Abstract
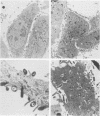
Bordetella pertussis produces extracytoplasmic adenylate cyclase toxin (AC toxin) which penetrates target cells and, upon activation by host calmodulin, generates high levels of intracellular cyclic AMP (cAMP). As a result, bactericidal functions of immune effector cells are impaired. Since a considerable amount of AC toxin is associated with the bacterium, it was proposed that the toxin may be delivered by direct interaction of the organism with the target cells (E. L. Hewlett, M. C. Gray, and R. D. Pearson, Clin. Res. 35:477A, 1987). Incubation of CHO cells with intact B. pertussis led to formation of intracellular cAMP at levels comparable to those produced in CHO cells by equivalent activities of isolated AC toxin. cAMP accumulation induced by the whole bacteria appeared after a lag of 40 to 60 min and reached high levels within 2 to 3 h, whereas adherence of the bacteria proceeded rapidly and reached a maximal level within 80 min. Sera of pertussis patients completely blocked cAMP accumulation induced by the whole bacteria without having a major effect on either bacterial adherence or cAMP production by the AC toxin. Cytochalasins B and D, inhibitors of bacterial invasion, abrogated the cAMP response to the whole bacteria but not the response to the AC toxin. These agents did not affect bacterial adherence. Transmission electron micrographs revealed that B. pertussis, within the time course of cAMP induction, invaded CHO cells. We suggest that cAMP induction by B. pertussis is caused by the entry of the whole bacteria into CHO cells rather than by delivery of AC toxin during bacterial adherence. This route of cell intoxication may be relevant to the pathogenesis of whooping cough.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlie R. M., Coote J. G., Parton R., Schultz J. E., Rogel A., Hanski E. Cloning of the adenylate cyclase genetic determinant of Bordetella pertussis and its expression in Escherichia coli and B. pertussis. Microb Pathog. 1988 May;4(5):335–344. doi: 10.1016/0882-4010(88)90061-7. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Sherburne R. K., Man S. F., Peppler M. S. Bordetella parapertussis invasion of HeLa 229 cells and human respiratory epithelial cells in primary culture. Infect Immun. 1989 Apr;57(4):1240–1247. doi: 10.1128/iai.57.4.1240-1247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel Z., Friedman E., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Intracellular localization and kinetics of penetration into various cells. Biochem J. 1987 Apr 1;243(1):153–158. doi: 10.1042/bj2430153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel Z., Könen S., Wiertz E., Klapmuts R., Addy P. A., Hanski E. Antibodies to Bordetella pertussis adenylate cyclase are produced in man during pertussis infection and after vaccination. J Med Microbiol. 1990 Jul;32(3):173–177. doi: 10.1099/00222615-32-3-173. [DOI] [PubMed] [Google Scholar]
- Friedman R. L. Pertussis: the disease and new diagnostic methods. Clin Microbiol Rev. 1988 Oct;1(4):365–376. doi: 10.1128/cmr.1.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Elmaoglou-Lazaridou A., Krin E., Ladant D., Bârzu O., Danchin A. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989 Mar;8(3):967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hanski E. Invasive adenylate cyclase toxin of Bordetella pertussis. Trends Biochem Sci. 1989 Nov;14(11):459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Gordon V. M., McCaffery J. D., Sutherland W. M., Gray M. C. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem. 1989 Nov 15;264(32):19379–19384. [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986 Dec 5;261(34):16264–16269. [PubMed] [Google Scholar]
- Masure H. R., Storm D. R. Characterization of the bacterial cell associated calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1989 Jan 24;28(2):438–442. doi: 10.1021/bi00428a005. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Farfel Z., Goldschmidt S., Shiloach J., Hanski E. Bordetella pertussis adenylate cyclase. Identification of multiple forms of the enzyme by antibodies. J Biol Chem. 1988 Sep 15;263(26):13310–13316. [PubMed] [Google Scholar]
- Rogel A., Schultz J. E., Brownlie R. M., Coote J. G., Parton R., Hanski E. Bordetella pertussis adenylate cyclase: purification and characterization of the toxic form of the enzyme. EMBO J. 1989 Sep;8(9):2755–2760. doi: 10.1002/j.1460-2075.1989.tb08417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Steinman L., Weiss A., Adelman N., Lim M., Zuniga R., Oehlert J., Hewlett E., Falkow S. Pertussis toxin is required for pertussis vaccine encephalopathy. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8733–8736. doi: 10.1073/pnas.82.24.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. I., Zapiain L. A., Galvan P., Hewlett E. L. Characterization of antibody inhibiting adherence of Bordetella pertussis to human respiratory epithelial cells. J Clin Microbiol. 1984 Aug;20(2):167–170. doi: 10.1128/jcm.20.2.167-170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]