Abstract
Nicotine is the principle addictive component that drives continued tobacco use despite users’ knowledge of the harmful consequences. The initiation of addiction involves the mesocorticolimbic dopamine system, which contributes to the processing of rewarding sensory stimuli during the overall shaping of successful behaviors. Acting mainly through nicotinic receptors containing the α4 and β2 subunits, often in combination with the α6 subunit, nicotine increases the firing rate and the phasic bursts by midbrain dopamine neurons. Neuroadaptations arise during chronic exposure to nicotine, producing an altered brain condition that requires the continued presence of nicotine to be maintained. When nicotine is removed, a withdrawal syndrome develops. The expression of somatic withdrawal symptoms depends mainly on the α5, α2, and β4 nicotinic subunits involving the epithalamic habenular complex and its targets. Thus, nicotine taps into diverse neural systems and an array of nicotinic acetylcholine receptor (nAChR) subtypes to influence reward, addiction, and withdrawal.
Keywords: synaptic plasticity, dopamine, ventral tegmental area, nucleus accumbens, habenula, interpeduncular nucleus
INTRODUCTION AND SUMMARY
Tobacco use is the leading cause of preventable death in developed countries (Benowitz 2008, Mathers & Loncar 2006, Peto et al. 1996), and nicotine is the main addictive component (Benowitz 2009, Dani et al. 2009, Dani & Heinemann 1996, Mansvelder & McGehee 2002). Nicotine is a tertiary amine alkaloid. In its charged form, nicotine binds to diverse nicotinic acetylcholine receptor (nAChR) subtypes that have unique expression patterns in the central nervous system. Like the cholinergic neurotransmitter systems, nAChRs are widely distributed, and they participate in cholinergic signaling in nearly every neural area (Changeux & Edelstein 2005, Dani & Bertrand 2007, Woolf 1991). In its uncharged form, nicotine is membrane permeable. Therefore, nicotine can influence intracellular processes indirectly via nAChRs and directly by entering the cytoplasm (Lester et al. 2009, Rezvani et al. 2007).
Nicotine initiates addiction by impinging directly on neural circuitry that normally reinforces behaviors that lead to rewarding goals. Since the earliest studies of intracranial electrical self-stimulation, investigators have identified cortical and limbic brain structures as mediating reward (Olds 1958, Olds & Milner 1954). In particular, the mesocorticolimbic dopamine (DA) system plays an important role in self-stimulation and in processing environmental reward. Likewise, this DA system plays a critical role in the acquisition of behaviors that are inappropriately reinforced by psychostimulant drugs, including nicotine (Balfour 2004, Corrigall 1999, Dani et al. 2009, Dani & Heinemann 1996, Di Chiara 2000, Mansvelder & McGehee 2002). One important dopaminergic pathway originates in the ventral tegmental area (VTA) of the midbrain and projects to the prefrontal cortex (PFC) as well as to limbic and striatal structures, including the nucleus accumbens (NAc). Acting mainly via midbrain nAChRs composed of the β2 subunit in combination with the α4 and/or α6 subunits, nicotine increases the firing rate and phasic burst activity of midbrain DA neurons and elevates DA in the PFC, NAc, and other targets (Imperato et al. 1986, Grenhoff et al. 1986, Mameli-Engvall et al. 2006, Pons et al. 2008, Zhang et al. 2009b). Those decisive mechanistic actions underlie nicotine’s ability to enhance reward from brain stimulation, reinforce conditioned place preference, and support self-administration (Corrigall 1999, Dani et al. 2009, Dani & Harris 2005, Mameli-Engvall et al. 2006, Picciotto et al. 1998, Stolerman & Shoaib 1991).
After chronic use of nicotine, removal of nicotine produces a withdrawal syndrome that can be relieved by nicotine replacement therapy (Dani et al. 2009, Dani & Heinemann 1996, De Biasi & Salas 2008, Di Chiara 2000, Mansvelder & McGehee 2002, Stolerman & Shoaib 1991). The withdrawal syndrome is not mediated exactly by the same mechanisms or by the same neural circuits that initiate addiction or dependency. The epithalamic habenular complex and its targets appear to be critical for the withdrawal syndrome. The medial habenula (MHb) and one of its primary targets, the interpeduncular nucleus (IPN), richly express β4 and α5 nAChR subunits (and α2 only in the rodent IPN), which are necessary for the neuroadaptations that lead to somatic withdrawal symptoms during nicotine abstinence (Salas et al. 2004b, 2009). Recent research shows that nicotine-induced reward, addiction, and withdrawal involve a wide range of nAChR subtypes expressed in diverse neural systems.
REWARD AND INITIATION OF ADDICTION
Rewarding stimuli promote learning of goal-directed behaviors, generate positive emotions, and subsequently stimulate repetition of those learned behaviors (Schultz 2010, Thorndike 1911). Intrinsic neural responses to reward evolved to ensure successful behaviors that perpetuate the genetic material of the individual and its species. Therefore, reward mechanisms are observed across species, from insects to primates (Hodos 1961, Lau et al. 2006, McClure et al. 2007, Qin & Wheeler 2007, Wise 2006). For humans, rewards can be more complex and abstract than food and sex and can take the form of monetary, aesthetic, cognitive, or social stimuli (Montague et al. 2006, O’Doherty et al. 2001). The effectiveness or value of rewards can be estimated by measuring the effort a subject expends on behaviors that achieve the rewarded goal (Hodos 1961, Schultz 2006).
The overall circuitry that mediates reward is broad and diverse, but particular fiber tracts and nuclei are now recognized as being especially important. The study of the brain reward circuitry was spearheaded in the 1950s by Olds and Milner (Olds 1958, Olds & Milner 1954), who showed that rats work incessantly to self-administer intracranial electrical stimulation to certain regions of the brain (Conover et al. 1994, Routtenberg & Lindy 1965, Spies 1965). Early investigations showed that the midbrain dopamine (DA) systems have an important role in the response to salient stimuli and to brain self-stimulation (Phillips & Olds 1969; Fibiger 1978, Wise 1978). Self-stimulation of the medial forebrain bundle facilitates DA release (Garris et al. 1999, Gratton et al. 1988, Phillips et al. 1989), and DA receptor antagonists or DA neuron lesions inhibit brain self-stimulation (Fouriezos & Wise 1976, Lippa et al. 1973, Stellar & Corbett 1989). Those early studies emphasized the importance of the mesocorticolimbic DA pathways during reward-motivated behaviors. The DA efferents originating from the midbrain VTA and particularly targeting the PFC and NAc became recognized as paramount neural structures shaping reward-related behavior (Schultz et al. 1997; Wise 2004, 2009; Wise & Rompre 1989). However, the role of the DA projections from the substantia nigra compacta (SNc) and wide-ranging DA targets have important, but less well-defined, roles (e.g., see Packard & Knowlton 2002).
The midbrain DA area expresses diverse nAChR subtypes (Grady et al. 2010, Klink et al. 2001, Pidoplichko et al. 1997, Wooltorton et al. 2003) and receives afferent cholinergic innervation from the nearby pedunculopontine tegmentum (PPT) and the laterodorsal tegmentum (LDT), which are a loose collection of cholinergic neurons interspersed with γ-aminobutyric acid (GABA)ergic and glutamatergic neurons (Omelchenko & Sesack 2005). Although the VTA receives a strong excitatory glutamate input from the PFC, that excitation is mainly onto DA neurons that project back to the cortex, not to the NAc (Carr & Sesack 2000). Rather, the PPT/LDT neurons provide potent glutamatergic excitation to the DA neurons projecting to the NAc (Omelchenko & Sesack 2005). In addition, the PPT/LDT cholinergic afferents have important endogenous influences over DA neurons by acting via nAChRs (Maskos 2008, 2010). The PPT/LDT cholinergic afferents boost glutamate afferent transmission via presynaptic nAChRs (Pidoplichko et al. 2004, Schilstrom et al. 2000) and provide some excitatory drive to GABA projection neurons and interneurons, mainly via β2-containing (β2*) nAChRs (Mansvelder et al. 2002, Pidoplichko et al. 2004, Pons et al. 2008). The PPT/LDT contribute to events associated with drug taking (Picciotto & Corrigall 2002), as exemplified by lesions in the PPT reducing nicotine self-administration (Lanca et al. 2000). This influence of the PPT/LDT arises, at least in part, because these areas contribute to the phasic burst firing of DA neurons (Floresco et al. 2003, Lodge & Grace 2006).
In the midbrain, nAChRs comprising the β2 nAChR subunit in combination with α4 and/or α6 subunits are essential for nicotine-induced reinforcement and the DA signal (Drenan et al. 2010, Mameli-Engvall et al. 2006, Picciotto et al. 1998, Pons et al. 2008, Tapper et al. 2004). Nicotine directly activates DA neurons via those and other nAChR subtypes (Pidoplichko et al. 1997), and nicotine modifies the firing modes and firing frequency of DA neurons through excitatory and inhibitory inputs and synaptic plasticity (Mameli-Engvall et al. 2006; Schilstrom et al. 2000, 2003; Zhang et al. 2009b).
DA neurons display a number of different firing modes that for convenience will be grouped into two different contributions: tonic and phasic. Tonic firing is at a relatively low frequency (roughly 4 Hz) that generally consists of individual action potentials. Phasic firing is usually at a higher average frequency, and it includes high-frequency burst discharges (roughly 20–80 Hz) (Grace et al. 2007; Grace & Bunney 1983, 1984; Hyland et al. 2002; Robinson et al. 2004). Although phasic bursts can be rather short, sometimes containing only one or two extra spikes, they are thought to convey or differentiate behaviorally relevant information. For example, phasic burst firing is induced by unpredicted reward or unanticipated cues that have been conditioned to a known reward (Schultz 2007b, Schultz et al. 1997). Disrupting those bursts impairs conditioned behavioral responses, diminishes the ability to learn cues about reward and salience, and disrupts the overall processing of reward (Schultz 2007b). Phasic burst firing by DA neurons and the consequent downstream phasic DA signals are vital for processing reward-related information and, thus, are important for the initial phase of addiction.
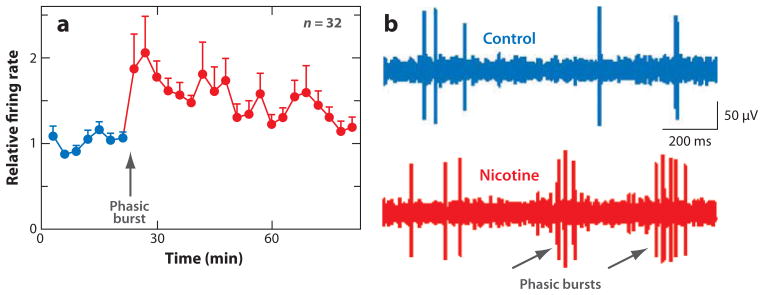
While increasing the firing of DA neurons, nicotine potently increases the number and length of phasic bursts that mediate the processing of reward (Figure 1) (Grenhoff et al. 1986, Mameli-Engvall et al. 2006, Zhang et al. 2009b). The phasic burst firing induced by nicotine causes greater extracellular DA release compared with tonic, single-spike firing (Floresco et al. 2003, Gonon 1988, Grace 1991, Zhang et al. 2009b). This burst firing mode normally mediates the phasic behavioral response to salient environmental stimuli (Maskos et al. 2005, Schultz et al. 1997). Nicotine, however, acts directly on this circuitry, as if a reward-related sensory input has been received. In mice lacking the β2 nAChR subunit (β2 −/−), nicotine does not induce phasic bursts by DA neurons (Mameli-Engvall et al. 2006), nicotine does not induce DA release, and nicotine does not support self-administration (Picciotto et al. 1998).
Figure 1.
Nicotine increases the action potential firing rate and phasic burst firing of putative midbrain dopamine (DA) neurons in freely moving rats. (a) The normalized average firing rate of DA neurons in response to administration of nicotine (0.4–0.5 mg/kg, i.p., free-base equivalent). (b) In vivo, chronic tetrode recording from a putative DA neuron, indicating that the DA neuron fires more phasic bursts (gray arrows) after nicotine administration (red trace) than before (blue control trace). Adapted from Zhang et al. (2009b).
The irrefutable role of β2* nAChRs in the reinforcing properties of nicotine is reflected in the analysis of nAChR null mice in combination with nAChR subunit re-expression with lentiviral techniques (Mameli-Engvall et al. 2006, Pons et al. 2008). Nicotine self-administration is reinstated by re-expression of the β2 subunit in the VTA but not in the neighboring SNc (Avale et al. 2008), confirming the specific role of the VTA in reward. Nicotine self-administration is also reinstated by the re-expression of either the α4 or the α6 nAChR subunit, consistent with the expression of α4β2 and α4α6β2 nAChRs in the VTA (Pons et al. 2008). Thus, β2* nAChRs in combinations with both α4 and α6 subunits are important for the reinforcing properties of nicotine, and the α4 and α6 subunits cannot completely substitute for each other even though they are abundantly expressed in VTA neurons (Champtiaux et al. 2003, Salminen et al. 2007).
Interestingly, local re-expression of α7 in the VTA did not reinstate self-administration in α7 −/− mice (Pons et al. 2008), consistent with the pharmacological evidence indicating that the α7 subunit does not influence chronic nicotine self-administration in trained rats (Grottick & Higgins 2000) nor nicotine-induced conditioned place preference (Stolerman et al. 2004, Walters et al. 2006). The roles of α7* nAChRs in nicotine addiction may be more subtle and widely distributed in the CNS, and these receptors may work appropriately only in combination with other nAChR subtypes. For instance, the α7 nAChRs seem to fine-tune nicotine-induced DA neuron firing only after β2* nAChRs have been activated (Mameli-Engvall et al. 2006).
DOWNSTREAM FROM DOPAMINE NEURON PHASIC BURSTS
If phasic burst firing by DA neurons is critical for nicotine self-administration, what are the downstream consequences of those bursts? The targets innervated by DA projections are broad and diverse, and their influences on reward-related behaviors have focused attention onto limbic and cortical sites, including the ventral and dorsal striatum, the amygdala, the hippocampus, the PFC, and the orbitofrontal cortex. Those DA projection target areas are involved in reinforcement (ventral striatum), learning and declarative memory (hippocampus), emotional memory (amygdala), and habit formation (dorsal striatum), as well as executive functions and working memory (PFC and orbitofrontal cortex) (Schultz W. 2007b, Laviolette 2007, Seamans & Yang 2004). Therefore, the dopaminergic system broadly influences neural processing that underlies reward-based, as well as other, mechanisms for memory and behavior. The NAc of the ventral striatum has garnered special interest in processing reward, beginning with the early intracranial self-stimulation experiments. Electrodes placed along fibers leading to the NAc potently mediate self-stimulation (Corbett & Wise 1979, Olds & Olds 1969).
The NAc and portions of the olfactory tubercle comprise the ventral striatum, which is mainly limbic related. It receives extensive excitatory innervation from the PFC, the hippocampus, and the amygdala (Haber et al. 2000, Heimer 2000, Pennartz et al. 1994). The primary DA innervation arises from the VTA and, to a much lesser degree, from the SNc (van Domburg & ten Donkelaar 1991). Among its functions, the NAc serves as the limbic-motor or motivation-action interface, and those functions are part of the NAc participation in reward-based learning and addiction (Haber et al. 2000, Mogenson et al. 1980). Addictive drugs (including nicotine) increase the basal DA concentration in the NAc shell as measured by microdialysis, but that background increase is smaller or not detected in the dorsolateral striatum (Di Chiara 1999, Pidoplichko et al. 2004, Pontieri et al. 1996, Zhang et al. 2009b). This increase in basal or background DA measured by microdialysis in the NAc shell is considered indicative of a drug’s addictive influence.
Nicotine, at the concentration obtained from tobacco, induces phasic bursts from VTA/SNc DA neurons (Figure 1b) that innervate much of the dorsal to ventral extent of the striatum (Grenhoff et al. 1986, Pidoplichko et al. 1997, Schilstrom et al. 2003, Zhang et al. 2009b), suggesting that the DA signal should be comparable throughout the striatum. However, nicotinic and other local striatal mechanisms regulate the frequency dependence of DA release (Cragg 2003, Rice & Cragg 2004, Zhang et al. 2009a, Zhang & Sulzer 2004, Zhou et al. 2001). Nicotinic receptors are expressed on DA neuron fibers and terminals, and the activity of those nAChRs, particularly containing the β2, α4, and α6 subunits (Pons et al. 2008), is highly regulated by ongoing striatal cholinergic interneuron discharges and dense acetylcholine esterase activity (Zhou et al. 2001, 2003).
Although only ~2% of the striatal neurons are cholinergic, they have extremely dense axonal arbors that provide denser cholinergic innervation than is seen anywhere else in the brain (Butcher & Woolf 1984, Zhou et al. 2001, 2002). The background firing rate of striatal cholinergic neurons is ~6 Hz, providing ongoing acetylcholine (ACh) release via direct synaptic transmission and volume transmission (Dani & Bertrand 2007, de Rover et al. 2002, Koos & Tepper 2002, Wilson 2004). The dense striatal acetylcholine esterase rapidly hydrolyzes the released ACh, preventing the appearance of long-lasting ACh signals that would strongly desensitize the high-affinity subtypes of nAChRs (Dani et al. 2000, Grady et al. 1994, Wooltorton et al. 2003). This ongoing ACh release and breakdown produce a background of cholinergic activity in the striatum that has a number of functions, including regulation of DA release (Exley & Cragg 2008, Zhang et al. 2009a, Zhang & Sulzer 2004).
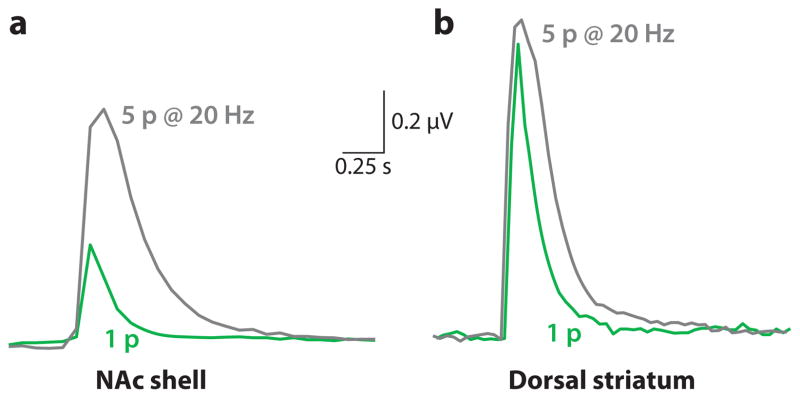
The frequency dependence and nicotinic modulation of DA release differ in the dorsolateral striatum and NAc shell (Chergui et al. 1994; Cragg 2003; Cragg et al. 2002; Zhang et al. 2009a,b). Direct measures of DA release using fast-scan cyclic voltammetry indicate that the transfer function relating DA neuron activity and DA release differs in those (and likely other) locations (Figure 2) (Cragg 2003, Rice & Cragg 2004, Zhang et al. 2009a, Zhang & Sulzer 2004, Zhou et al. 2001). Presynaptic or axonal nAChRs enhance DA release evoked at low firing frequencies (Zhou et al. 2001). The ongoing activity of striatal cholinergic interneurons (Bennett & Wilson 1999) excites presynaptic nAChRs on the DA terminals (Jones et al. 2001), producing an increase of intraterminal calcium that enhances DA release (Grady et al. 1997, Wonnacott et al. 2000). However, when nicotine is added to striatal brain slices, a significant proportion of presynaptic and axonal nAChRs is desensitized. As a consequence, low-frequency, tonic DA release is inhibited (Rice & Cragg 2004, Zhang & Sulzer 2004, Zhou et al. 2001), but phasic DA release is regulated differently, such that phasic bursts induce large DA signals (Cragg 2003; Rice & Cragg 2004; Zhang et al. 2009a,b).
Figure 2.
Different dopamine (DA) release properties in the dorsal striatum and NAc shell. DA release was evoked by a single electrical stimulus pulse (1p) or by a stimulus train of 5 pulses delivered at 20 Hz (5p @ 20Hz) to brain slices. The traces represent DA concentration measured in brain slices by carbon-fiber voltammetry. Example measurements are shown of the DA signal evoked by 1p (gray traces) or by 5p at 20 Hz (green traces). In the NAc shell, the DA signal evoked by 1p is much smaller than that evoked by 5p (as measured by the area under the curve) curve). In the dorsal striatum, the DA signal evoked by 1p is only slightly slight smaller than that evoked by a 5p train. Adapted from data within Zhang et al (2009b).
The difference in DA release between the NAc shell and the dorsal striatum is exemplified in Figure 2, which shows DA-concentration traces arising from electrical stimulation applied to DA afferents (Zhang et al. 2009a,b). In the NAc shell, phasic burst discharges produce a greater increase in DA release than in the dorsal striatum, where the DA release from a single-pulse stimulus is comparable to the release from a phasic-burst stimulus. These results arise, in part, because the probability of DA release to a single-action potential is greater in the dorsal striatum than the NAc shell (Zhang et al. 2009a,b; Zhang & Sulzer 2004). In the dorsal striatum, DA release probability is already high in response to a single-tonic stimulus. Therefore, a phasic burst is not able to increase DA release much further. On the other hand, the NAc shell has a low probability of release in response to a single-tonic stimulus and, thus, has further to increase DA release in response to a phasic burst.
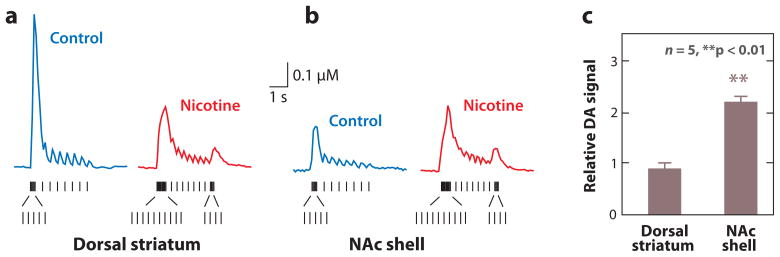
More telling was the experiment examining the effect of nicotine over DA release (Figure 3). A stimulus train representing in vivo DA neuron firing in the absence or presence of nicotine evokes DA release differently from a dorsal striatal slice (Figure 3a) and from a NAc shell slice (Figure 3b). The stimulus trains are shown below the DA-concentration traces. With identical DA neuron bursting, nicotine increases the average DA release more in the NAc shell than in the dorsal striatum (Figure 3c). The details of the DA signaling change in both anatomical areas, but as judged by the area under the curves (Figure 3a,b), the data suggest that the background DA concentration would increase in the NAc shell and not the dorsal striatum (Zhang et al. 2009b). These results are consistent with the direct measurements of background DA concentrations made by microdialysis (Zhang et al. 2009b).
Figure 3.
The burst firing by dopamine (DA) neurons measured in vivo that is induced by nicotine causes a greater increase of DA release in the NAc shell than in the dorsal striatum. The traces represent DA concentration measured in brain slices by carbon-fiber voltammetry. Electrical stimulus patterns used to evoke DA release were designed to mimic the in vivo firing patterns (vertical tick marks below) of DA neurons measured from freely moving rats before (control) and after nicotine injections. Patterned stimulus trains based on the in vivo DA-unit recordings are shown below the evoked DA release in the absence (control) or presence of nicotine in the dorsal striatum (a) or the NAc shell (b). (c) The relative DA signal (calculated as the area under the curve) was unchanged by nicotine in the dorsal striatum dorsal striatum but was increased in the NAc shell. Adapted from Zhang et al. (2009b).
Measured in vivo from the VTA, nicotine administration to a rat causes (some) DA neurons to fire at a higher rate and to fire more and longer bursts (Figure 1). The downstream DA signaling is altered, but the transfer function from DA neuron spikes to DA release is dependent on the target and time because regulatory mechanisms change with local conditions (Zhang et al. 2009a). DA axon terminals in the dorsolateral striatum and NAc shell respond differently to incoming action potentials, consistent with phasic bursts inducing a greater increase of DA release in the NAc shell (Cragg 2003; Rice & Cragg 2004; Zhang et al. 2009a,b; Zhang & Sulzer 2004), which has a more important role in mediating the rewarding properties of nicotine that drive the early phases of addiction. Given the properties and topology of DA fiber tracts, it is likely that DA release in cortical areas commonly responds to DA firing frequency more as is seen in the NAc shell rather than as is seen in the dorsal striatum.
Despite these potent effects of nicotine in the striatum, it is the nAChRs and nicotinic mechanisms within and impinging on the midbrain DA centers that are required for the addictive drive of nicotine (Mameli-Engvall et al. 2006, Maskos et al. 2005, Pons et al. 2008). Synaptic plasticity within the midbrain and onto DA neurons is an important early step in the nicotine addiction process (Dani et al. 2001, Mansvelder et al. 2002, Mansvelder & McGehee 2000, Pidoplichko et al. 2004). The extensive cholinergic innervation and nicotinic effects throughout the striatum suggest, however, that the direct and indirect actions of nicotine on the striatal (and other) targets also serve underappreciated roles in the overall nicotine addiction process.
AVERSIVE SENSORY SIGNALING IN THE MESOCORTICOLIMBIC SYSTEM
Nicotine in both humans and animals follows an inverted U-shaped dose-response curve, with aversive effects coming into play at higher nicotine concentrations (Ashton et al. 1980, Fattore et al. 2002, Herskovic et al. 1986, Paterson et al. 2003). Aside from effects on the peripheral nervous system, this dose-response relationship arises from nicotine acting on a heterogeneous population of nAChR subtypes in different neuronal circuits that mediate rewarding and aversive effects. Smokers titrate their nicotine intake to experience the rewarding while avoiding the aversive actions (Benowitz 2001, Benowitz & Jacob 1985, Dani et al. 2009, Hutchison & Riley 2008, Kassel et al. 2007, Simpson & Riley 2005, Wise et al. 1976).
Although investigators originally postulated that the DA released from the VTA mediates only the hedonic or pleasurable effects of natural reinforcers and drugs in the NAc, evidence indicates that DA can signal events of opposite hedonic valence based on novelty or salience. Reward omission induces phasic inhibition of VTA neurons (Schultz 2007a,b), and aversive stimuli excite ventral (Brischoux et al. 2009) and inhibit dorsal (Ungless et al. 2004) VTA neurons. VTA inhibition has been proposed to signal the motivational value of unexpected, aversive events whereas VTA excitation is thought to signal salience to help respond to environmental changes (Bromberg-Martin et al. 2010). In the NAc of rats, intraoral sucrose (i.e., rewarding) infusion increased DA levels, whereas quinine (i.e., aversive) infusion decreased DA levels (Roitman et al. 2008). Owing to afferents mediating sensory input, NAc neurons decrease their firing to appetitive stimuli and increase their firing to aversive stimuli (Roitman et al. 2005). Because the activity of DA neurons and the resulting DA release on its targets depend on the balance between excitatory and inhibitory inputs (Marinelli et al. 2006), understanding how DA neurons are inhibited provides insight into behavior and the complex effects of nicotine.
Aversive, negative sensory input is processed by the habenular complex, an epithalamic structure involved in fear, anxiety, depression, and stress (Geisler et al. 2008, Ikemoto 2010, Winter et al. 2010). The habenula is divided into the MHb and the two divisions of the lateral nucleus (LHb). It receives massive afferents from the medial frontal cortex, the NAc, the olfactory bulb, the septum, and the striatum via the stria medullaris thalami and sends projections to the IPN, the VTA, the SNc, the medial raphe complex, the locus coeruleus, and the periaqueductal gray (Geisler et al. 2008; Greatrex & Phillipson 1982; Herkenham & Nauta 1977, 1979; Kim & Chang 2005; Scheibel 1997; Sutherland 1982). Whereas the MHb receives inputs primarily from the limbic system and sends outputs mainly to the IPN, the LHb receives inputs primarily from the basal ganglia and sends outputs mainly to dopaminergic and serotonergic neurons (Hikosaka 2010).
An aversive sensory input administered to monkeys elicits LHb activity that indirectly inhibits DA neurons (Hikosaka et al. 2008; Ikemoto 2010; Matsumoto & Hikosaka 2007, 2009). Functional MRI studies in humans are consistent with those results. A human decision-making error that causes a negative sensory feedback or the absence of an expected sensory reward produces strong activity within the habenular complex (Salas et al. 2010, Shepard et al. 2006, Ullsperger & von Cramon 2003). The firing patterns of glutamatergic LHb neurons negatively correlate with the firing patterns of DA neurons. For example, LHb neurons increase their firing in the absence of predicted reward and decrease their firing upon delivery of reward, which is opposite from the usual firing of DA neurons (Hikosaka et al. 2008, Matsumoto & Hikosaka 2007). Furthermore, electrical stimulation of the LHb inhibits most DA neurons (Christoph et al. 1986, Ji & Shepard 2007, Matsumoto & Hikosaka 2007). In contrast, habenular lesions increase DA turnover in the NAc and PFC, reflecting an activation of the dopaminergic system (Lisoprawski et al. 1980, Nishikawa et al. 1986).
Recent studies suggest that the LHb, rather than projecting directly to the VTA, exerts its effects through the stimulation of neurons in the newly characterized rostromedial tegmental nucleus (RMTg), a region at the tail of the VTA (Perrotti et al. 2005). The RMTg sends GABAergic projections to the VTA and SNc and inhibits DA cells (Ikemoto 2010, Jhou et al. 2009a). As in the LHb, RMTg neurons are phasically activated by aversive stimuli and inhibited by natural rewards such as food or reward-predictive stimuli, and conversely, RMTg neurons are stimulated by aversive stimuli (Jhou et al. 2009b). RMTg neurons are also modulated by drugs of abuse, such as cocaine and methamphetamine. Nicotine increases the RMTg neuron activity (Lecca et al. 2010). Because physiological doses of nicotine commonly stimulate DA neurons (Grenhoff et al. 1986, Mameli-Engvall et al. 2006, Pidoplichko et al. 1997, Zhang et al. 2009b), nicotine-induced RMTg activity may contribute to the inverted U-shaped dose-response curve. Furthermore, a transient inhibition of some DA neurons can be detected before the longer-lasting, nicotine-induced excitation (Erhardt et al. 2002).
The MHb densely expresses nAChRs and receives rich cholinergic innervation, but its role in regulating catecholamine transmission is not known. Although projections have been identified from the MHb to the LHb (Kim & Chang 2005), the MHb sends most of its projections to the IPN (Klemm 2004). The IPN, in turn, sends projections to the serotonergic raphé nuclei and to the dopaminergic VTA (Groenewegen et al. 1986, Klemm 2004, Montone et al. 1988). Therefore, the MHb, at least, influences monoaminergic transmission via its connections to the IPN.
NICOTINE-INDUCED NEUROADAPTATIONS
The addiction process produces cellular adaptations that influence widely distributed neural circuits, particularly including the mesocorticolimbic system (Koob & Le Moal 2001). Acting via distinct anatomical distributions and cellular locations of nAChR subtypes, nicotine produces neuroadaptations through its influence over intracellular pathways (Wonnacott et al. 2005). Diverse nAChR subtypes are expressed on DA, GABA, and Glu neurons of the midbrain, and nAChRs are expressed on afferent projections arriving from diverse areas, including the cortex, the PPT/LDT, and the NAc (Kalivas 1993, Steffensen et al. 1998, Walaas & Fonnum 1980). Each of these neuronal subpopulations expresses nAChRs of differing subunit compositions and in differing cellular localizations (Calabresi et al. 1989, Klink et al. 2001, Wooltorton et al. 2003).
One of the most direct and important neuroadaptations induced by chronic nicotine use is the subtype-specific upregulation of nAChRs (Buisson & Bertrand 2001; Changeux et al. 1984; Dani & Heinemann 1996; Flores et al. 1997; Mansvelder et al. 2002; Marks et al. 1983, 1992; Perry et al. 1999; Rezvani et al. 2007, 2009; Rowell & Wonnacott 1990; Schwartz & Kellar 1983). The mechanisms invoked for nAChR upregulation are multiple and likely act in parallel. Because nicotine is not hydrolyzed by acetylcholine esterase, as ACh is, nicotine’s long-lasting presence favors excessive nAChR desensitization. The homeostatic response to desensitized (turned-off) receptors is upregulation (Dani & Heinemann 1996, Fenster et al. 1999, Picciotto et al. 2008). Other mechanisms that likely contribute to upregulation are decreased surface receptor turnover (Peng et al. 1997) and increased receptor assembly at the endoplasmic reticulum (Corringer et al. 2006; Darsow et al. 2005; Rezvani et al. 2007, 2010; Sallette et al. 2005). Isomerization of surface nAChRs to high-affinity nicotinic sites has also been proposed (Buisson & Bertrand 2002, Vallejo et al. 2005).
Nicotinic receptor upregulation differs among the diverse subtypes, varies among brain regions for the same nAChR subtype, and depends on the contingency of nicotine administration (Gaimarri et al. 2007, Marks et al. 1983, McCallum et al. 2006, Metaxas et al. 2010, Nguyen et al. 2004, Pauly et al. 1996, Schwartz & Kellar 1983). Because nicotinic mechanisms are distributed throughout the brain, changes in nAChR subtype expression densities could have influences over diverse neuronal circuitry.
In parallel with and, in part, arising from nAChR upregulation, nicotine also produces heterologous neuroadaptations. Nicotine-induced synaptic plasticity increases the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid/N-methyl-D-aspartate current ratio of glutamate receptors (GluRs) at locations mediating drug-associated memory (Dani et al. 2001, Kauer & Malenka 2007, Tang & Dani 2009), including at DA neurons (Gao et al. 2010, Placzek et al. 2009, Saal et al. 2003). Nicotine exposure also increases high-affinity DA D2 receptors in the NAc (Novak et al. 2010), an effect, also produced by cocaine, that causes DA hypersensitivity (Briand et al. 2008, Grigoriadis & Seeman 1986). Beyond the well-studied events of synaptic plasticity, chronic nicotine exposure leads to further remodeling of synapses, producing changes in scaffolding proteins, such as PSD95 and Shank (Hwang & Li 2006, Rezvani et al. 2007). These changes derive, at least in part, from nicotine-induced changes in protein turnover and from partial inhibition of proteasomal function (Rezvani et al. 2007). Because proteasomes are a fundamental component of the protein degradation machinery, nicotine entrance into the cytoplasm that influences proteasomes has far-reaching effects even on cells that do not express nAChRs (Rezvani et al. 2007).
Chronic nicotine exposure also has indirect effects on motivational systems by altering synthesis and release of opioid peptides in a time-dependent and peptide-specific fashion (Berrendero et al. 2010). The endogenous opioid system influences positive and negative motivational and affective states (Steiner & Gerfen 1998) and participates in the behavioral effects of several addictive drugs, including nicotine (Berrendero et al. 2005, Hadjiconstantinou & Neff 2010, Trigo et al. 2009). Opioid peptides affect DA function in the VTA and the striatum. In particular, dynorphins decrease and enkephalins increase the release of DA (Devine et al. 1993; Di Chiara & Imperato 1988; Longoni et al. 1991; Pentney & Gratton 1991; Spanagel et al. 1990a,b, 1992). In addition, DA controls the synthesis of striatal dynorphin and enkephalin by affecting mRNA levels (Angulo & McEwen 1994, Hadjiconstantinou & Neff 2010). These changes could have broad influence over circuits influencing reward-related processes and, thus, drug use and withdrawal.
More generally, nAChRs affect the release of virtually every major neurotransmitter (Alkondon et al. 1997, Dani & Bertrand 2007, Hadjiconstantinou & Neff 2010, Kenny 2010, McGehee et al. 1995, Gray et al. 1996, Role & Berg 1996). Therefore, neuroadaptations arising from chronic nicotine exposure may cause widespread alterations in brain neurotransmission. Consequently, the concept that only certain brain areas narrowly and rigidly mediate reward, aversion, and addiction to nicotine is becoming obsolete. The overall neuroadaptations contribute to the mechanisms that maintain nicotine consumption, including mechanisms underlying associative learning (Tang & Dani, 2009), and they also participate in the nicotine-withdrawal syndrome (De Biasi & Salas 2008, Hadjiconstantinou & Neff 2010).
BEHAVIORAL MANIFESTATIONS OF NICOTINE WITHDRAWAL
As the brain adapts to chronic nicotine exposure, a new homeostatic condition is achieved by the brain’s circuitry that requires the presence of nicotine to be maintained. When access to nicotine is not available, these homeostatic neuroadaptations are no longer appropriate, which contributes to the withdrawal syndrome (Koob & Volkow 2009).
Nicotine withdrawal is a collection of somatic and affective symptoms that is observed within a few hours after discontinuation of nicotine intake (De Biasi & Salas 2008). The symptoms reflect the imbalance in brain neurochemistry created by the absence of nicotine in the addicted brain. The most prominent withdrawal symptoms are irritability, anxiety, anger, difficulty concentrating, sleep disturbance, increased appetite, and weight gain (APA 2000, Hogle et al. 2010, Hughes 2007). Other quantifiable manifestations include reduced heart rates, altered neurohormonal profiles, disrupted electroencephalographic theta power, and perturbations of learned behaviors (Buchhalter et al. 2008, Koob & Le Moal 2005). Powerful cravings also accompany withdrawal, and they may be precipitated by drug-reinforced memories, such as the sight of a cigarette and the place associated with smoking (Dalley & Everitt 2009, Dani & Montague 2007, Shiffman et al. 2002, Smolka et al. 2005, Volkow et al. 2002).
Withdrawal is also associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and the stress-response system (Koob 2008). Acute withdrawal from many drugs elevates corticosterone and corticotropin-releasing factor (CRF) (Koob 2008, Koob & Kreek 2007). During nicotine withdrawal, corticotropin-releasing factor receptor CRF1 influences anxiety and brain reward functions (Bruijnzeel et al. 2009, George et al. 2007). The endogenous opioid system, dynorphin in particular, engages (Hadjiconstantinou & Neff 2010). Nicotine cessation increases the levels of prodynorphin mRNA in NAc dynorphinergic neurons, possibly as a compensatory mechanism to increased dynorphinergic tone (Isola et al. 2008).
The emergence of negative affective symptoms such as dysphoria, anxiety, and irritability (Koob & Le Moal 2001) and, to a lesser extent, the somatic manifestations of withdrawal, signal dependency and underlie drug-seeking behavior (Koob & Le Moal 2005, Koob & Volkow 2009). The negative emotional state produced by the combination of withdrawal symptoms causes enough distress to become a deterrent to abstinence and a drive to relapse (Bruijnzeel & Gold 2005, Koob & Le Moal 2005, West et al. 1989). Drug taking is then maintained through negative reinforcement mechanisms.
Withdrawal symptoms can be assessed in animals chronically exposed to nicotine by the sudden discontinuation of nicotine treatment or administration of nAChR antagonists (De Biasi & Salas 2008). Mecamylamine, which has a slightly higher affinity for α3β4* nAChRs than for β2* receptors, is the best antagonist at precipitating nicotine withdrawal (Damaj et al. 2003). Like humans, rodents manifest both somatic and nonsomatic signs of withdrawal (Malin & Goyarzu 2009). In rats, somatic signs of physical dependency include teeth-chattering, chewing, gasps, palpebral ptosis, tremors, shakes, and yawns (Malin et al. 1992). Similar symptoms are observed in mice, including shaking, grooming, and scratching, which become repetitive and long lived. Jumping, which is not normally observed, also appears in mice undergoing nicotine withdrawal (Damaj et al. 2003, De Biasi & Salas 2008, Isola et al. 1999).
Anhedonia, conditioned place aversion, anxiety-related behavior, and conditioned fear are four major affective manifestations of nicotine withdrawal. Anhedonia is quantifiable in animals as an increase in stimulus threshold needed to induce intracranial electrical self-stimulation to reward pathways (Paterson & Markou 2007). Increased brain-stimulation reward thresholds reflect a decreased sensitivity to the rewarding effect of the electrical stimulation. Both spontaneous (Epping-Jordan et al. 1998, Harrison et al. 2001) and mecamylamine-precipitated (Watkins et al. 2000) nicotine withdrawal induce increases in self-stimulation thresholds. In the conditioned place-aversion paradigm, the animal learns to avoid the compartment in which nicotine withdrawal is experienced (Jackson et al. 2009, Kenny & Markou 2001). A previously neutral environment becomes aversive because of the association of the place with the negative affective state of withdrawal (Suzuki et al. 1996).
Another nonsomatic manifestation of nicotine withdrawal is increased anxiety-like behavior in the elevated plus maze (Pellow et al. 1985). Mice and rats undergoing nicotine withdrawal show increased anxiety-like behavior in the elevated plus maze (Damaj et al. 2003, Irvine et al. 2001). This phenomenon is similar to the anxiety reported by humans experiencing nicotine abstinence and suggests that the sensitivity to anxiety influences the degree of withdrawal signs. These properties may contribute to the more severe withdrawal symptoms commonly experienced by those suffering from depression or anxiety disorders (Dani & Harris 2005, Pomerleau et al. 2005). The conditioned fear paradigm can be used to measure the cognitive symptoms of withdrawal (Davis et al. 2005) as nicotine withdrawal produces deficits in contextual fear conditioning and affects the acquisition of learned responses (Portugal et al. 2008).
MOLECULAR MECHANISMS OF WITHDRAWAL
Withdrawal reflects the abrupt disruption of the equilibrium maintained in the presence of nicotine, and withdrawal triggers new neuroadaptations that counteract the negative state. Spontaneous or mecamylamine-precipitated nicotine withdrawal is associated with a decrease in extracellular DA levels in the NAc (Carboni et al. 2000, Gaddnas et al. 2002, Hildebrand et al. 1998, Rada et al. 2001, Rahman et al. 2004). This phenomenon is also observed during ethanol, morphine, cocaine, and amphetamine withdrawal (Rossetti et al. 1992, Weiss et al. 1992) and is a trigger of the withdrawal syndrome. The decrease in extracellular DA concentrations reflects changes in both DA release and reuptake (Duchemin et al. 2009). Nicotine abstinence is accompanied by increased DA uptake into synaptosomes and elevated DA transporters (DATs) in the SNc/VTA (Hadjiconstantinou & Neff 2010, Rahman et al. 2004). Changes in DA release and uptake are short lived and normalize within 48 h after nicotine cessation (Hadjiconstantinou & Neff 2010).
DAT upregulation and decreased DA overflow may contribute to withdrawal symptoms because those DA signaling changes temporally coincide with the emergence of somatic and affective signs of withdrawal (Hadjiconstantinou & Neff 2010). Although buproprion inhibits nAChRs, it is mainly a norepinephrine/DA re-uptake blocker. Buproprion may aid smoking cessation, in part, by normalizing NAc DA levels, and those DA levels may in turn attenuate anhedonia and somatic withdrawal signs (Cryan et al. 2003, Paterson & Markou 2007).
During withdrawal, the NAc displays a DA deficit, whereas the PFC has an increase in DA output (Carboni et al. 2000). Because enhanced DA transmission in the PFC occurs during stressful and aversive stimuli and is implicated in anxiety, the elevated PFC DA during nicotine withdrawal may contribute to the aversive manifestations (Bradberry et al. 1991, Broersen et al. 2000, Inglis & Moghaddam 1999, Kawasaki et al. 2001, Thierry et al. 1976). Besides DA, other neurotransmitters, such as serotonin and norepinephrine, known to mediate the manifestations of withdrawal from other drugs may play a role (Bruijnzeel et al. 2010, Fletcher et al. 2008, Semenova & Markou 2010, Slotkin & Seidler 2007).
DISTINCT NICOTINIC RECEPTOR SUBTYPES MEDIATE WITHDRAWAL
The use of mouse models has clarified the specific brain area that mediates physical dependency. Salas et al. (2003a, 2004a) observed that mice null for the α5 and β4 nAChR subunits are less sensitive to the acute effects of nicotine measured as hypolocomotion in the open field arena and nicotine-induced seizure activity. Attenuation of nicotine-induced seizure is also present in mice heterozygous for the α3 subunit, which is found in the same gene cluster that contains α5 and β4 (Boulter et al. 1990, Salas et al. 2004a). Absence of β4 abolishes the somatic signs of withdrawal precipitated by systemic injection of mecamylamine (Salas et al. 2004b) and prevents withdrawal-induced hyperalgesia (Salas et al. 2004b). This phenotype is in sharp contrast with that of β2 −/− mice, which display normal somatic signs of withdrawal (Besson et al. 2006, Salas et al. 2004b). Physical signs of dependency are also absent in α5 −/− mice in which nicotine withdrawal occurs either spontaneously or upon nicotine cessation or is precipitated by mecamylamine injection (Salas et al. 2009). Both α5 −/− and β4 −/− strains also display reduced anxiety-related behaviors (Gangitano et al. 2009, Salas et al. 2003b), in contrast with β2 −/− mice, which show normal anxiety-like responses (Maskos et al. 2005). The latter is an important result, considering the role of anxiety and stress in nicotine withdrawal and relapse (De Biasi & Salas 2008). Another nicotinic subunit that contributes to the somatic signs of withdrawal is the α2 subunit (Salas et al. 2009), which is selectively expressed in rodent IPN and olfactory bulb (Ishii et al. 2005, Whiteaker et al. 1999). The α7 subunit also plays some role in mecamylamine-precipitated somatic signs of withdrawal as α7 −/− mice showed an intermediate withdrawal phenotype (Salas et al. 2007). The same mice manifested decreased hyperalgesia upon spontaneous nicotine withdrawal (Grabus et al. 2005).
In the mouse, the α5, α2, and β4 nAChR subunits are expressed at high levels in either MHb and/or IPN (De Biasi & Salas 2008, Grady et al. 2009), and no mRNA encoding for nAChR subunits can be detected in the LHb by in situ hybridization techniques. Therefore, investigators postulated that the MHb/IPN axis may be involved in the somatic signs of withdrawal. Indeed, microinjection of the nAChR antagonist mecamylamine into the Hb and IPN was sufficient to precipitate nicotine withdrawal symptoms in mice chronically treated with nicotine. Conversely, mecamylamine injection into the VTA, the hippocampus, or the cortex did not trigger somatic signs of withdrawal (Salas et al. 2009), establishing the MHb and IPN as mediators of somatic withdrawal from nicotine.
Other data in the literature suggest additional involvements of the MHb/IPN axis with brain reward areas. First, electrical stimulation of the MHb and the fasciculus retroflexus produces rewarding effects (Sutherland & Nakajima 1981). Second, most stimulant drugs of abuse cause axonal degeneration in the LHb and the fasciculus retroflexus, and nicotine causes degeneration of neurons in the portion of the fasciculus retroflexus that connects the MHb to the IPN (Ellison 2002, Ellison et al. 1996). Third, a derivative of the alkaloid ibogaine, 18-methoxycoronaridine (18-MC) (Vastag 2005), is a potent antagonist of β4* nAChRs (Glick et al. 2002) with significant antiaddictive properties (Maisonneuve & Glick 2003). In animal models, 18-MC reduced self-administration of nicotine, morphine, cocaine, and methamphetamine; reduced oral intake of alcohol and nicotine; and decreased signs of opioid withdrawal (Maisonneuve & Glick 2003). Fourth, a recent report shows that α5 −/− mice self-administer nicotine at doses that elicit strong aversion in wild-type mice. This result suggests that α5-containing nAChRs in the MHb are key to controlling the amounts of nicotine self-administered (Fowler et al. 2011).
The MHb/IPN axis has a prominent role mediating the aversive effects of nicotine and the somatic symptoms of withdrawal. The data suggest the existence of reward and withdrawal circuits with partially overlapping functions. Smokers finely control nicotine intake by regulating how they puff on a cigarette and how deeply they inhale. In that way, they achieve the most desirable nicotine dose, at the top of the inverted U dose-response curve. The primary circuits underlying this exquisitely regulated dosing are mainly the mesocorticolimbic DA system and the epithalamic habenular circuits: One supports the rising arm, the other the falling arm of the inverted U dose-response curve.
Recent findings on the influence of gene variants in the CHRNA5-CHRNA3-CHRNB4 gene cluster on nicotine addiction (Bierut et al. 2008, Thorgeirsson et al. 2008) show that a single nucleotide polymorphism (SNP) within CHRNA5, rs16969968, seems to correlate with nicotine dependency risk, heavy smoking, and the pleasurable sensation produced by a cigarette (Berrettini et al. 2008, Bierut et al. 2008, Saccone et al. 2007, Thorgeirsson et al. 2008). This nonsynonymous SNP, located in the second intracellular loop of the α5 subunit, leads to a reduction in receptor function (Bierut et al. 2008). Because the nAChR subunits comprised in the CHRNA5-CHRNA3-CHRNB4 reduce the aversive effects of nicotine and drive its consumption, it is tempting to speculate that people with SNP rs16969968 smoke more and become addicted at a younger age because they lack sufficient functional α5* nAChRs. The presence of fewer aversive effects (even at higher nicotine doses) during the initial contact with the drug would promote the hedonic drive, thereby promoting the transition from use to abuse and dependency. New mouse models examining the role of nAChR gene variants will help investigators explore the role of gene polymorphisms in nicotine addiction and will provide insight into cessation therapies tailored for specific subpopulations of smokers.
Acknowledgments
We thank William Doyon and Michael Paolini for helpful comments and Dang Q. Dao and Erika E. Perez for assistance with the figures. We are supported by grants from the Cancer Prevention and Research Institute of Texas and the National Institutes of Health (NINDS NS21229 and NIDA DA09411, DA017173, DA029157).
Glossary
- nAChR
nicotinic acetylcholine receptor
- DA
dopamine
- VTA
ventral tegmental area
- PFC
prefrontal cortex
- NAc
nucleus accumbens
- IPN
interpeduncular nucleus
- SNc
substantia nigra compacta
- PPT
pedunculopontine tegmentum
- LDT
laterodorsal tegmentum
- GABA
γ-aminobutyric acid
- ACh
acetylcholine
- LHb
lateral habenula
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–411. [PubMed] [Google Scholar]
- Am. Psychiatr. Assoc. (APA) American Psychiatric Association, Diagnostic and Statistical Manual—IVTR. Washington, DC: APA; 2000. [Google Scholar]
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Ashton H, Marsh VR, Millman JE, Rawlins MD, Telford R, Thompson JW. Biphasic dose-related responses of the CNV (contingent negative variation) to I.V. nicotine in man. Br J Clin Pharmacol. 1980;10:579–89. doi: 10.1111/j.1365-2125.1980.tb00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale ME, Faure P, Pons S, Robledo P, Deltheil T, et al. Interplay of beta2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc Natl Acad Sci USA. 2008;105:15991–96. doi: 10.1073/pnas.0807635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour DJ. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus. Nicotine Tob Res. 2004;6:899–912. doi: 10.1080/14622200412331324965. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci. 1999;19:5586–96. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Compensatory smoking of low yield cigarettes. In: Shopland DR, editor. Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. Bethesda, MD: NIH; 2001. pp. 39–64. NIH Publ. No. 02–5074. [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–41. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Nicotine renal excretion rate influences nicotine intake during cigarette smoking. J Pharmacol Exp Ther. 1985;234:153–55. [PubMed] [Google Scholar]
- Berrendero F, Mendizabal V, Robledo P, Galeote L, Bilkei-Gorzo A, et al. Nicotine-induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci. 2005;25:1103–12. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Robledo P, Trigo JM, Martin-Garcia E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010;35:220–31. doi: 10.1016/j.neubiorev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–73. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, et al. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology. 2006;187:189–99. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–71. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, et al. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990;265:4472–82. [PubMed] [Google Scholar]
- Bradberry CW, Lory JD, Roth RH. The anxiogenic beta-carboline FG 7142 selectively increases dopamine release in rat prefrontal cortex as measured by microdialysis. J Neurochem. 1991;56:748–52. doi: 10.1111/j.1471-4159.1991.tb01987.x. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Seeman P, Robinson TE. Cocaine self-administration produces a persistent increase in dopamine D2 High receptors. Eur Neuropsychopharmacol. 2008;18:551–56. doi: 10.1016/j.euroneuro.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA. 2009;106:4894–99. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM, Abbate F, Feenstra MG, de Bruin JP, Heinsbroek RP, Olivier B. Prefrontal dopamine is directly involved in the anxiogenic interoceptive cue of pentylenetetrazol but not in the interoceptive cue of chlordiazepoxide in the rat. Psychopharmacology. 2000;149:366–76. doi: 10.1007/s002130000390. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KF, Yavarovich KR, et al. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology. 2010;212:485–99. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–28. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–17. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter AR, Fant RV, Henningfield JE. Novel pharmacological approaches for treating tobacco dependence and withdrawal: current status. Drugs. 2008;68:1067–88. doi: 10.2165/00003495-200868080-00005. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–29. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–36. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- Butcher LL, Woolf NJ. Histochemical distribution of acetylcholinesterase in the central nervous system: clues to the localization of cholinergic neurons. In: Björklund A, Hökfelt T, Kuhar MJ, editors. Handbook of Chemical Neuroanatomy, Vol. 3: Classical Transmitters and Transmitter Receptors in the CNS, Part II. Amsterdam: Elsevier Biomed; 1984. pp. 1–50. [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–40. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–29. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiery A, Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984;225:1335–45. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Edelstein SJ. Nicotinic Acetylcholine Receptors: From Molecular Biology to Cognition. New York: Odile Jacob; 2005. [Google Scholar]
- Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–45. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–19. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover KL, Woodside B, Shizgal P. Effects of sodium depletion on competition and summation between rewarding effects of salt and lateral hypothalamic stimulation in the rat. Behav Neurosci. 1994;108:549–58. doi: 10.1037//0735-7044.108.3.549. [DOI] [PubMed] [Google Scholar]
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending noradrenergic fiber systems of the pontine tegmentum and caudal midbrain: a moveable electrode mapping study. Brain Res. 1979;177:423–36. doi: 10.1016/0006-8993(79)90461-x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Sallette J, Changeux JP. Nicotine enhances intracellular nicotinic receptor maturation: a novel mechanism of neural plasticity? J Physiol. 2006;99:162–71. doi: 10.1016/j.jphysparis.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J Neurosci. 2003;23:4378–85. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Hille CJ, Greenfield SA. Functional domains in dorsal striatum of the nonhuman primate are defined by the dynamic behavior of dopamine. J Neurosci. 2002;22:5705–12. doi: 10.1523/JNEUROSCI.22-13-05705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology. 2003;168:347–58. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ. Dopamine receptors in the learning, memory and drug reward circuitry. Semin Cell Dev Biol. 2009;20:403–10. doi: 10.1016/j.semcdb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–34. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–8. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–52. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Dani JA, Kosten TR, Benowitz NL. The pharmacology of nicotine and tobacco. In: Ries RK, Fiellin DA, Miller SC, Saitz R, editors. Principles of Addiction Medicine. Philadelphia, PA: Wolters Kluwer/Lippincott, Williams & Wilkins; 2009. pp. 179–91. [Google Scholar]
- Dani JA, Montague PR. Disrupting addiction through the loss of drug-associated internal states. Nat Neurosci. 2007;10:403–4. doi: 10.1038/nn0407-403. [DOI] [PubMed] [Google Scholar]
- Dani JA, Radcliffe KA, Pidoplichko VI. Variations in desensitization of nicotinic acetylcholine receptors from hippocampus and midbrain dopamine areas. Eur J Pharmacol. 2000;393:31–38. doi: 10.1016/s0014-2999(00)00003-0. [DOI] [PubMed] [Google Scholar]
- Darsow T, Booker TK, Pina-Crespo JC, Heinemann SF. Exocytic trafficking is required for nicotine-induced up-regulation of alpha 4 beta 2 nicotinic acetylcholine receptors. J Biol Chem. 2005;280:18311–20. doi: 10.1074/jbc.M501157200. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med. 2008;233:917–29. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB. Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci. 2002;16:2279–90. doi: 10.1046/j.1460-9568.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–46. [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–80. [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, et al. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci. 2010;30:9877–89. doi: 10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchemin AM, Zhang H, Neff NH, Hadjiconstantinou M. Increased expression of VMAT2 in dopaminergic neurons during nicotine withdrawal. Neurosci Lett. 2009;467:182–86. doi: 10.1016/j.neulet.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Ellison G. Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur Neuropsychopharmacol. 2002;12:287–97. doi: 10.1016/s0924-977x(02)00020-2. [DOI] [PubMed] [Google Scholar]
- Ellison G, Irwin S, Keys A, Noguchi K, Sulur G. The neurotoxic effects of continuous cocaine and amphetamine in Habenula: implications for the substrates of psychosis. NIDA Res Monogr. 1996;163:117–45. [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Engberg G. Excitatory and inhibitory responses of dopamine neurons in the ventral tegmental area to nicotine. Synapse. 2002;43:227–37. doi: 10.1002/syn.10044. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta MC, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–98. doi: 10.1093/alcalc/37.5.495. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–14. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger HC. Drugs and reinforcement mechanisms: a critical review of the catecholamine theory. Annu Rev Pharmacol Toxicol. 1978;18:37–56. doi: 10.1146/annurev.pa.18.040178.000345. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Le AD, Higgins GA. Serotonin receptors as potential targets for modulation of nicotine use and dependence. Prog Brain Res. 2008;172:361–83. doi: 10.1016/S0079-6123(08)00918-7. [DOI] [PubMed] [Google Scholar]
- Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem. 1997;69:2216–19. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fouriezos G, Wise RA. Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res. 1976;103:377–80. doi: 10.1016/0006-8993(76)90809-x. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011 doi: 10.1038/nature09797. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddnas H, Piepponen TP, Ahtee L. Mecamylamine decreases accumbal dopamine output in mice treated chronically with nicotine. Neurosci Lett. 2002;330:219–22. doi: 10.1016/s0304-3940(02)00734-6. [DOI] [PubMed] [Google Scholar]
- Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C. Regulation of neuronal nicotinic receptor traffic and expression. Brain Res Rev. 2007;55:134–43. doi: 10.1016/j.brainresrev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8:398–406. doi: 10.1111/j.1601-183X.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci. 2010;30:13814–25. doi: 10.1523/JNEUROSCI.1943-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, et al. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33:2688–700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur J Pharmacol. 2002;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Imad Damaj M. Nicotine physical dependence in the mouse: involvement of the alpha7 nicotinic receptor subtype. Eur J Pharmacol. 2005;515:90–93. doi: 10.1016/j.ejphar.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience. 1983;10:301–15. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4:2866–76. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–27. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grady SR, Grun EU, Marks MJ, Collins AC. Pharmacological comparison of transient and persistent [3H]dopamine release from mouse striatal synaptosomes and response to chronic L-nicotine treatment. J Pharmacol Exp Ther. 1997;282:32–43. [PubMed] [Google Scholar]
- Grady SR, Marks MJ, Collins AC. Desensitization of nicotine-stimulated [3H]dopamine release from mouse striatal synaptosomes. J Neurochem. 1994;62:1390–98. doi: 10.1046/j.1471-4159.1994.62041390.x. [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, et al. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–82. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, McIntosh JM, Marks MJ, Collins AC. Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. J Mol Neurosci. 2010;40:91–95. doi: 10.1007/s12031-009-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton A, Hoffer BJ, Gerhardt GA. Effects of electrical stimulation of brain reward sites on release of dopamine in rat: an in vivo electrochemical study. Brain Res Bull. 1988;21:319–24. doi: 10.1016/0361-9230(88)90247-x. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;83:713–16. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Greatrex RM, Phillipson OT. Demonstration of synaptic input from prefrontal cortex to the habenula in the rat. Brain Res. 1982;238:192–97. doi: 10.1016/0006-8993(82)90782-x. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–58. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Grigoriadis D, Seeman P. [3H]-domperidone labels only a single population of receptors which convert from high to low affinity for dopamine in rat brain. Naunyn-Schmiedebergs Arch Pharmakol. 1986;332:21–25. doi: 10.1007/BF00633192. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJ. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Neff NH. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2010.11.010. In Press. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Heimer L. Basal forebrain in the context of schizophrenia. Brain Res Brain Res Rev. 2000;31:205–35. doi: 10.1016/s0165-0173(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–46. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Herskovic JE, Rose JE, Jarvik ME. Cigarette desirability and nicotine preference in smokers. Pharmacol Biochem Behav. 1986;24:171–75. doi: 10.1016/0091-3057(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–13. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Bromberg-Martin E, Hong S, Matsumoto M. New insights on the subcortical representation of reward. Curr Opin Neurobiol. 2008;18:203–8. doi: 10.1016/j.conb.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–25. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–44. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: neuroadaptation in human addiction. Biol Psychiatry. 2010;68:719–25. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hutchison MA, Riley AL. Adolescent exposure to nicotine alters the aversive effects of cocaine in adult rats. Neurotoxicol Teratol. 2008;30:404–11. doi: 10.1016/j.ntt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Hwang YY, Li MD. Proteins differentially expressed in response to nicotine in five rat brain regions: identification using a 2-DE/MS-based proteomics approach. Proteomics. 2006;6:3138–53. doi: 10.1002/pmic.200500745. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–92. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–50. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–38. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–94. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Tolerance to nicotine’s effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol Biochem Behav. 2001;68:319–25. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. Comparison of alpha2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J Comp Neurol. 2005;493:241–60. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola R, Vogelsberg V, Wemlinger TA, Neff NH, Hadjiconstantinou M. Nicotine abstinence in the mouse. Brain Res. 1999;850:189–96. doi: 10.1016/s0006-8993(99)02131-9. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse. 2008;62:448–55. doi: 10.1002/syn.20515. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR, Damaj MI. The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology. 2009;56:970–74. doi: 10.1016/j.neuropharm.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–96. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–30. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Bolam JP, Wonnacott S. Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J Comp Neurol. 2001;439:235–47. doi: 10.1002/cne.1345. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Greenstein JE, Evatt DP, Wardle MC, Yates MC, et al. Smoking topography in response to denicotinized and high-yield nicotine cigarettes in adolescent smokers. J Adolesc Health. 2007;40:54–60. doi: 10.1016/j.jadohealth.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Kaufman O, Damasio H, Damasio AR, Granner M, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nat Neurosci. 2001;4:15–16. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Tobacco dependence, the insular cortex and the hypocretin connection. Pharmacol Biochem Behav. 2010;97:700–7. doi: 10.1016/j.pbb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–49. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol. 2005;483:236–50. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–73. [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–63. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–44. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–35. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanca AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–42. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Lau B, Bretaud S, Huang Y, Lin E, Guo S. Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav. 2006;5:497–505. doi: 10.1111/j.1601-183X.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33:971–81. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, et al. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2010;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, et al. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J. 2009;11:167–77. doi: 10.1208/s12248-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa AS, Antelman SM, Fisher AE, Canfield DR. Neurochemical mediation of reward: a significant role for dopamine? Pharmacol Biochem Behav. 1973;1:23–28. doi: 10.1016/0091-3057(73)90050-6. [DOI] [PubMed] [Google Scholar]
- Lisoprawski A, Herve D, Blanc G, Glowinski J, Tassin JP. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183:229–34. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci USA. 2006;103:5167–72. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni R, Spina L, Mulas A, Carboni E, Garau L, et al. (D-Ala2)deltorphin II: D1-dependent stereo-typies and stimulation of dopamine release in the nucleus accumbens. J Neurosci. 1991;11:1565–76. doi: 10.1523/JNEUROSCI.11-06-01565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–18. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Malin DH, Goyarzu P. Rodent models of nicotine withdrawal syndrome. Handb Exp Pharmacol. 2009;2009:401–34. doi: 10.1007/978-3-540-69248-5_14. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–84. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–21. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–57. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–17. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–25. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–84. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153(Suppl 1):S438–45. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U. Role of endogenous acetylcholine in the control of the dopaminergic system via nicotinic receptors. J Neurochem. 2010;114:641–6. doi: 10.1111/j.1471-4159.2010.06798.x. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–7. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–15. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–41. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic alpha 3/alpha 6 beta 2 and alpha 4 beta 2 nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J Pharmacol Exp Ther. 2006;318:381–88. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–96. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Metaxas A, Bailey A, Barbano MF, Galeote L, Maldonado R, Kitchen I. Differential region-specific regulation of alpha4beta2* nAChRs by self-administered and non-contingent nicotine in C57BL/6J mice. Addict Biol. 2010;15:464–79. doi: 10.1111/j.1369-1600.2010.00246.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Montague PR, King-Casas B, Cohen JD. Imaging valuation models in human choice. Annu Rev Neurosci. 2006;29:417–48. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- Montone KT, Fass B, Hamill GS. Serotonergic and nonserotonergic projections from the rat interpeduncular nucleus to the septum, hippocampal formation and raphe: a combined immunocytochemical and fluorescent retrograde labelling study of neurons in the apical subnucleus. Brain Res Bull. 1988;20:233–40. doi: 10.1016/0361-9230(88)90183-9. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–49. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–36. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Novak G, Seeman P, Le Foll B. Exposure to nicotine produces an increase in dopamine D2(High) receptors: a possible mechanism for dopamine hypersensitivity. Int J Neurosci. 2010;120:691–97. doi: 10.3109/00207454.2010.513462. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–24. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Olds ME, Olds J. Effects of lesions in medial forebrain bundle on self-stimulation behavior. Am J Physiol. 1969;217:1253–64. doi: 10.1152/ajplegacy.1969.217.5.1253. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–35. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–64. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing alpha 4 or beta 2 mRNA levels. J Pharmacol Exp Ther. 1996;278:361–69. [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol. 1997;51:776–84. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–61. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pentney RJ, Gratton A. Effects of local delta and mu opioid receptor activation on basal and stimulated dopamine release in striatum and nucleus accumbens of rat: an in vivo electrochemical study. Neuroscience. 1991;45:95–102. doi: 10.1016/0306-4522(91)90106-x. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, et al. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–24. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Blaha CD, Fibiger HC. Neurochemical correlates of brain-stimulation reward measured by ex vivo and in vivo analyses. Neurosci Biobehav Rev. 1989;13:99–104. doi: 10.1016/s0149-7634(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Olds J. Unit activity: motiviation-dependent responses from midbrain neurons. Science. 1969;165:1269–71. doi: 10.1126/science.165.3899.1269. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–41. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–77. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–4. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, et al. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–92. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine Tob Res. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–57. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–13. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Wheeler AR. Maze exploration and learning in C. elegans. Lab Chip. 2007;7:186–92. doi: 10.1039/b613414a. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–10. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience. 2004;129:415–24. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Slade S, Wells C, Petro A, Lumeng L, et al. Effects of sazetidine-A, a selective alpha4beta2 nicotinic acetylcholine receptor desensitizing agent on alcohol and nicotine self-administration in selectively bred alcohol-preferring (P) rats. Psychopharmacology. 2010;211:161–74. doi: 10.1007/s00213-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, De Biasi M. The ubiquitin-proteasome system regulates the stability of neuronal nicotinic acetylcholine receptors. J Mol Neurosci. 2009;40:177–84. doi: 10.1007/s12031-009-9272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–19. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–84. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robinson S, Smith DM, Mizumori SJ, Palmiter RD. Firing properties of dopamine neurons in freely moving dopamine-deficient mice: effects of dopamine receptor activation and anesthesia. Proc Natl Acad Sci USA. 2004;101:13329–34. doi: 10.1073/pnas.0405084101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–97. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–77. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–85. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–34. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Lindy J. Effects of the availability of rewarding septal and hypothalamic stimulation on bar pressing for food under conditions of deprivation. J Comp Physiol Psychol. 1965;60:158–61. doi: 10.1037/h0022365. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Wonnacott S. Evidence for functional activity of up-regulated nicotine binding sites in rat striatal synaptosomes. J Neurochem. 1990;55:2105–10. doi: 10.1111/j.1471-4159.1990.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Baldwin P, De Biasi M, Montague PR. BOLD responses to negative reward prediction errors in human habenula. Front Hum Neurosci. 2010;4:36. doi: 10.3389/fnhum.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004a;47:401–7. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–69. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003a;63:1059–66. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004b;24:10035–39. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci. 2003b;23:6255–63. doi: 10.1523/JNEUROSCI.23-15-06255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–18. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–71. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Scheibel AB. The thalamus and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci. 1997;9:342–53. doi: 10.1176/jnp.9.3.342. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, et al. Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–83. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Rawal N, Mameli-Engvall M, Nomikos GG, Svensson TH. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int J Neuropsychopharmacol. 2003;6:1–11. doi: 10.1017/S1461145702003188. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007a;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007b;30:259–88. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–99. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–16. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. The alpha2 adrenergic receptor antagonist idazoxan, but not the serotonin-2A receptor antagonist M100907, partially attenuated reward deficits associated with nicotine, but not amphetamine, withdrawal in rats. Eur Neuropsychopharmacol. 2010;20:731–46. doi: 10.1016/j.euroneuro.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr Bull. 2006;32:417–21. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, et al. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–45. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–79. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. A unique role for striatal serotonergic systems in the withdrawal from adolescent nicotine administration. Neurotoxicol Teratol. 2007;29:10–16. doi: 10.1016/j.ntt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Bühler M, Klein S, Zimmermann U, Mann K, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2005;184:1–12. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990a;55:1734–40. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Identification of the opioid receptor types mediating beta-endorphin-induced alterations in dopamine release in the nucleus accumbens. Eur J Pharmacol. 1990b;190:177–84. doi: 10.1016/0014-2999(90)94124-g. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–50. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G. Food versus intracranial self-stimulation reinforcement in food-deprived rats. J Comp Physiol Psychol. 1965;60:153–57. doi: 10.1037/h0022367. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–15. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Corbett D. Regional neuroleptic microinjections indicate a role for nucleus accumbens in lateral hypothalamic self-stimulation reward. Brain Res. 1989;477:126–43. doi: 10.1016/0006-8993(89)91400-5. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–71. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12:467–73. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J Comp Physiol Psychol. 1981;95:781–91. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol. 1996;314:281–84. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Tang J, Dani JA. Dopamine enables in vivo synaptic plasticity associated with the addictive drug nicotine. Neuron. 2009;63:673–82. doi: 10.1016/j.neuron.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–44. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–42. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL, editor. Animal Intelligence. New York: Macmillan; 1911. [Google Scholar]
- Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2009;108:183–94. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–14. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–42. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–72. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Domburg PHMF, ten Donkelaar HJ. The human substantia nigra and ventral tegmental area. Adv Anat Embryol Cell Biol. 1991;121:1–132. [PubMed] [Google Scholar]
- Vastag B. Addiction research. Ibogaine therapy: a ‘vast, uncontrolled experiment’. Science. 2005;308:345–46. doi: 10.1126/science.308.5720.345. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Walaas I, Fonnum F. Biochemical evidence for gamma-aminobutyrate containing fibres from the nucleus accumbens to the substantia nigra and ventral tegmental area in the rat. Neuroscience. 1980;5:63–72. doi: 10.1016/0306-4522(80)90071-8. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184:339–44. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–64. [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–18. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med. 1989;19:981–85. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Davies AR, Marks MJ, Blagbrough IS, Potter BV, et al. An autoradiographic study of the distribution of binding sites for the novel alpha7-selective nicotinic radioligand [3H]-methyllycaconitine in the mouse brain. Eur J Neurosci. 1999;11:2689–96. doi: 10.1046/j.1460-9568.1999.00685.x. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford, UK: Oxford Univ. Press; 2004. pp. 361–413. [Google Scholar]
- Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav Brain Res. 2010;216:463–65. doi: 10.1016/j.bbr.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–47. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B. 2006;361:1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci. 2009;32:517–24. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191:1273–75. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–85. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–82. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009a;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009b;29:4035–43. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–29. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson C, Dani JA. Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist. 2003;9:23–36. doi: 10.1177/1073858402239588. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]