated from the genus of Impatiens including I. balsamina L., I. bicolor and I. glandulifera Royl [7–10]. The plant Swertia calycina (Gentianaceae) was also found to contain this compound [11]. Some bioactivities were demonstrated in MeONQ including antipruritic [12], antiinflammatory, antiallergic [13] and anticancer [14] activities. Furthermore, reducing oral lesion recurrence in human immunodeficiency virus patients was also reported for this compound [15]. Antimicrobial activity against fungal and bacterial pathogens were also published [7, 16].
Figure 1.
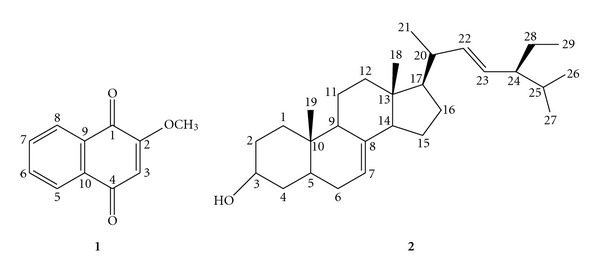
Chemical structures of compounds (1) and (2) from I. balsamina L.: (1) 2-methoxy-1,4-naphthoquinone (MeONQ); (2) and stigmasta-7,22-diene-3β-ol (spinasterol).
Spinasterol (Figure 1) was isolated from the plants of I. balsamina, Adenophora tetraphylla, Medicago sativa, Polyalthia cerasoides Bedd and Pueraria mirifica [8, 17–20]. The bioactivity reported in spinasterol includes antibacterial action against Streptococcus mutans and S. sorbrinus [19] and antitumor effect against breast, ovarian and skin cancer cells [20].
In our previous study, we revealed the whole plant of I. balsamina L. exhibiting strong bactericidal H. pylori activity, especially, the acetone and ethyl acetate pod extracts. This activity was higher than any previously reported natural products [21].
Therefore, in the current study, we isolated anti-H. pylori compounds from I. balsamina L., in which a silica gel chromatography was used. Minimum inhibiory concentrations (MICs), minimum bactericidal concentrations (MBCs), time-kill assay, effect of environmental pH, and thermal stability of the isolated compounds were examined. Multiple antibiotic-resistant H. pylori strains were used in the tests.
2. Methods
2.1. Plant Materials
Impatiens balsamina L. (white flower) were collected from a local farm in Erhshuei township between May 2007 and August 2007, which were identified by Professor Yang-Shiun Chang from the Institute of the Chinese Pharmaceutical Science China Medical University. Voucher specimens (No. 258526) were deposited at the Institute of Ecology and Evolutionary Biology, College of Life Science, National Taiwan University. The parts of pods and mixed roots/stems/leaves were dried by a warm stream of air (below 60°C) and ground (0.25 mm particle size) for the anti-H. pylori compounds isolation.
2.2. Extraction and Isolation
The I. balsamina L. pod extract was prepared according to Wang and Huang [22]. In brief, a mixture of 100 g of pod sample and 700 mL of acetone was stirred at room temperature for 1 h, and then was centrifuged at 13,666 g for 15 min at 4°C. The residue was extracted twice more with 700 mL of acetone of each time. All the supernatants were collected and concentrated in a rotary vacuum evaporator at less than 40°C, and thus the pod extract (7.28 g) was obtained. This extract was subjected to a silica gel column [Kiesel gel 60 (40–63 μm), Merck, Germany; 2.5 cm i.d. × 33 cm] chromatography eluted with dichloromethane-methanol (150 : 1, v/v) to develop two bands in the column. The upper band was collected and subjected to the second silica gel column chromatography eluted with ethyl acetate-dichloromethane (1 : 1); one band was developed in the column and was collected. The collected fraction was subjected to the third silica gel column chromatography orderly eluted with n-hexane-ethyl acetate (10 : 0, 9 : 1, 8 : 2). From the 8 : 2 elution, one band was developed in the column and was collected to yield compound (1) (2.61 g).
Samples (100 g) of roots/stems/leaves were extracted with 95% ethanol according to the aforementioned method and 13.11 g of extract were obtained. This extract was fractionated with water and n-hexane. The n-hexane fraction was collected (1.88 g) and subjected to a silica gel column chromatography orderly eluted with n-hexane-ethyl acetate (10 : 0, 9 : 1 and 8 : 2) to yield two bands in the column from the 8 : 2 elution. The lower band was collected (0.638 g) and then subjected to the second silica gel column chromatography orderly eluted with n-hexane–ethyl acetate (100 : 0, 95 : 5, 90 : 10, 85 : 15). From the 85 : 15 elution, two bands were developed in the column. The upper band was collected and subjected to the third silica gel column chromatography eluted with n-hexane–ethyl acetate (75 : 25) to give three bands in the column. The middle band was collected to yield compound (2) (0.089 g).
2.3. Instrumentations
UV and IR spectra were recorded on a UV/Visible Hitachi U-2800 (Japan) spectrophotometer and a Bruker Equinox 55 (USA) FTIR, respectively. 1H and 13C NMR spectra were recorded at 600 and 150 MHz, respectively, on a Varian Inova 600 (USA) spectrometer. Chemical shifts are reported in δ units (ppm) and coupling constants (J) in HZ. CDCl3 (Sigma-Aldrich, St. Louis, MO, USA) was used as the solvent and TMS (Sigma-Aldrich, St. Louis, MO, USA) as the internal standard, in which chemical shifts for CDCl3 were δ H 7.266 and δ C 77.335. MS spectra were measured on a EI-Finnigan/Thermo Quest MAT 95 XL (Germany) mass spectrometer. Melting point was performed on the Mel-Temp (USA).
2.4. Bacterial Strains and Cultivation
Helicobacter pylori strains of ATCC 700824, 43504, and 43526 were obtained from American Type Culture Collection (ATCC). Helicobacter pylori strains of KMUH 4917, 4952, and 4967 were isolated from the stomach of patients from Chung-Ho Memorial Hospital, Kaohsiung Medical University. Of these H. pylori strains, the three ATCC strains have resistance to MTZ, the three clinical strains are cross-resistance to CLR, MTZ and levofloxacin (LVX); and all the test strains are sensitive to AMX.
A volume of 0.1 mL of H. pylori suspension was added in 5 mL tryptic soy broth (TSB, Difco, USA, pH 7.3), with Columbia agar (bioMérieux, France, pH 7.3) slant containing 5% (v/v) defibrinated sheep blood formed at the bottom of the test tube. The broth was incubated in a microaerophilic jar system (BBL, USA; 5% O2 and 10% CO2 in air, an OXOID BR 056A gas-generating kit was used) at 37°C for 72 h to produce 0.5–2.0 × 107 cfu mL−1 of the bacterial counts.
2.5. MIC and MBC Testing
The MICs and MBCs were determined using an agar-dilution method according to Wang and Huang [22]. The test compound was dissolved in dimethyl sulfoxide (DMSO) and 2-fold diluted in Columbia agar containing 5% (v/v) defibrinated sheep blood. A volume of 0.1 mL of H. pylori suspension (0.5–2.0 × 107 cfu mL−1) was spread onto a Columbia agar plate containing 5% (v/v) defibrinated sheep blood. After incubation in a microaerophilic jar system (5% O2 and 10% CO2 in air) at 37°C for 72 h, the colonies formed on the plate were enumerated. AMX and MTZ (Sigma-Aldrich, St. Louis, MO, USA) were used as the positive controls. Experiments were performed in triplicate. The MIC was defined as the lowest concentration of the test sample at which no colonies of the test H. pylori on the plate were formed.
For MBC testing, the test compound was dissolved in DMSO and 2-fold diluted in Columbia agar containing 5% (v/v) defibrinated sheep blood or TSB, and was then individually added to test tubes, with Columbia agar slant formed at the bottom of the test tube and the TSB layer to be covered on the slant, both the slant and broth containing the same concentrations of the test compound. Helicobacter pylori suspensions were added to the TSB layer to produce 2–8 × 105 cfu mL−1 of the initial bacterial counts. The suspension was then incubated in a microaerophilic jar system (5% O2 and 10% CO2 in air) at 37°C for 72 h. A volume of 0.1 mL of each suspension was spread onto a Columbia agar plate containing 5% (v/v) defibrinated sheep blood, without the test compound. After being incubated in a microaerophilic jar system (5% O2 and 10% CO2 in air) at 37°C for another 72 h, the colonies formed were subsequently enumerated. AMX and MTZ were used as the positive controls. Experiments were performed in triplicate. The MBC was defined as the lowest concentration of the test sample giving complete inhibition of colony formation of the test H. pylori at the latter cultivation.
2.6. Time-Kill Assay of MeONQ
Forty milliliters of Columbia agar containing 5% (v/v) defibrinated sheep blood and 0–2.5 μg mL−1 (control, 1, 2 and 4 times MBC) MeONQ were added to a 250 mL flask to create a plate at the bottom of the flask. Fifty milliliters of TSB containing the same concentrations of MeONQ were poured onto the plate. A volume of 1 mL of the H. pylori ATCC 700824 suspension was added to the TSB layer to produce the initial bacterial concentrations of 2–8 × 105 cfu mL−1. The broth was incubated in a microaerophilic jar system (5% O2 and 10% CO2 in air) at 37°C for 72 h. At various intervals during the incubation period, the broths were taken and diluted by 10-fold in 0.1% peptone solution. A volume of 0.1 mL of each suspension was spread onto Columbia agar plate containing 5% (v/v) defibrinated sheep blood, without MeONQ. After incubation in a microaerophilic jar system (5% O2 and 10% CO2 in air) at 37°C for 72 h, the colonies formed on the plate were enumerated. Experiments were performed in triplicate.
2.7. Effect of pH of MeONQ
The effects of pH of MeONQ on the bactericidal activity against H. pylori was determined. Five milliliters of the Columbia agar containing 5% (v/v) defibrinated sheep blood at the final concentrations of 0–1.25 μg mL−1 (control, MIC, and 1 and 2 times MBC) of MeONQ was added to test tubes to create slants at the bottom. Four milliliters of TSB (adjusted to pH 2–8 with 3 N HCl or 3 N NaOH) containing the same concentrations of MeONQ was poured onto the slants. A volume of 0.1 mL of the H. pylori ATCC 700824 suspension was added to the TSB layer to produce the initial bacterial concentrations of 2–8 × 105 cfu mL−1. The broth was incubated in a microaerophilic jar system (5% O2 and 10% CO2 in air) at 37°C for 24 h. Each of the broth was sampled and diluted by 10-fold in 0.1% peptone solution. A volume of 0.1 mL from each of the diluted sample was spread onto Columbia agar plates containing 5% (v/v) defibrinated sheep blood, without MeONQ. After incubation in a microaerophilic jar system (5% O2 and 10% CO2 in air) at 37°C for 72 h, the colonies formed on the Columbia agar plate were enumerated. Experiments were performed in triplicate.
2.8. Thermal Stability of MeONQ
The thermal stability of MeONQ on bactericidal activity against H. pylori ATCC 700824 was determined. MeONQ was prepared with DMSO at the concentration of 7.5 mg mL−1 and heated at 50, 70, and 100°C for 30 min; and 121°C for 15 min, then, immediately cooled in an ice bath. The treated MeONQ was subjected to the MIC testing. Unheated MeONQ was used as the control. Experiments were performed in triplicate.
2.9. MeONQ Levels in I. balsamina
L. MeONQ levels in the different parts (pods, flowers, seeds, roots, stems and leaves) of I. balsamina L. were determined by a Hitachi HPLC system (Tokyo, Japan) consisting of a Model L-7100 pump equipped with a multi-solvent delivery system and an L-7455 photodiode array detector. The column was a Mightysil RP-18GP, 5 μm, 4.6 mm in internal diameter (i.d.), and 250 mm in length (Kanto, Tokyo, Japan). The sample preparation was the same as the aforementioned, and 95% ethanol was used as the extraction solvent. The mobile phase was composed of a mixture of 2% acetic acid aqueous-methanol (1/1, v/v). An isocratic elution mode with 1.0 mL min−1 of flow rate was done. The UV absorbance was detected at 248 nm. The operating temperature was maintained at room temperature. Experiments were performed in triplicate.
2.10. Statistical Analysis
Data for time-kill assay, effect of pH for MeONQ, and MeONQ levels in I. balsamina L. were subjected to analysis of variance, and a t-test was used to identify significant differences among the means (P < .05).
3. Results
3.1. Isolation and Structure Elucidation of Anti-H. pylori Compounds
The pod extract of I. balsamina L. was subjected to silica gel column chromatography to produce compound (1). Compound (1) was identified as MeONQ by comparison of the spectroscopic data with those of the known compound [8, 16]. The n-hexane fraction of roots/stems/leaves extract of I. balsamina L. was subjected to silica gel column chromatography to give compound (2). Compound (2) was identified as stigmasta-7,22-diene-3β-ol (spinasterol) by comparison of the spectroscopic data with those of the known compound [8, 16, 20]. The chemical structures of MeONQ and spinasterol are shown in Figure 1.
3.1.1. MeONQ (Compound 1)
Yellow powder; m.p. 182–184°C; UV (MeOH) λ max 243, 248, 278, 338 nm; FTIR (KBr) v max 1682 (C=O), 1648 (C=O), 1605 (C=C, Ar), 1244 (C–O) cm−1; for 1H and 13C NMR, see Table 1; EI-MS (+70 eV) m/z 188 [M]+ (31), 173 [M−CH3]+ (18), 158 [M−OCH2]+ (14), 102 (46), 89 (100).
Table 1.
1H and 13C NMR (600 and 150 MHZ, δ ppm, CDCl3) spectral data for compounds (1) and (2).
| C/H | (1) | (2) | ||
|---|---|---|---|---|
| 1H | 13C NMR | 1H | 13C NMR | |
| 1 | 180.1 | 37.1 | ||
| 2 | 160.4 | 31.5 | ||
| 3 | 6.19 s | 109.9 | 3.60 (1H, m) | 71.0 |
| 4 | 184.9 | 38.0 | ||
| 5 | 8.13 dd (1.2, 7.2) | 126.7 | 40.2 | |
| 6 | 7.76 td (1.2, 7.2) | 134.4 | 29.6 | |
| 7 | 7.73 td (1.2, 7.2) | 133.4 | 5.15 (1H, t, J = 12.0 HZ) | 117.4 |
| 8 | 8.10 dd (1.2, 7.2) | 126.2 | 139.5 | |
| 9 | 131.0 | 49.4 | ||
| 10 | 132.0 | 34.2 | ||
| 11 | 3.92 s | 56.4 | 21.5 | |
| 12 | 39.4 | |||
| 13 | 43.3 | |||
| 14 | 55.1 | |||
| 15 | 23.0 | |||
| 16 | 28.5 | |||
| 17 | 55.8 | |||
| 18 | 0.55 (3H, s) | 12.0 | ||
| 19 | 0.79 (3H, s) | 13.0 | ||
| 20 | 40.8 | |||
| 21 | 1.02 (3H, d, J = 6.6 HZ) | 21.1 | ||
| 22 | 5.15 (1H, dd, J = 9.0, 15.0 HZ) | 138.2 | ||
| 23 | 5.02 (1H, dd, J = 8.7, 15.3 HZ) | 129.4 | ||
| 24 | 51.2 | |||
| 25 | 31.9 | |||
| 26 | 0.80 (3H, d, J = 4.2 HZ) | 19.0 | ||
| 27 | 0.85 (3H, d, J = 5.4 HZ) | 21.4 | ||
| 28 | 25.4 | |||
| 29 | 0.80 (3H, t, J = 6.0 HZ) | 12.3 | ||
3.1.2. Spinasterol (Compound 2)
White powder; m.p. 164–166°C; UV (EtOH) λ max 203, 274 nm; FTIR (KBr) v max 3448 (O–H), 2941 (C–H), 2869 (C–H), 1654 (C=C) cm−1; for 1H and 13C NMR, see Table 1; EI-MS (+70 eV) m/z 412 [M]+ (32), 397 [M−CH3]+ (13), 394 [M − H2O]+ (2), 379 [M−CH3−H2O]+ (2), 369 [M−C3H7]+ (18), 351 (7), 300 [M−C3H12O]+ (16), 271 [M−C10H19−2H]+ (100), 255 [M−C10H19−H2O]+ (50), 253 [M−C10H19−H2O−2H]+ (19), 246 (23), 231 [M−C10H19−C3H6]+ (18), 229 [M−0C10H19−C3H8]+ (22), 213 [M−0C10H19−C3H6−H2O]+ (19), 211 [M−C10H19−C3H8−H2O]+ (3).
3.2. MICs and MBCs for MeONQ and Spinasterol
The MICs and MBCs for MeONQ and spinasterol are listed in Table 2. Both MICs and MBCs for MeONQ were relatively low, in the ranges of 0.156–0.625 and 0.313–0.625 μg mL−1, respectively. Compared with the positive controls, the MICs and MBCs of MeONQ were 25–400% of AMX (0.078–2.5 and 0.156–2.5 μg mL−1 of MICs and MBCs, resp.); and much lower than those of MTZ (160–5120 μg mL−1 of both MICs and MBCs). MeONQ demonstrated very good anti- and bactericidal H. pylori activity against CLR, MTZ, and LVX-resistant H. pylori, which was equivalent to that of AMX.
Table 2.
MICs and MBCs for MeONQ and spinasterol against H. pylori.
| Strain | MIC (μg mL−1) | MBC (μg mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| MeONQ | Spinasterol | AMX | MTZ | MeONQ | Spinasterol | AMX | MTZ | |
| ATCC 700824 | 0.156 | 20 | 0.078 | 160 | 0.313 | 20 | 0.156 | 320 |
| ATCC 43504 | 0.313 | 20 | 0.078 | 2560 | 0.625 | 40 | 0.156 | 2560 |
| ATCC 43526 | 0.625 | 80 | 0.156 | 160 | 0.625 | 80 | 0.156 | 160 |
| KMUH 4917 | 0.313 | 20 | 0.313 | 160 | 0.625 | 40 | 0.313 | 160 |
| KMUH 4952 | 0.625 | 80 | 2.5 | 5120 | 0.625 | 80 | 2.5 | 5120 |
| KMUH 4967 | 0.625 | 80 | 0.625 | 160 | 0.625 | 80 | 0.625 | 160 |
Both MICs and MBCs for spinasterol against six strains of CLR, MTZ and LVX-resistant H. pylori were 20–80 μg mL−1 (Table 2). Compared with the positive controls, both the MICs and MBCs for spinasterol were higher than AMX and lower than MTZ.
3.3. Time-Kill Assay of MeONQ
As Figure 2 illustrates, three treatments (0.313, 0.625, and 1.25 μg mL−1) initially reduced survival counts significantly by 0.37–0.53 log cfu mL−1 compared with the control. The 0.313 and 0.625 μg mL−1 treatments (1 and 2 times MBC, resp.) reduced H. pylori survival counts from 5.10 ± 0.18–5.18 ± 0.16 log cfu mL−1 to 0 after 24 h cultivation; when the treated dose increased to 1.25 μg mL−1 (four times MBC), the survival counts were reduced to 0 after 8 h. The bactericidal action between the three treated does was significantly different (P < .05), indicating that the bactericidal H. pylori ATCC 700824 action of MeONQ was dose-dependent.
Figure 2.
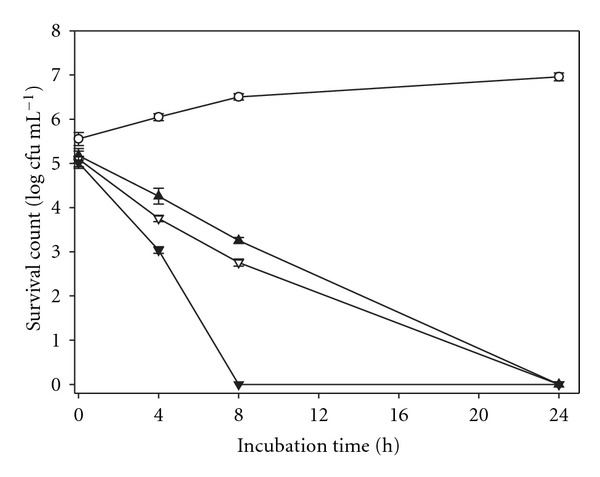
Time-kill assay for MeONQ against H. pylori ATCC 700824: control (open circles), 0.313 μg mL−1 (filled triangles), 0.625 μg mL−1 (inverted open triangles), 1.25 μg mL−1 (inverted filled triangles). Data points presented are in mean ± standard deviation (SD) (n = 3).
3.4. Effect of pH of MeONQ
In Figure 3, at pH ≤ 3, the survival H. pylori ATCC 700824 counts were zero for all of the treatments including the control because those pH values stopped the bacterial growth. At pH 4–8, when the treated dose (0.156 μg mL−1) was below the MBC (0.313 μg mL−1), MeONQ reduced H. pylori ATCC 700824 survival counts by 1.84–2.91 cfu mL−1 after 24 h cultivation; while the treated doses were equal to or higher than the MBC, survival counts in all the treatments (pH 4–8) were reduced to zero after 24 h cultivation. The bactericidal H. pylori activity of MeONQ was not influenced by the environmental pH values (4–8).
Figure 3.
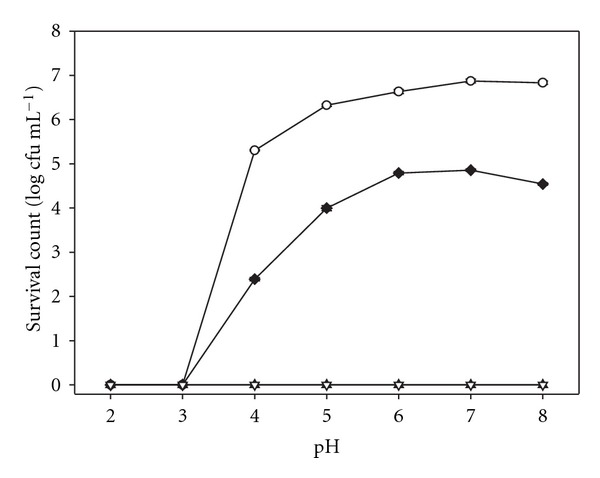
Effect of pH of MeONQ on the bactericidal activity against H. pylori ATCC 700824: control (open circles), 0.156 μg mL−1 (filled diamonds), 0.313 μg mL−1 (filled triangles), 0.625 μg mL−1 (inverted open triangles). Data points presented are in mean ± SD (n = 3).
3.5. Thermal Stability of MeONQ
After heat treatments (heating at 50, 70, and 100°C for 30 min; and 121°C for 15 min) of MeONQ, the MICs for the heat-treated MeONQ against H. pylori ATCC 700824 and KMUH 4917 (0.156 and 0.313 μg mL−1, resp.) were the same as the untreated MeONQ, which indicated that MeONQ's anti-H. pylori activity was not altered even after strong heat treatment.
3.6. MeONQ Levels in the Parts of I. balsamina
L. As shown in Table 3, the I. balsamina L. pods had the greatest amount of MeONQ (43.92 ± 1.56 mg g−1 db) which was 8–150 times that of the other parts. The MeONQ level in the flowers then followed at 5.45 ± 0.11 mg g−1 db. The levels in the parts of roots, stems, leaves and seeds were low (0.29 ± 0.00 to 0.56 ± 0.02 mg g−1 db) (P < .05).
Table 3.
MeONQ levels in the different parts of I. balsamina L.
| Sample | MeONQ (mg g−1 dba) |
|---|---|
| Pod | 43.92 ± 1.56a |
| Flower | 5.45 ± 0.11b |
| Root | 0.35 ± 0.01c |
| Stem | 0.56 ± 0.02c |
| Leaf | 0.29 ± 0.00c |
| Seed | 0.37 ± 0.00c |
Values presented are in mean ± SD (n = 3). Means followed by different letters are significantly different (P < .05).
aDried base.
4. Discussion
In this study, anti-H. pylori compounds, MeONQ and spinasterol, were isolated from I. balsamina L. Such anti- and bactericidal H. pylori activity had never been reported in those compounds. Especially, MeONQ exhibited relatively low MICs and MBCs against CLR, MTZ and LVX-resistant H. pylori. This activity was neither influenced by the environmental pH value nor strong heat treatment.
Numerous anti-H. pylori compounds including phenolics [23], flavonoids [24], triterpenoids [25], quinones [26] and others [27] were isolated from natural products. The MICs for those compounds varied from 1.3 to 200 μg mL−1, however, none of them were lower than 1 μg mL−1. Compared with our compounds, MICs for MeONQ against six H. pylori strains ranged from 0.156 to 0.625 μg mL−1 which were much lower than those of the aforementioned compounds. In addition, those values were similar to AMX and much lower than MTZ (Table 2). MeONQ is the most effective bactericidal H. pylori natural compound which has thus far been reported.
We suppose that the mechanisms for the bactericidal H. pylori activity of MeONQ is due to the high redox potential of its hydroquinone form [28]. MeONQ is a quinone. Quinones are metabolized by flavoenzymes (such as NADPH-cytochrome P-450 reductase and xanthine oxidase) to form hydroquinones. The hydroquinones are quite unstable, which react with molecular oxygen to form semiquinones and superoxide anion radicals at physiological pH. Under aerobic conditions, the semiquinone free radicals are most likely to react with molecular oxygen to form superoxide anion radicals and quinones. The regenerated quinones react with anion form of hydroquinones resulting in large amounts of reactive oxygen species (ROS). These ROS can damage a number of cellular macromolecules and may lead to cell or microorganism death [28–30]. The hypothetical diagram is presented in Figure 4.
Figure 4.

The hypothetical diagram for MeONQ that leads H. pylori death. MeONQ: 2-methoy-1,4-naphthoquinone; MeONQH2: hydroquinone derivative of MeONQ; MeONQH−: anion form of MeONQH2; MeONQ−•: semiquinone derivative of MeONQ; Re-MeONQ: regenerated MeONQ; ROS: reactive oxyge species.
Resistance to antibiotics are the greatest factor in H. pylori eradication failure, especially to MTZ and CLR. Resistance rates have increased annually over the last 15 years [4, 31]. For instance, the rates of 7.7% cross-resistance between MTZ and CLR in Poland and 21.7% between erythromycin and CLR in Ireland were reported [32, 33]. Thus, killing H. pylori is becoming more and more difficult. In our study, MeONQ not only exhibited relatively good anti- and bactericidal H. pylori activity but was also an effective bactericide against CLR, MTZ and LVX-resistant H. pylori.
AMX and CLR were not stable in aqueous solutions of pH lower than 4.0 and 5.0, respectively [34], and therefore degrade rapidly at normal gastric pH (1.0–2.0). From our results (Figure 3), both anti- and bactericidal H. pylori activity of MeONQ were not influenced by the environmental pH values (4, 8). Moreover, MeONQ exhibited very good thermal stability, even when heated at 121°C for 15 min. These physical properties highly increased MeONQ's efficiency in H. pylori eradication.
In this study, we first revealed that MeONQ abound in the I. balsamina L. pod as high as 43.92 ± 1.56 mg g−1 db (Table 3). As previously reported, MeONQ levels in the leaves, stems and flowers of I. glandulifera Royle were 1.98, 1.33, and 4.86 mg g−1 db, respectively [10]; and that of levels in 40 I. balsamina L. leaf samples were in a range of 0.3–1.9 mg g−1 db [8]. Our MeONQ level in the leaves of I. balsamina L. was similar to Panichayupakaranant et al. [8], lower than Lobstein et al. [10], and the level of that in the flowers was similar to Lobstein et al. [11].
Funding
National Science Council, Taiwan, R.O.C. (grant no.: NSC 95-2313-B-005-057-MY3).
References
- 1.Forman D, Newekk DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. British Medical Journal. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worrel JA, Stoner SC. Eradication of Helicobacter pylori . Medical Update for Psychiatrists. 1998;3(4):99–104. [Google Scholar]
- 3.Debets-Ossenkopp YJ, Herscheid AJ, Pot RGJ, Kuipers EJ, Kusters JG, Vandenbroucke-Grauls CMJE. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxycillin, tetracycline and trovafloxacin in The Netherlands. Journal of Antimicrobial Chemotherapy. 1999;43(4):511–515. doi: 10.1093/jac/43.4.511. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. Journal of Clinical Microbiology. 2007;45(12):4006–4010. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oku H, Ishiguro K. Antipruritic and antidermatitic effect of extract and compounds of Impatients balsamina L. in atopic dermatitis model NC mice. Phytotherapy Research. 2001;15:506–510. doi: 10.1002/ptr.964. [DOI] [PubMed] [Google Scholar]
- 6.Lee DG, Shin SY, Kim D-H, et al. Antifungal mechanism of a cysteine-rich antimicrobial peptide, Ib-AMP1, from Impatiens balsamina against Candida albicans . Biotechnology Letters. 1999;21(12):1047–1050. [Google Scholar]
- 7.Little JE, Sproston TJ, Foote MW. Isolation and antifungal action of naturally occurring 2-methoxy-1,4-naphthoquinone. Journal of Biological Chemistry. 1948;174:335–342. [PubMed] [Google Scholar]
- 8.Panichayupakaranant P, Noguchi H, De-Eknamkul W, Sankawa U. Naphthoquinones and coumarins from Impatiens balsamina root cultures. Phytochemistry. 1995;40(4):1141–1143. [Google Scholar]
- 9.Hasan A, Tahir MN. Flavonoids from the leaves of Impatiens bicolor . Turkish Journal of Chemistry. 2005;29(1):65–70. [Google Scholar]
- 10.Lobstein A, Brenne X, Feist E, Metz N, Weniger B, Anton R. Quantitative determination of naphthoquinones of Impatiens species. Phytochemical Analysis. 2001;12(3):202–205. doi: 10.1002/pca.574. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez S, Wolfender J-L, Hakizamungu E, Hostettmann K. An antifungal naphthoquinone, xanthones and secoiridoids from Swertia calycina . Planta Medica. 1995;61(4):362–364. doi: 10.1055/s-2006-958102. [DOI] [PubMed] [Google Scholar]
- 12.Oku H, Kato T, Ishiguro K. Antipruritic effects of 1,4-naphthoquinones and related compounds. Biological & Pharmaceutical Bulletin. 2002;25(1):137–139. doi: 10.1248/bpb.25.137. [DOI] [PubMed] [Google Scholar]
- 13.Lien J-C, Huang L-J, Wang J-P, Teng C-M, Lee K-H, Kuo S-C. Synthesis and antiplatelet, antiinflammatory and antiallergic activities of 2,3-disubstituted 1,4-naphthoquinones. Chemical and Pharmaceutical Bulletin. 1996;44(6):1181–1187. doi: 10.1248/cpb.44.1181. [DOI] [PubMed] [Google Scholar]
- 14.Ding Z-S, Jiang F-S, Chen N-P, Lv G-Y, Zhu C-G. Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina . Molecules. 2008;13(2):220–229. doi: 10.3390/molecules13020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blignaut E, Patton LL, Nittayananta W, Ramirez-Amador V, Ranganathan K, Chattopadhyay A. (A3) HIV phenotypes, oral lesions, and management of HIV-related disease. Advances in Dental Research. 2006;19(1):122–129. doi: 10.1177/154407370601900123. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Summerhurst DK, Koval SF, Ficker C, Smith ML, Bernards MA. Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fractionation. Phytotherapy Research. 2001;15(8):676–680. doi: 10.1002/ptr.906. [DOI] [PubMed] [Google Scholar]
- 17.Huang LS, Grunwald C. Effect of light on sterol changes in Medicago sativa . Plant Physiology. 1988;88:1403–1406. doi: 10.1104/pp.88.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dan S, Dan SS, Mukhopadhyay P, Mukherjee MK. Chemical investigation of some Annonaceae species. International Journal of Crude Drug Research. 1985;23:73–76. [Google Scholar]
- 19.Salvador MJ, Zucchi OLAD, Candido RC, Ito IY, Dias DA. In vitro antimicrobial activity of crude extracts and isolated constituents of Alternanthera maritima . Pharmaceutical Biology. 2004;42(2):138–148. [Google Scholar]
- 20.Jeon G-C, Park M-S, Yoon D-Y, Shin C-H, Sin H-S, Um S-J. Antitumor activity of spinasterol isolated from Pueraria roots. Experimental and Molecular Medicine. 2006;37(2):111–120. doi: 10.1038/emm.2005.15. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y-C, Wu D-C, Liao J-J, Wu C-H, Li W-Y, Weng B-C. In vitro activity of Impatiens balsamina L. against multiple antibiotic-resistant Helicobacter pylori . American Journal of Chinese Medicine. 2009;37(4):713–722. doi: 10.1142/S0192415X09007181. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y-C, Huang T-L. Anti-Helicobacter pylori activity of Plumbago zeylanica L. FEMS Immunology and Medical Microbiology. 2005;43(3):407–412. doi: 10.1016/j.femsim.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Rüegg T, Calderón AI, Queiroz EF, et al. 3-Farnesyl-2-hydroxybenzoic acid is a new anti-Helicobacter pylori compound from Piper multiplinervium . Journal of Ethnopharmacology. 2006;103(3):461–467. doi: 10.1016/j.jep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Ustün O, Ozçelik B, Akyön Y, Abbasoglu U, Yesilada E. Flavonoids with anti-Helicobacter pylori activity from Cistus laurifolius leaves. Journal of Ethnopharmacology. 2006;108(3):457–461. doi: 10.1016/j.jep.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Leo MD, Tommasi ND, Sanogo R, et al. Triterpenoid saponins from Pteleopsis suberosa stem bark. Phytochemistry. 2006;67(24):2623–2629. doi: 10.1016/j.phytochem.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Park B-S, Lee H-K, Lee S-E, et al. Antibacterial activity of Tabebuia impetiginosa Martius ex DC (Taheebo) against Helicobacter pylori . Journal of Ethnopharmacology. 2006;105(1-2):255–262. doi: 10.1016/j.jep.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.O’Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori . Applied and Environmental Microbiology. 2000;66(5):2269–2273. doi: 10.1128/aem.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munday R. Autooxidation of naphthaquinones: effect of pH, naphthoquinones and superoxide dimutase. Free Radical Research. 2000;32:245–253. doi: 10.1080/10715760000300251. [DOI] [PubMed] [Google Scholar]
- 29.Osman AM, van Noort PCM. Evidence for redox cycling of lawsone (2-hydroxy-1,4-naphthoquinone) in the presence of the hypoxanthine/xanthine oxidase system. Journal of Applied Toxicology. 2003;23(4):209–212. doi: 10.1002/jat.908. [DOI] [PubMed] [Google Scholar]
- 30.McMillan DC, Sarvete SD, Oatis JE, Jr., Jollow DJ. Role of oxidant stress in lawsone-induced hemolytic anemia. Toxicological Sciences. 2004;82(2):647–655. doi: 10.1093/toxsci/kfh288. [DOI] [PubMed] [Google Scholar]
- 31.De Francesco V, Margiotta M, Zullo A, et al. Prevalence of primary clarithromycin resistance in Helicobacter pylori strains over a 15 year period in Italy. Journal of Antimicrobial Chemotherapy. 2007;59(4):783–785. doi: 10.1093/jac/dkm005. [DOI] [PubMed] [Google Scholar]
- 32.Rozynek E, Dzierzanowska-Fangrat K, Celińska-Cedro D, Joźźwiak P, Madaliński K, Dzierzanowska D. Primary resistance of Helicobacter pylori to antimicrobial agents in Polish children. Acta Microbiologica Polonica. 2002;51(3):255–263. [PubMed] [Google Scholar]
- 33.Xia H-X, Buckley M, Keane CT, O’Morain CA. Clarithromycin resistance in Helicobacter pylori: prevalence in untreated dyspeptic patients and stability in vitro. Journal of Antimicrobial Chemotherapy. 1996;37(3):473–481. doi: 10.1093/jac/37.3.473. [DOI] [PubMed] [Google Scholar]
- 34.Erah PO, Goddard AF, Barrett DA, Shaw PN, Spiller RC. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. Journal of Antimicrobial Chemotherapy. 1997;39(1):5–12. doi: 10.1093/jac/39.1.5. [DOI] [PubMed] [Google Scholar]


