Abstract
Background
Aspirin reduces myocardial infarction but increases gastrointestinal bleeding. Proton pump inhibitors (PPIs) may reduce upper gastrointestinal bleed. We estimate the cost-utility of aspirin treatment with or without PPI for coronary heart disease (CHD) prevention among men at different risks for CHD and gastrointestinal bleed.
Methods
We updated a Markov model to compare costs and outcomes of low-dose aspirin+PPI (omeprazole 20-mg daily), low-dose aspirin alone, or no treatment for CHD prevention. We performed lifetime analyses in men with different risks for cardiovascular events and gastrointestinal bleed. Aspirin reduced nonfatal myocardial infarction by 30%, increased total stroke by 6%, and increased gastrointestinal bleed risk 2-fold. Adding PPI reduced upper gastrointestinal bleed by 80%. Annual aspirin cost was $13.99; generic PPI was $200.
Results
In 45-year-old men with 10-year CHD risk of 10% and 0.8/1,000 annual gastrointestinal bleed risk, aspirin ($17,571 and 18.67 quality-adjusted life years [QALYs]) was more effective and less costly than no treatment ($18,483 and 18.44 QALYs). Compared with aspirin alone, aspirin+PPI ($21,037 and 18.68 QALYs) had an incremental cost/QALY of $447,077. Results were similar in 55- and 65-year-old men. The incremental cost/QALY of adding PPI was less than $50,000/QALY at annual gastrointestinal bleed probabilities greater than 4–6/1,000.
Conclusion
Aspirin for CHD prevention is less costly and more effective than no treatment in men over 45 with greater than 10-year, 10% CHD risks. Adding PPI is not cost-effective for men with average gastrointestinal bleed risk but may be cost-effective for selected men at increased risk for gastrointestinal bleed.
INTRODUCTION
The value of aspirin for primary prevention in men depends on trade-offs between its ability to reduce nonfatal myocardial infarction and its potential to increase risk of hemorrhagic stroke and extracranial (primarily gastrointestinal) bleeding.1 Although the increased risk of hemorrhagic stroke cannot be mitigated, risk of upper gastrointestinal bleeding can be reduced by acid suppressive therapy.2,3 A recent model suggests that the addition of generic, low-cost omeprazole in patients aged 65 years and older using aspirin for secondary prevention may be cost-effective due to its impact on reducing serious gastrointestinal adverse events.4
Our previous modeling has found aspirin to be cost-effective for primary prevention in men with increased coronary heart disease (CHD) risk but used relatively crude measures of gastrointestinal bleed.5 Men were assumed to have a fixed excess rate of gastrointestinal bleed with aspirin, with estimates of excess risk drawn from randomized trials of aspirin that enrolled somewhat selected populations. Recent data from large observational cohort studies have found higher rates of gastrointestinal bleed and larger relative risks (approximately 2.0) associated with aspirin use.6 In addition, recent meta-analysis from the Antithrombotic Trialists’ Collaboration suggested that gastrointestinal bleed risk from aspirin increased with other CHD risk factors.7 Previous models also have not modeled the risk of fatal gastrointestinal bleed. Although fatal bleeds from aspirin are rare,7 such an outcome should be considered in order to provide conservative estimates of aspirin’s net effect. Finally, to our knowledge, no primary prevention models have examined the effectiveness and cost-effectiveness of routine use of gastrointestinal prophylactic agents to mitigate aspirin-related risk of gastrointestinal bleed.
We sought to examine the cost-effectiveness of aspirin with and without the addition of routine use of proton pump inhibitors (PPIs) for primary CHD prevention in men with a range of underlying CHD and upper gastrointestinal bleed risks.
METHODS
Overview and Model Structure
We updated a previously developed a Markov model, programmed in Microsoft Excel5,8 to examine the costs and outcomes of primary prevention treatment with aspirin alone or aspirin plus PPI (aspirin+PPI) for men (see Appendix Figure A-1).
In the model, men begin treatment in the healthy state and transition through the model annually. In each cycle, men remain in the healthy state; progress to initial, nonfatal cardiovascular events such as angina, myocardial infarction, or stroke; have upper gastrointestinal bleed; or die. Men who have cardiovascular events are assumed to stay in the subacute state for the remainder of that cycle then enter a post-event health state where they receive optimal secondary prevention. Because we are interested in primary prevention, we did not simulate or examine the additional treatment course of patients after primary, nonfatal events. Instead, we assigned them an increased risk for mortality, increased costs, and decreased utilities, using data from published literature on the average experience of patients after an initial event.
Men who have gastrointestinal bleed discontinue aspirin use but do not receive PPIs if they are not in the aspirin+PPI arm. They then enter a post-event health state where they progress though the model as healthy men. However, these men are at greater risk for subsequent gastrointestinal bleed.6
Men were followed for the remainder of their lifetimes to estimate costs, cardiovascular and adverse events, life-years, and quality-adjusted life-years (QALYs). Efficacy data were taken from published literature. Resource use and costs were drawn from published literature and standard United States (US) costing sources and updated to 2009 dollars. The analysis was performed from a third-party payer perspective. All costs and outcomes were discounted at 3% per annum.
Patient Population
Men in the base-case analysis were assumed to be healthy, middle-aged men with starting age of 45 years, no history of CHD events, and 10%, 10-year CHD risk (e.g., systolic blood pressure 120, total cholesterol 220, high-density lipoprotein 30, nonsmoker, and no diabetes).
Comparators
Men in the aspirin arm received 81 mg of generic aspirin daily. Patients taking aspirin+PPI were treated with combination of aspirin as 81mg daily and generic omeprazole 20 mg daily. We did not consider use of other CHD preventive strategies (smoking cessation, hypertension treatment, or statin use) in this analysis, nor did we examine the effect of aspirin in patients with diabetes or previous cardiovascular events. Treatment efficacy was based on actual effects observed in clinical trials; we did not model effects of different levels of adherence.
Model Parameters
Baseline Event Probabilities
Baseline risks of initial CHD events (myocardial infarction, angina, and CHD death) and stroke were drawn from Framingham risk equations, using hypothetical scenarios of nonsmoking adults without diabetes and with different sets of other risk factors.9 We examined scenarios for men aged 45, 55, and 65 years with up to six risk levels: 2.5%,5.0%, 7.5%, 10.0%, 15.0%, and 25.0%. Assuming an exponential distribution, these 10-year risks were translated into annual, event-related transition probabilities. These probabilities were allowed to change annually to reflect increasing CHD and stroke risk over the time horizon of the analysis.
Age-dependent, noncardiovascular mortality was estimated from National Vital Statistics life tables.10 Probabilities were adjusted as the cohort aged over the analysis time horizon. Baseline risk of upper gastrointestinal bleed (not taking aspirin) was obtained from Hernandez-Diaz et al.6 Annual risks were estimated as 0.0008, 0.0024, 0.0024, and 0.0036 for men aged 45, 55, 65, and over 65 years, respectively.6 In the model, a man starts with the baseline gastrointestinal bleed risk for his age and his gastrointestinal bleed risk increases as he ages.
Treatment Efficacy
The relative risks as drawn from published meta-analyses and clinical trials are presented in Table 1. Aspirin was assumed to reduce men’s risk of nonfatal myocardial infarction by 30%, CHD death by 13%, and to have no effect on angina. It increased the combined risk of stroke (ischemic and hemorrhagic considered together) by 6%.11 More recent gender-specific analyses have found similar estimates for men.12 We did not model the effect of aspirin on stroke-related mortality or on initial use of revascularization procedures. The changes in each parameter were tested extensively in sensitivity analyses.
Table 1.
Clinical Parameters, Values, and Plausible Ranges
| Parameter | Base-Case Value (Range) |
Source |
|---|---|---|
| Relative Risk of Events | ||
| Aspirin Alone | ||
| Angina | 1.00 (95% CI: 0.80, 1.20) | Assumption |
| Stroke | 1.06 (95% CI: 0.91, 1.24) | 11 |
| Myocardial infarction | 0.70 (95% CI: 0.62, 0.79) | 11 |
| CHD death, males | 0.87 (95% CI: 0.70, 1.09) | 16 |
| Gastrointestinal bleed (no history of gastrointestinal bleed) | 2.00 | 6 |
| Gastrointestinal bleed (with history of gastrointestinal bleed) | 10.00 | 6 |
| Aspirin+PPI | ||
| Gastrointestinal bleed | 0.20 (95% CI: 0.10, 0.90) | 13 |
| Mortality | ||
| Proportion of hemorrhage strokes that are fatal | 0.33 (95% CI: 0.10, 0.50) | Expert clinical opinion |
| Death due to gastrointestinal bleed | 0.001 (range: 0.00001, 0.1) | Expert clinical opinion |
| Increase in Risk of Mortality | ||
| After Initial CHD Event | ||
| After myocardial infarction | 3.7 (95% CI: 3.0, 4.7) | 14 |
| After angina | 3.0 (95% CI: 2.1, 4.2) | 14 |
| After stroke | 2.3 (95% CI: 1.0, 4.6) | 15 |
| Reduction of death due to aspirin therapy after a CV event | 0.85 (95% CI: 0.80, 0.90) | 17 |
CHD = coronary heart disease; CI = confidence interval; CV = cardiovascular; PPI = proton pump inhibitor.
PPI’s primary benefit was assumed to be an 80% reduction in upper gastrointestinal bleed (i.e., occurrence of hospitalization due to gastrointestinal bleed) (relative risk [RR]: 0.20; range: 0.10, 0.90), based mainly on one small randomized controlled trial in patients at high-risk for gastrointestinal bleed.3,13 The efficacy of combination use of aspirin+PPI was assumed to be independent: PPIs added no additional benefit and did not reduce the benefit of aspirin for avoiding CHD events. We did not assume any direct adverse effects from PPIs, nor did we include benefits of PPIs for treating symptomatic conditions such as dyspepsia or reflux; our analysis applied to men without symptomatic upper gastrointestinal conditions, thus we assumed that patients would adopt such treatments equally in both groups if they became symptomatic.
All men who survived an initial CHD event received optimal secondary prevention including aspirin, or alternate antiplatelet agents if they were not able to tolerate aspirin. The effect of optimal treatment after initial events was the same between groups in the model.
Adverse Effects
Aspirin increased stroke risk by 6%.11 Increase in gastrointestinal bleed risk in aspirin users with and without history of gastrointestinal bleed was 10.0 and 2.0, respectively.6 Although some data suggested no increased risk of fatal gastrointestinal bleed with low-dose aspirin,7 we conservatively assumed a risk of gastrointestinal bleed mortality of 1 out of 1,000 patients, and we tested a range of values in sensitivity analyses. Men who had adverse effects were assumed to stop the offending agent and were not placed on alternate agents for primary prevention.
Mortality
The effect of optimal secondary prevention on all-cause mortality was estimated as RRs after initial cardiovascular events (Table 1), drawn from population-based meta-analyses and from mortality in US life tables.14,15
Costs
Costs are presented in Table 2 and include outpatient physician visits, events, and drug costs. Costs were similar to those in our previous analyses5,8 and updated to 2009 US dollars using the Medical Consumer Price Index.18 Additional cost details are presented in the Appendix.
Table 2.
Cost and Utility Parameters, Values, and Plausible Ranges
| Parameter | Base-Case Value (Range) |
|---|---|
| Cost Data (Annual) | |
| Aspirin | $13.99 |
| Generic PPI | $199.79 |
| Outpatient physician visit | $62.76 |
| Health-State Costs (Annual) | |
| Healthy | $62.76 |
| Gastrointestinal bleed | $13,342 |
| Post gastrointestinal bleed | $62.76 |
| Angina | $13,372 |
| Post angina | $5,993 |
| Stroke | $21,706 |
| Post stroke | $1,835 |
| Myocardial infarction | $32,625 |
| Post myocardial infarction | $3,590 |
| Utility Data | |
| Healthy | 1.000 |
| Gastrointestinal bleed | 0.94 (95% CI: 0.880, 1.000) |
| Post gastrointestinal bleed | 1.000 |
| Dyspepsia | 0.996 (95% CI: 0.997, 1.000) |
| Angina | 0.929 (95% CI: 0.923, 1.000) |
| Post angina | 0.997 (95% CI: 0.997, 1.000) |
| Stroke | 0.610 (95% CI: 0.480, 0.830) |
| Post stroke | 0.830 |
| Myocardial infarction | 0.870 (95% CI: 0.820, 0.920) |
| Post myocardial infarction | 0.910 (95% CI: 0.860, 0.960) |
| Healthy | 1.000 |
CI = confidence interval; PPI = proton pump inhibitor.
Utilities
The utilities for the model were drawn from the literature.5,8 In most cases, they were estimated using time trade-off techniques in original studies (see Table 2 and Appendix Table A-1). Where no data existed, we made estimates and examined a wide range of values in sensitivity analyses.
Outcome Measures
The model estimated the following outcomes: number of myocardial infarctions, strokes, and gastrointestinal bleed events; costs; total costs; patient survival (life-years); QALYs; and the incremental cost per QALY gained.
We examined the effect of treatment on different 10-year CHD risk levels (2.5%, 5.0%, 7.5%, 15.0%, and 25.0%), different model time horizons (5 year, 10 year, or lifetime), and different starting ages (45, 55, and 65 years). Younger (under 45 years) and older (over 75 years) starting ages were not examined because sufficient treatment efficacy data were not available.
Sensitivity Analyses
To test the robustness of model assumptions and specific parameters, we examined the effect of changing several parameters in one-way sensitivity analyses. Parameters analyzed included the relative risk of myocardial infarction, stroke, and CHD death for patients on aspirin; increase in baseline risk of gastrointestinal bleed; relative risk of gastrointestinal bleed, given treatment with aspirin, PPI, or aspirin+PPI; relative risk of cardiovascular death with optimal secondary prevention; increase in mortality due to hemorrhagic stroke and gastrointestinal bleed; costs of events and treatment; and utility weights for each health state. The effect of varying individual parameters was examined using plausible ranges of values (Table 1) from the literature, where 95% confidence intervals (CIs) were available, or by varying the estimates by 20% in each direction.
In addition to one-way sensitivity analyses, we performed probabilistic sensitivity analysis (second-order Monte Carlo simulation). The parameters varied in these analyses were similar to those in one-way sensitivity analysis. We assumed that parameter estimates followed a gamma distribution for all relative risks of events, increases in gastrointestinal bleed, and increases in mortality, health-state costs, and drug costs. A beta distribution was assumed for all health-state utilities. Analyses were run 10,000 times in order to capture stability in results. Scatter plots were developed to represent uncertainty, and cost-effectiveness acceptability curves were created.
RESULTS
Base Case
For 45-year-old men with 10-year, 10% CHD risk and annual gastrointestinal bleed risk of 8/10,000, men on aspirin gained more QALYs in their remaining lifetime than men on no treatment (18.67 vs. 18.44). Treating with aspirin also was less costly ($17,571) over a man’s remaining lifetime than no treatment ($18,483). Men on aspirin+PPI gained more QALYs (18.68) but incurred higher costs ($21,037) than men assigned to aspirin alone. As a result, the incremental cost per QALY with aspirin+PPI compared with aspirin alone was $447,077, suggesting that addition of PPI prophylaxis was not cost-effective.
Aspirin remained more effective and less costly than no treatment at 5% 10-year CHD risk and above (Table 3). However, across CHD risks levels, aspirin+PPI remained expensive compared with aspirin alone. The patterns were similar for 55- and 65-year-old men with base-case gastrointestinal bleed risk of 24/10,000 (Table 4). Shorter time horizons (5, 10, and 20 years) had no appreciable effect on the cost-effectiveness of aspirin alone and led to even higher costs per QALY gained with aspirin+PPI.
Table 3.
Effect of 10-Year CHD Risk on Lifetime Cost-Utility Ratio for 45-Year-Old Men
| Low (2.5%) Risk |
Low- Moderate (5.0%) Risk |
Moderate (7.5%) Risk |
High- Moderate (10%) Risk |
High (15%) Risk |
Very High (25%) Risk |
|
|---|---|---|---|---|---|---|
| Results for 45-Year-Old Men | ||||||
| Aspirin vs. no treatment | $5,714 | More effective, less costly | More effective, less costly | More effective, less costly | More effective, less costly | More effective, less costly |
| Aspirin+PPI vs. aspirin alone | $782,929 | $627,863 | $502,362 | $447,077 | $379,096 | $343,448 |
| Results for 65-Year-Old Men | ||||||
| Aspirin vs. no treatment | — | $11,039 | $3,634 | $50 | More effective, less costly | More effective, less costly |
| Aspirin+PPI vs. aspirin alone | — | $360,273 | $320,421 | $276,984 | $236,420 | $180,793 |
CHD = coronary heart disease; PPI = proton pump inhibitor
Table 4.
Effect of Time Horizon and Age on the Cost-effectiveness of Aspirin and Aspirin+PPI in Men With 10-Year, 10%, CHD
| Time Horizon | ||||||
|---|---|---|---|---|---|---|
| Age (Years) | 5 Years | 10 Years | 25 Years | Lifetime | ||
| Aspirin vs. No Treatment | ||||||
| 45 | More effective, less costly | More effective, less costly | More effective, less costly | More effective, less costly | ||
| 55 | More effective, less costly | More effective, less costly | More effective, less costly | More effective, less costly | ||
| 65 | $1,120 | More effective, less costly | More effective, less costly | $50 | ||
| Aspirin+PPI vs. Aspirin Alone | ||||||
| 45 | $2,513,270 | $1,974,339 | $1,010,770 | $447,077 | ||
| 55a | $702,561 | $531,451 | $250,420 | $175,641 | ||
| 65 | $712,499 | $537,048 | $290,968 | $276,984 | ||
CHD = coronary heart disease; PPI = proton pump inhibitor; QALY = quality-adjusted life year.
Incremental cost per QALY from 55 to 65 years of age reduces, although the risks of myocardial infarction and CHD deaths increase, because the cost of the PPI outweighs the benefit of including the PPI.
Effect of Gastrointestinal Bleeding Risk
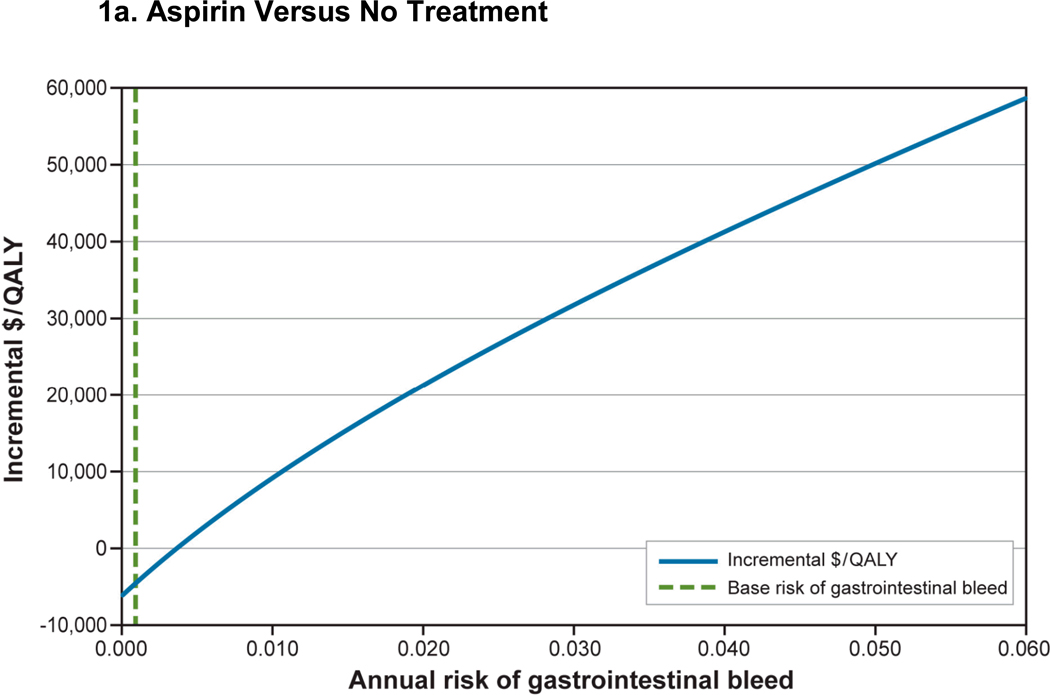
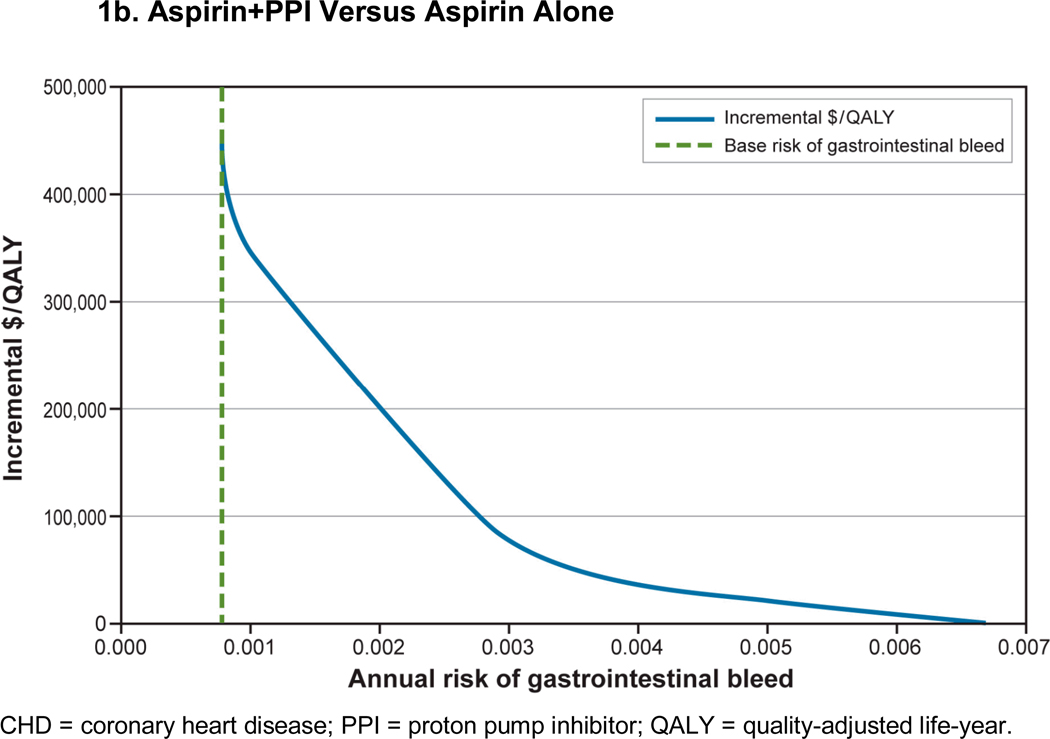
We examined the effects of men’s baseline risk of gastrointestinal bleed on the incremental cost per QALY (Figure 1). In 45-year-old men with 10%, 10-year CHD risk, aspirin compared with no treatment remained cost-saving and cost-effective until a man’s risk of gastrointestinal bleed was greater than 5%. Although use of aspirin+PPI is expensive, the incremental cost per QALY of aspirin+PPI versus aspirin alone is less than $50,000 when annual gastrointestinal bleed risk is 4/1000 and cost-saving when gastrointestinal bleed risk is greater than 7/1,000.
Figure 1. Effect of Change in Baseline Gastrointestinal Bleed Risk in a 45-Year-Old Man With a 10-Year, 10% CHD Risk.
Figure 1a: Aspirin Versus No Treatment
Figure 1b: Aspirin+PPI Versus Aspirin Alone
CHD = coronary heart disease; PPI = proton pump inhibitor; QALY = quality-adjusted life-year.
In 55- and 65-year-old men, the incremental cost per QALY of aspirin+PPI was less than $50,000 when baseline gastrointestinal bleed risk increased as little as 2- to 3-fold above base case (Appendix Figures A-2 and A-3). Overall, as gastrointestinal bleed risk increased, the incremental cost per QALY of aspirin+PPI compared with aspirin alone was lower in younger men because they received cardiovascular benefits of aspirin over longer periods of time (their lifetime).
Sensitivity Analyses
One-Way Sensitivity Analysis
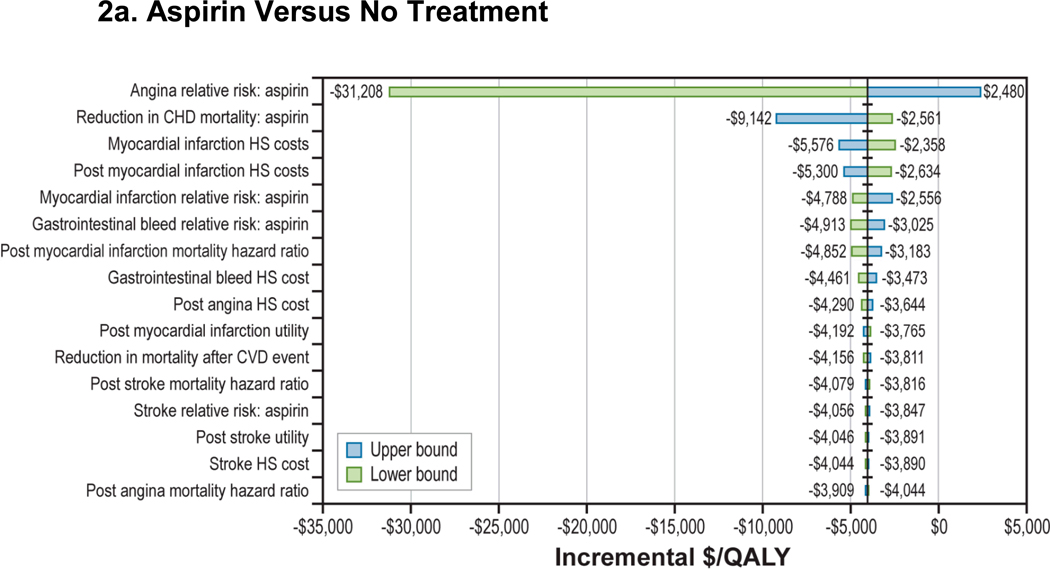
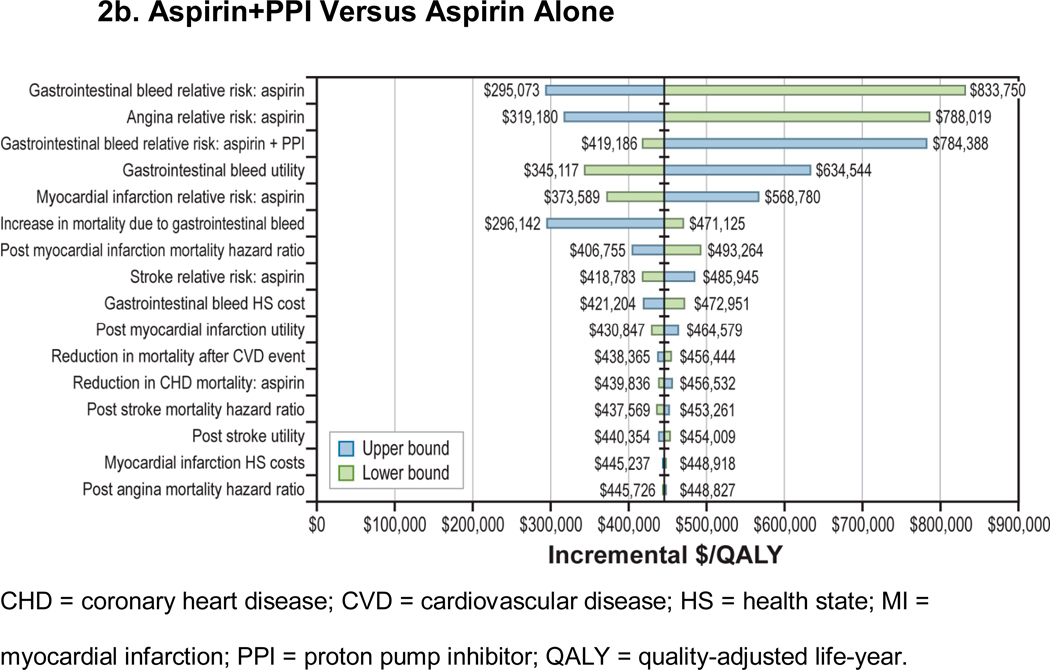
Sensitivity analyses (Figure 2) showed that the cost-effectiveness of aspirin compared with no treatment was robust to changes to all key parameters within their plausible ranges. In particular, aspirin’s effect on nonfatal myocardial infarction and CHD deaths had minimal impact on cost-effectiveness of aspirin compared with no treatment. The cost-effectiveness of aspirin+PPI compared with aspirin alone was sensitive to changes in gastrointestinal bleed-related parameters, such as aspirin and aspirin+PPI’s relative effect on gastrointestinal bleed and utility of gastrointestinal bleed (Figure 2b). However, within their plausible ranges, the incremental costs per QALY remained in the $300,000 to $800,000 range. Incremental cost per QALY also was insensitive to changes in risk of mortality due to gastrointestinal bleed until risk increased to more than 1% (Appendix Figure A-4). We found that lowering the annual cost of PPIs to under $50 (less than 20% of the base-case value) resulted in an incremental cost per QALY of less than $100,000 for aspirin+PPI compared with aspirin alone; in contrast, at higher PPI prices, aspirin was not cost-effective even for those at increased bleeding risk (Appendix Figure A-5). Two-way sensitivity analyses that used newer meta-analysis with gender-specific RRs of stroke (RR: 1.13; 95% CI: 0.96, 1.33) and MI (RR: 0.68; 95% CI: 0.54, 0.86) for aspirin showed that results remained unchanged (Appendix Table A-2).12
Figure 2. One-Way Sensitivity Analysis: 45-Year-Old Man With a 10-Year, 10% CHD Risk.
Figure 2a: Aspirin Versus No Treatment
Figure 2b: Aspirin+PPI Versus Aspirin Alone
CHD = coronary heart disease; CVD = cardiovascular disease; HS = health state; MI = myocardial infarction; PPI = proton pump inhibitor; QALY = quality-adjusted life-year.
Probabilistic Sensitivity Analysis
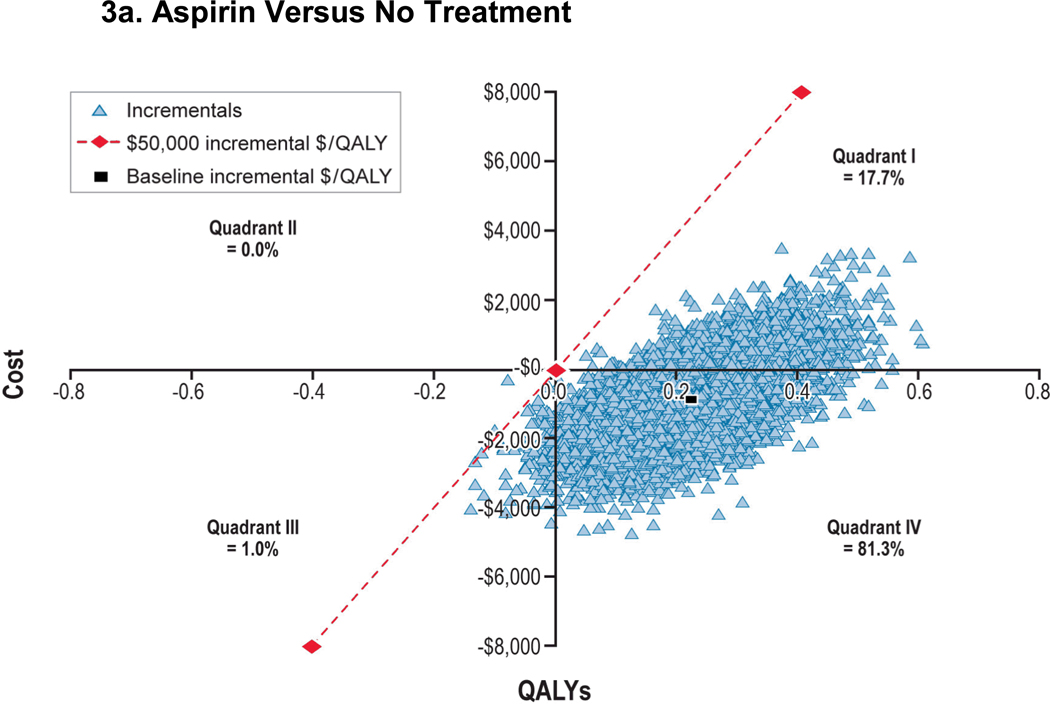
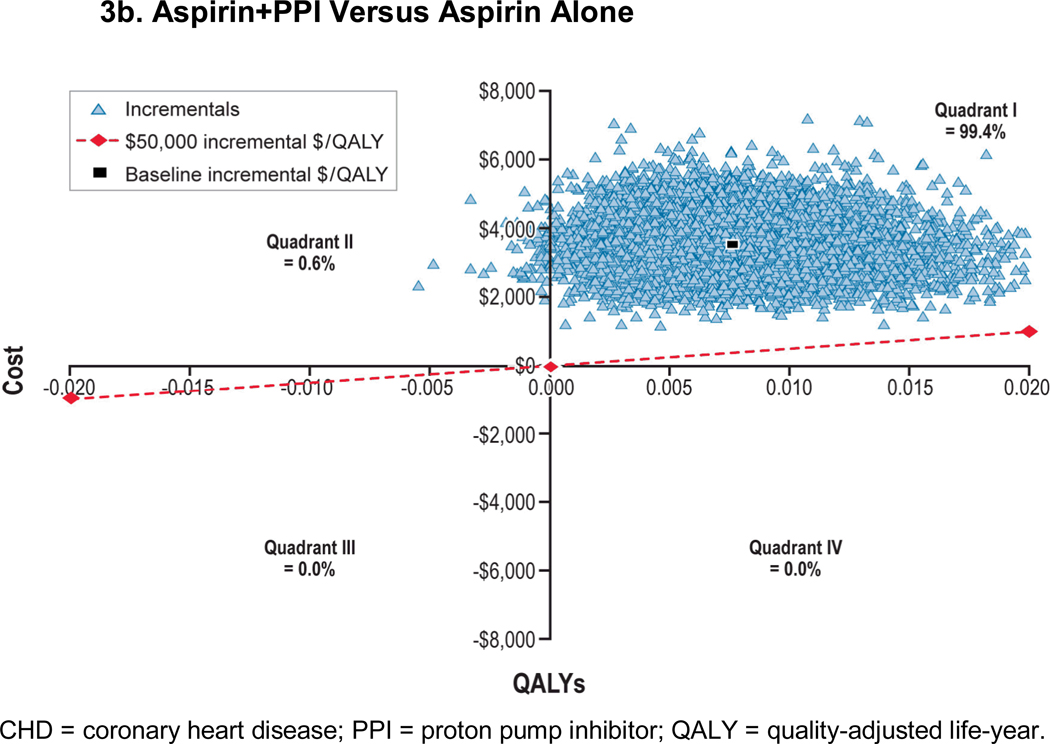
We performed probabilistic sensitivity analyses to examine the collective effect of parameter uncertainty (Figure 3). We observed incremental costs per QALY of aspirin versus no treatment of less than $50,000 in 99.8% and 97.4% of men aged 45 and 65 years, respectively. Compared with aspirin alone, incremental costs per QALY of aspirin+PPI versus aspirin alone was less than $50,000 for 0.0% and 0.5% of the time in 45- and 65-year-old men, respectively. However, incremental costs per QALY crossed all four quadrants. Scatter plots and cost-effectiveness acceptability curves for men aged 45 and 65 years with 10-year, 10% CHD risk are presented in Appendix Figures A-6 through A-8.
Figure 3. Probabilistic Sensitivity Analysis: 45-Year-Old Man With a 10-Year, 10% CHD Risk.
Figure 3a: Aspirin Versus No Treatment
Figure 3b: Aspirin+PPI Versus Aspirin Alone
CHD = coronary heart disease; PPI = proton pump inhibitor; QALY = quality-adjusted life-year.
DISCUSSION
Decisions about which men should receive low-dose aspirin for CHD prevention involves trade-offs between aspirin’s adverse effects and its beneficial effects. Some investigators have attempted to estimate the benefit/harm trade-off by determining relative risks of each event through meta-analyses and then informally “weighing up” likely benefits and disadvantages for patient populations with different CHD and gastrointestinal bleed risk profiles.7,11 Such informal processes, however, are difficult to perform because main outcomes of interest (stroke, myocardial infarction, and gastrointestinal bleed) each have different health effects.1 We and others have attempted to rigorously model effects of aspirin and have found aspirin to be cost-saving or cost-effective for men with moderate and greater CHD risk levels.5,19
Recent data, however, have suggested that gastrointestinal bleed risk may be higher than levels observed in clinical trials6 and that patients with elevated CHD risk may be at higher risk for gastrointestinal bleed.7 Some decision makers have advocated using PPI therapy concurrently with aspirin to reduce the risk of bleeding, after considering clinical trial evidence from patients at high risk for gastrointestinal bleed3 and decision-analytic modeling studies suggesting PPI use is beneficial in secondary CHD prevention.4 In this updated analysis, we attempted to address some of these concerns and better estimate the net effect of aspirin with or without PPI for patients at different levels of CHD and gastrointestinal bleed risk. In modeling an age-dependent gastrointestinal bleed risk, we found that aspirin remained cost-saving compared with no treatment across a wide range of CHD and gastrointestinal bleed risk levels for men aged 45 to 65 considering initiation of aspirin. We also found that adding generic PPI therapy to aspirin for all men was not cost-effective in most cases because risk of gastrointestinal bleed was not large enough to warrant routine prophylaxis. However, under favorable assumptions about PPI efficacy and pricing, adding PPI when gastrointestinal bleed risk is 5/1,000 per year for men aged 45 (and slightly higher for older men), had a favorable cost-effectiveness ratio of $22,000 per QALY gained. This represents about a 4-fold increase in baseline bleeding risk. Assuming a branded cost of a PPI at $1,951 per year, a man’s gastrointestinal bleed risk would need to be 6.7/100 per year (over 10 times higher than our base case) in order for the addition of a PPI to be cost-effective in a man with the same CHD risk.
Our analysis highlights that some men who are at increased gastrointestinal bleeding risk may benefit from adding PPI when using low-dose aspirin for CHD risk reduction. Providers should assess the risk of gastrointestinal bleeding by considering the patients age, prior history of gastrointestinal bleeding, and use of other medications that increase bleeding risk (Table 5).6,20 For example, a 55-year-old man taking naproxen (a nonsteroidal anti-inflammatory drug) for arthritis and a selective serotonin reuptake inhibitor for depression would have a 4.8 per 1000 annual risk of bleeding and would be a reasonable candidate for PPI if those medications could not be discontinued.20
Table 5.
Estimated Baseline Risk per 1,000 Patient-Years of Gastrointestinal Bleeding in Men With No History of Ulcer
| Age (Years) | No NSAID or SSRIa |
SSRI Use, No NSAIDb |
NSAID Use, No SSRIa |
NSAID and SSRI Usec |
|---|---|---|---|---|
| Less than 60 | 0.8 | 1.9 | 3.2 | 4.8 |
| 60–69 | 2.4 | 5.8 | 9.6 | 14.4 |
| 70–79 | 3.6 | 8.6 | 14.4 | 21.6 |
| 80–89 | 6.0 | 14.4 | 24.0 | 36.0 |
NSAID = nonsteroidal anti-inflammatory drug; SSRI= selective serotonin reuptake inhibitor.
From Hernandez-Diaz et al.6
Odds ratio = 2.4.20
Odds ratio = 6.0.20
The risk in this table is for men with no ulcer-related bleeding and assumes that the relative risks approximate the odds ratios and that the relative risks are independent. For estimating gastrointestinal bleeding risk for patient with previous history of uncomplicated ulcer, multiply by 6; multiply by 10 for history of complicated ulcer.
Our findings were robust across a range of CHD risk levels and were not affected by factors such as a reduced relative benefit of aspirin on nonfatal CHD or an increased risk of fatal gastrointestinal bleed. In addition, results were insensitive to changes in aspirin risk of MI and stroke, as obtained from a gender-specific meta-analysis.12 However, if PPI’s actual gastrointestinal bleed risk reduction is much lower than the modeled effect or its pricing is much higher than $200 per year, the benefits of adding PPI to aspirin will be small or negligible, even for those at increased risk.
Other modeling studies have reached similar results when considering the effect of aspirin in men at different CHD risk levels. Specifically, Greving and colleagues developed a Markov model to evaluate the cost-effectiveness of aspirin for primary prevention in the Netherlands.19 They used a 10-year time horizon and drew baseline event probabilities and costs from Dutch data. Relative risk reductions were based on available meta-analyses and were similar to those used in our model. Utility of taking aspirin was assumed to be 0.999, and annual cost of aspirin was €97, to account for pharmacist and practitioner prescribing and dispensing costs. They found aspirin to be cost-effective (incremental cost per QALY < €20,000) for men 55 to 75 years old with 10-year CHD risk over 10%. However, the study found an incremental cost per QALY of greater than €100,000 for men younger than 55 years old with CHD risk of between 5% and 6%. Results were sensitive to aspirin cost assumptions and disutility associated with aspirin use. Lamotte and colleagues developed models of effect of aspirin for primary prevention and reached conclusions similar to our current and previous analyses.21 None of the previous models, however, have examined the cost-effectiveness of adding routine PPI plus aspirin compared with aspirin alone or no treatment for primary prevention. Our study has a number of limitations. Given the lack of trials of PPIs in aspirin users without prior history of ulcer bleeding, we estimated benefit of PPI therapy among aspirin users on the basis of a single, randomized controlled trial among high-risk users.3 This estimate is supported by a large body of observational data that informed the recommendation that PPIs be given to patients with high gastrointestinal bleed risk.2
We used data on upper gastrointestinal bleeding for our analysis; aspirin may increase the risk of lower gastrointestinal bleeding, as well as other extracranial bleeds, but we did not model these directly. However, aspirin remains cost-effective compared with no therapy, even if the annual risk of gastrointestinal bleeding is 5%, so not including other, less common sources of gastrointestinal bleeding is unlikely to change our results.
Although PPI therapy may increase the risk of various adverse clinical outcomes among long-term users, we did not include such effects in our model. Specifically, observational studies have demonstrated a modest but significantly increased risk for community-acquired pneumonia as well as enteric infections, particularly involving Clostridium difficile.22 In addition, high-dose, twice-daily PPI therapy has been proposed to increase risk of osteoporotic fractures. Existing data on these effects remain controversial, and robust data to model their risk are limited.2,23,24
CONCLUSIONS
This updated analysis supports the role of aspirin for primary prevention of CHD events in middle-aged men across a range of CHD and gastrointestinal bleeding risk levels. Increased risk of gastrointestinal bleeding does not reduce aspirin’s net benefit until gastrointestinal bleeding risk becomes quite high, such as the level seen in men with previous gastrointestinal bleeds. Adding PPI therapy does not appear to be cost-effective for those patients at low or average risk for gastrointestinal bleed but may be valuable for those with a gastrointestinal bleed risk over 4/1,000 per year. Further efforts to include gastrointestinal bleeding risk assessment when prescribing low-dose aspirin for CHD protection are warranted.
Supplementary Material
Footnotes
The funding for this study was provided by Bayer Inc.
Conflicts of Interest
Dr. Scheiman is Professor of Medicine in the Division of Gastroenterology at the University of Michigan. Dr. Fendrick is Professor of Internal Medicine and Health Management and Policy at the University of Michigan. Drs. Scheiman and Fendrick are paid consultants to RTI Health Solutions. Dr. Scheiman also is a consultant to AstraZeneca, Bayer Inc., Pozen, Inc., as well as other pharmaceutical companies with products not relevant to this work. Dr. Earnshaw and Ms. McDade are employees of RTI Health Solutions, an independent contract research organization that has received research funding for this and other studies from Bayer, Inc. and other pharmaceutical companies that market drugs to prevent cardiovascular events and other conditions. Dr. Pignone is Professor of Medicine and Division Chief for General Internal Medicine at the University of North Carolina at Chapel Hill. His effort was supported by the Foundation for Informed Medical Decision Making and a K05 Established Investigator Award from the National Cancer Institute.
Bayer, Inc. did not participate in the development of the model or in the collection, management, analysis, and interpretation of the data. The preparation and editing of the manuscript was performed solely by the authors. Bayer received a copy of the draft manuscript but had no role in decisions about submission and revision.
Contributor Information
Stephanie R. Earnshaw, RTI Health Solutions, Research Triangle Park, NC.
James Scheiman, University of Michigan, Ann Arbor, MI.
A. Mark Fendrick, University of Michigan, Ann Arbor, MI.
Cheryl McDade, RTI Health Solutions, Research Triangle Park, NC.
Michael Pignone, University of North Carolina at Chapel Hill, Chapel Hill, NC.
REFERENCES
- 1.US Preventive Services Task Force (USPSTF) Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Scheiman J, Abraham NS, et al. and the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118(18):1894–1909. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 3.Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346(26):2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 4.Saini SD, Schoenfeld P, Fendrick AM, Scheiman J. Cost-effectiveness of proton pump inhibitor cotherapy in patients taking long-term, low-dose aspirin for secondary cardiovascular prevention. Arch Intern Med. 2008;168(15):1684–1690. 1691. doi: 10.1001/archinte.168.15.1684. [DOI] [PubMed] [Google Scholar]
- 5.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med. 2006;144(5):326–336. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Diaz S, Garcia Rodriquez LA. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22. doi: 10.1186/1741-7015-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antithrombotic Trialists’ (ATT) Collaboration. Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pignone M, Earnshaw S, Pletcher MJ, et al. Aspirin for the primary prevention of cardiovascular disease in women. Arch Intern Med. 2007;167:290–295. doi: 10.1001/archinte.167.3.290. [DOI] [PubMed] [Google Scholar]
- 9.Anderson KM, Odell PM, Wilson PWF, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121((1)):293–298. doi: 10.1016/0002-8703(91)90861-b. (Part 2) [DOI] [PubMed] [Google Scholar]
- 10.Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56(10) [PubMed] [Google Scholar]
- 11.Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart. 2001;85:265–271. doi: 10.1136/heart.85.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials [Erratum in: JAMA. 2006;295(17):2002] JAMA. 2006;295(3):306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 13.Lanas A, Scheiman J. Low-dose aspirin and upper gastrointestinal damage: epidemiology, prevention and treatment. Curr Med Res Opin. 2007;23(1):163–173. doi: 10.1185/030079907X162656. [DOI] [PubMed] [Google Scholar]
- 14.Lampe FC, Whincup PH, Wannamethee SG, Shaper AG, Walker M, Ebrahim S. The natural history of prevalent ischaemic heart disease in middle-aged men. Eur Heart J. 2000;21:1052–1062. doi: 10.1053/euhj.1999.1866. [DOI] [PubMed] [Google Scholar]
- 15.Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 16.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;136:161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 17.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke. JAMA. 1998;280:1930–1935. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 18.US Dept of Labor, US Bureau of Labor Statistics. US city average, not seasonally adjusted medical care. [Accessed April 10, 2009]; Available at: http://data.bls.gov/PDQ/outside.jsp?survey=cu.
- 19.Greving JP, Buskens E, Koffijberg H, Algra A. Cost-effectiveness of aspirin treatment in the primary prevention of cardiovascular disease events in subgroups based on age, gender, and varying cardiovascular risk. Circulation. 2008;117(22):2875–2883. doi: 10.1161/CIRCULATIONAHA.107.735340. [DOI] [PubMed] [Google Scholar]
- 20.Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27(1):31–40. doi: 10.1111/j.1365-2036.2007.03541.x. [DOI] [PubMed] [Google Scholar]
- 21.Lamotte M, Annemans L, Evers T, Kubin M. A multi-country economic evaluation of low-dose aspirin in the primary prevention of cardiovascular disease. Pharmacoeconomics. 2006;59(8):807–815. doi: 10.2165/00019053-200624020-00005. [DOI] [PubMed] [Google Scholar]
- 22.Pant C, Madonia P, Minocha A. Does PPI therapy predispose to Clostridium difficile infection? Nat Rev Gastroenterol Hepatol. 2009;6(9):555–557. doi: 10.1038/nrgastro.2009.128. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 24.Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138(3):896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.