Abstract
Immunity and inflammation are key elements of the pathobiology of stroke, a devastating illness second only to cardiac ischemia as a cause of death worldwide. While the immune system participates in the brain damage produced by ischemia, the damaged brain, in turn, exerts a powerful immunosuppressive effect that promotes fatal intercurrent infections and threatens the survival of stroke patients. Inflammatory signaling is instrumental in all stages of the ischemic cascade, from the early damaging events triggered by arterial occlusion, to the late regenerative processes underlying post-ischemic tissue repair. Recent developments have revealed that stroke, like multiple sclerosis, engages both innate and adaptive immunity. But, unlike multiple sclerosis, adaptive immunity triggered by newly exposed brain antigens does not have an impact on the acute phase of the damage. Nevertheless, modulation of adaptive immunity exerts a remarkable protective effect on the ischemic brain and offers the prospect of new stroke therapies. However, immunomodulation is not devoid of deleterious side effects, and gaining a better understanding of the reciprocal interaction between the immune system and the ischemic brain is essential to harness the full therapeutic potential of the immunology of stroke.
Introduction
Inflammation has long been know to affect the brain after stroke, and cells of the immune system, such as neutrophils and macrophages, have traditionally been used by neuropathologists and forensic pathologists to determine the approximate age of cerebrovascular lesions1. Commonly thought to merely be a reaction to tissue damage, inflammation has been increasingly recognized as a key contributor to the pathophysiology of cerebrovascular diseases, especially stroke caused by arterial occlusion or ischemic stroke2. Recent evidence suggests that elements of the immune system are intimately involved in all stages of ischemic cascade (Box 1), from the acute intravascular events triggered by the interruption of the blood supply, to the parenchymal processes leading to brain damage and to the ensuing tissue repair. In turn, the ischemic brain, through the autonomic nervous system, exerts a potent suppressive effect on lymphoid organs, which promotes intercurrent infections, a major determinant of stroke morbidity and mortality3,4. Therefore, the immune system is closely related to critical events determining the fate of the ischemic brain and the survival of stroke patients. Like in multiple sclerosis (MS), the classical inflammatory disease of the central nervous system (CNS), elements of innate and adaptive immunity are engaged in the post-ischemic brain5. Thus, molecular cues generated by cerebral ischemia activate components of innate immunity, promote inflammatory signaling and contribute to tissue damage. At the same time, these processes stimulate a potentially damaging adaptive immune response directed at antigens previously sequestered behind the blood-brain barrier (BBB). These recent developments warrant a re-evaluation of the contribution of inflammation and immunity to stroke pathophysiology. In this brief review, we will focus on the involvement of innate and adaptive immunity in ischemic brain injury and assess their impact on tissue damage and repair. Furthermore, we will examine the evidence for an adaptive cytotoxic response against newly exposed brain antigens and assess their role in the acute and chronic phase of the injury. Finally, we will evaluate the therapeutic opportunities afforded by modulation of the immune system and their potential pitfalls.
Box 1: From ischemia to infarction: The ischemic cascade.
The brain is critically dependent on the continuous delivery of oxygen and glucose through blood flow, and interruption of the cerebral blood supply leads to irretrievable brain damage2. Ischemic damage results from a cascade of cellular and molecular events triggered by sudden lack of blood flow and subsequent reperfusion of the ischemic territory. Neurons are more vulnerable than glia and vascular cells, and when exposed to hypoxia-ischemia quickly become dysfunctional and die108. In ischemia produced by occlusion of the middle cerebral artery, the most common type of stroke, the damage is more rapid and severe in the center of the ischemic territory (ischemic core), where flow is lowest2. At the periphery of the ischemic region, the so called ischemic penumbra, neuronal damage develops more slowly because blood flow arising from adjacent vascular territories (collateral flow) keeps cerebral perfusion above the threshold for immediate cell death2. In the ischemic core the major mechanism of cell death is energy failure. Without oxygen and glucose neurons cannot generate the ATP needed to fuel the ionic pumps that maintain the ionic gradient across the neuronal membrane, mainly the Na+/K+ ATPase108. Consequently, massive Na+ and Ca2+ cytoplasmic accumulation leads to swelling and degeneration of the organelles, loss of membrane integrity and dissolution of the cell (necrotic cell death)108. In the ischemic penumbra, the flow reduction is not sufficient to cause energy failure, and neurons remain viable for a prolonged period of time after the insult. However, neurons are stressed and critically vulnerable to pathogenic events that may tip over their fragile metabolic balance2. Excessive extracellular accumulation of glutamate is a major factor contributing to the demise of the ischemic penumbra. The resulting overactivation of the NMDA subtype of glutamate receptors leads to cytoplasmic accumulation or Ca2+, which activates Ca2+ dependent enzymes, including the proteases calpain and caspase, and enzymes producing nitric oxide, free radicals and arachidonic acid metabolites108. These events lead to necrosis or programmed cell death depending on the intensity of the insult and the metabolic state of the neurons2,108. Injured and dying cells play a key role in post-ischemic inflammation because they release “danger signals” which activate the immune system (see text).
The mechanisms of ischemic stroke have been studied using in vitro and in vivo models of cerebral ischemia2,108. These models do not recapitulate all the features of human stroke and may introduce important confounders, such as anesthesia and surgical trauma. Furthermore, differences in vascularization and immune responses in the human brain (Box 2) may alter the initiation and evolution of the tissue damage. However, the core features of stroke pathophysiology are comparable between animals and humans and animal models of cerebral ischemia remain a mainstay of preclinical stroke research109.
Inflammatory signaling in the early post-ischemic period
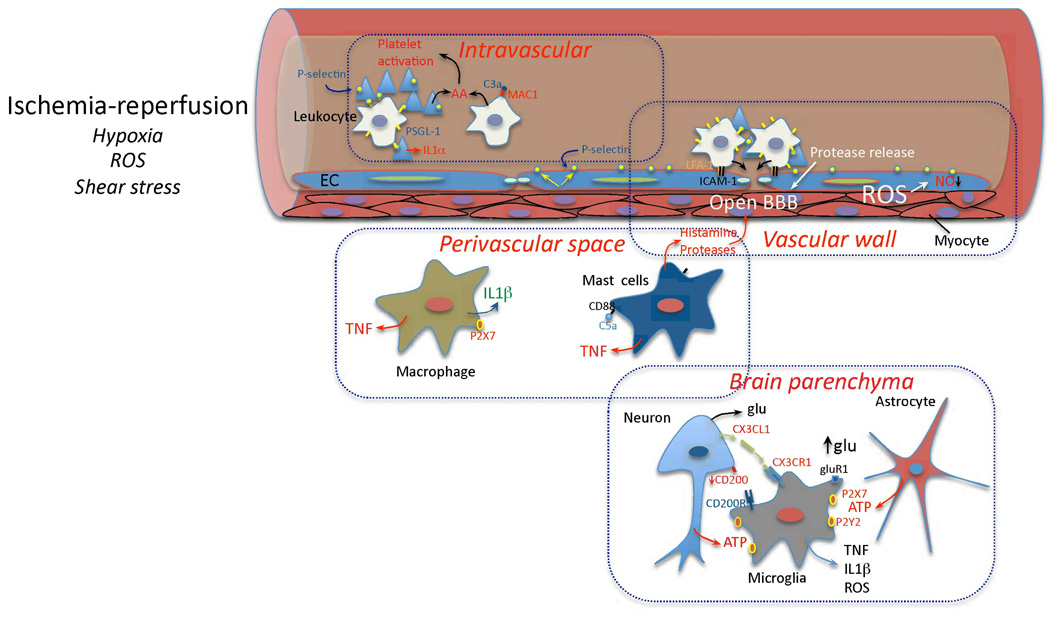
Post-ischemic inflammation is characterized by an orderly sequence of events involving the brain, its vessels, the circulating blood, and lymphoid organs. Inflammation is an integral part of the cascade of events triggered by ischemia and reperfusion (I/R) (Box 1). The inflammatory process begins in the intravascular compartment immediately after arterial occlusion, when the ensuing hypoxia, changes in shear stress, and production of reactive oxygen species (ROS) trigger the coagulation cascade, and lead to activation of complement, platelet and endothelial cells (EC)6–9(Fig. 1). Intravascular formation of fibrin, traps platelets and leukocytes leading to microvascular occlusions10,11. Within minutes after ischemia, the adhesion molecule P-selectin is translocated to the surface membrane of platelet and EC12, and proinflammatory signals are rapidly generated (Table 1). Oxidative stress in EC reduces the bioavailability of nitric oxide (NO), a potent vasodilator and an inhibitor of platelet aggregation and leukocyte adhesion12. Loss of the beneficial effects of NO exacerbates intravascular plugging and aggravates the ischemic insult by reducing blood flow to the ischemic territory13,14. Furthermore, oxidative stress leads to constriction of pericytes, contractile cells that replace myocytes in capillaries, producing microvascular occlusions15. Oxidative stress and inflammatory mediators also alter the permeability of the BBB. Pinocytotic vescicles increase in the cytoplasm of EC, enhancing trans-endothelial transport16. Proteases are expressed in vascular cells and released by leukocytes, whereas junctional proteins that seal adjacent EC are downregulated, facilitating extravasation of proteins and cells through the paracellular route (Fig. 1)16. In the perivascular space, I/R activates perivascular macrophages and mast cells. Mast cell degranulation releases vasoactive mediators (histamine), proteases, and TNF, while activated macrophages release proinflammatory cytokines17–20 (Box 2) (Fig. 1). These proinflammatory mediators contribute to the endothelial expression of adhesion molecules and to the BBB damage that promotes the infiltration of leukocytes (neutrophils, lymphocytes and monocytes)19.
Figure 1. Early vascular, perivascular and parenchymal events triggered by I/R.
Hypoxia, ROS and changes in shear stress initiate the cellular events induced by I/R. In the vessels lumen, I/R leads to blood clotting, platelet aggregation and cytokine release (IL-1α). Translocation of P-selectin on the surface of platelets and EC leads to platelet-leukocyte aggregation. Complement is activated and arachidonic acid metabolites are released. In the vascular wall, upregulation of E- and P-selectin on EC provides a platform for low affinity leukocyte binding through interaction with sialyl Lex moieties of glycoproteins expressed on leukocytes, e.g. PSGL-1. Firm adhesion is obtained after endothelial expression of ICAM-1 interacting with leukocyte β2 integrins (LFA-1 and Mac-1)144. Loss of NO promotes vasoconstriction and enhances leukocyte and platelet aggregation. MMP activation could lead to BBB breakdown and matrix proteolysis facilitating leukocyte extravasation. In the perivascular space, chemotactic complement subunits (C5a) acting on mast cell complement receptors (CD88) leads to degranulation and release of histamine and proteases, contributing to BBB leakiness. Cytokines (TNF, IL-1β) are produced by mast cells and perivascular macrophages, providing further signals to guide leukocyte migration across the vessel wall145,146. In the brain parenchyma, injured cells release purines (ATP), which act as early proinflammatory signals leading to production of cytokines and chemokines. Disruption of neuronal-microglial interaction (CX3CL1, CD200) and increases in extracellular glutamate (glu) acting on microglial GluR1 metabotropic receptor27 also contribute to the pro-inflammatory milieu.
Table 1.
Mediators of post-ischemic inflammation and their producing cells
| INITIATION non-transcriptional [cell type] |
AMPLIFICATION Transcriptional [cell type] |
RESOLUTION Transcriptional [cell type] |
|---|---|---|
| Adhesion molecules: | Adhesion molecules: | Growth factors: |
| P-selectin [EC, PLT] | ICAM1, VCAM1, P-selectin, E- selectin, Mac-1, VLA-1, [EC, Leuk, PVM, MG, AG] |
BDNF, EPO, FGF, G-CSF, GDNF, HB-EGF, IGF-1, NGF, VEGF [MG, AG, PVM, Macr EC, Neu] |
| Cytokines: | Cytokines: | Cytokines: |
| IL-1β [MG, PVM, MC] IL-1α [PLT] TNF [MC] |
IL-1, IL-6, IL-10, IL-17, IL-20, TNF [EC, PVM, MG, AG, Neu] |
TGFβ, IL-10, IL-17, IL-23, [T cells, MG, Macr, AG] |
| Chemokines: | Chemokines: | |
| CCL5 (RANTES), CXCL4, CXCL7 [PLT] CX3CL1 (Fractalkine) [Neu] |
CCL2 (MCP-1), CCL3 (MIP- 1α), CCL5 (RANTES), CXCL2/3 (MIP2), CXCL8 (IL-8) [EC, PVM, MG, AG, Neu] |
|
| Proteases: | Proteases: | Proteases: |
| Elastase, MMP8, MMP9, MT6-MMP [Leuk] Clotting factors [Circ] Complement [Circ, EC, AG Neu] |
MMP2, MMP9 [EC, Leuk] Complement [Circ, EC, AG Neu] |
MMP9 [AG, Neu] Complement [Circ, EC, AG Neu] |
| Small molecules: | Other: | Small molecules: |
| Prostanoids, Leukotrienes [EC,PLT,MG, Neu] ATP [Circ, Neu] Radicals [EC, PLT, Leuk PVM, MG, Neu] |
iNOS [MG, Leuk, EC] COX-2 [Neu, MG, Leuk, EC] LOX [Neu, Leuk] PTGES [Neu, MG, Leuk, EC] NADPH oxidase [MG, Leuk] |
Cyclopentenones prostaglandins Lipoxins Docosanoids (resolvins, protectins) |
AG: astroglia; Circ: Plasma; EC: endothelial cells; Leuk: leukocytes; Macr: macrophages; MC: mast cells; MG: microglia; Neu: neurons; PLT: platelets; PVM: perivascular macrophages.
ATP: adenosine triphosphate; BDNF: brain-derived growth factor; COX-2: cyclooxygenase-2; EPO: erythropoietin; FGF: fibroblast growth factor; G-CSF: granulocyte colony-stimulating factor; GDNF: glial cell derived neurotrophic factor; HB-EGF: heparin-binding epidermal growth factor-like growth factor; ICAM1: intercellular adhesion molecule 1; IGF-1: insulin-like growth factor 1; IL: interleukin; iNOS: inducible nitric oxide synthase; LOX: lipoxygenase; Mac-1: macrophage-1 antigen; MIP: macrophage inflammatory protein; MMP: matrix metalloproteinase; NGF: nerve growth factor; PTGES: prostaglandin E2 synthase-1; RANTES: regulated upon activation, normally T-expressed, and presumably secreted; TGFβ: transforming growth factor-β; TNF: tumor tecrosis tactor; VCAM1: vascular adhesion molecule 1; VEGF: vascular endothelial growth factor; VLA-1: very late activation antigen-1;
(see Supplemental Table 1 for references)
Box 2: Cells of the innate immune system.
Cerebral ischemia engages both innate and adaptive immunity, the two main braches of the vertebrate immune system51. The innate system is germline-encoded, rapidly activated, and relies on low affinity receptors to gain wide-ranging target recognition. The adaptive system is based on high-affinity receptors, i.e., T-cell receptors and immunoglobulins, which are randomly generated by somatic mutations. In contrast to the innate system, adaptive immunity needs antigen-driven clonal cell expansion, a process that requires several days, and retains a memory of this antigen exposure51. Although specific cell types are predominantly associated with one of the two branches, there is considerable overlap between the role of these cells in innate and adaptive immunity. Here we describe cells predominantly associated with innate immunity. Lymphocytes are described in Box 3.
Microglia derive from the hematopoietic system and constitute the innate immune cells of the CNS110,111. Resting microglia survey the brain parenchyma through continuous extension and retraction of their processes60,112. Microglia contribute to post-ischemic inflammation by producing TNF, IL-1β, ROS and other pro-inflammatory mediators (Table 1)(Fig. 1). However, they also contribute to the resolution of inflammation and tissue repair by producing IL-10 and TGF-β, as well as several growth factors, including IGF-1 (Table 1)(Fig. 4).
Perivascular macrophages are confined to the space between the vascular basement membrane and the brain surface (glia limitans), and, unlike microglia, are continuously replenished by hematogenous precursors110,113. Macrophages can be classified into two groups. M1 macrophages produce pro-inflammatory cytokines (IL-1β, IL-12, IL-23 and TNF), chemokines, ROS, and NO, thus promoting a Th1 immune response (Box 3). In contrast, M2 macrophages produce anti-inflammatory cytokines (IL-10 and TGF-β), IL-1ra, and arginase114. After cerebral ischemia, cytokines produced by perivascular macrophages are thought to drive the infiltration of inflammatory cells19.
Mast cells (MCs) are found in meninges and cerebral blood vessels. MC granules store vasoactive substances (histamine), cytokines (TNF), anticoagulants (heparin), and proteases (tryptase, chymase, MMP2, MMP9)17,18. In addition, MCs are capable of phagocytosis, antigen presentation and can modulate the adaptive immune response115.
Blood monocytes are direct precursors of tissue macrophages. At least two different monocyte populations have been described in humans and mice according to their surface markers and function. “Classical” monocytes produce the anti-inflammatory cytokine IL-10 when challenged with LPS, whereas “pro-inflammatory” monocytes produce TNF. After cerebral ischemia, inflammatory monocytes are rapidly recruited to the site of injury where they give raise to macrophages and DC35,116.
Dendritic cells are specialized APC and constitute the main interface between innate and adaptive immunity (Box 3). They originate from myeloid lineage through a common dendritic precursor117. In the normal brain, DCs are associated with meninges, choroid plexus and the CSF. Cells expressing DC markers (CD11c+, MHC Class II+) appear in the brain parenchyma after focal ischemia and originate from resident, as well as blood borne cells35,118.
Neutrophils are secretory and phagocytic cells of the innate immune system and carry different types of cytoplasmic granules and secretory vesicles. Major pro-inflammatory molecules stored in granules and vesicles include, iNOS, NADPH oxidase, myeloperoxidase, MMP8, MMP9, elastase, and cathepsins. After cerebral ischemia neutrophils adhere to the cerebral endothelium and transmigrate into the tissue12. Vesicle and granule exocytosis is induced after receptor engagement, e.g. binding to E-selectin on EC, IL-8 stimulation119.
Is the immune system comparable in rodents and humans? Circulating neutrophils predominate in humans (50–70%), while lymphocytes predominate in rodents (75–90%)120. Several immune-related molecules, i.e., iNOS, P2×7, TLR2, antigen presenting proteins, co-stimulatory molecules, immunoglobulins, and chemokines, are differentially expressed in mice and humans, or present in one species and not the other120. Human EC can present antigens to resting CD4+ and CD8+ T cells, while mice EC can only activate CD8+ T121. Consequently, the immune responses initiated by vascular pathologies may differ quantitatively and qualitatively between humans and mice. The impact of these differences needs to be considered when transposing findings obtained from rodent models of cerebral ischemia to human stroke.
Ischemic cell death activates innate immunity and sets the stage for adaptive immunity
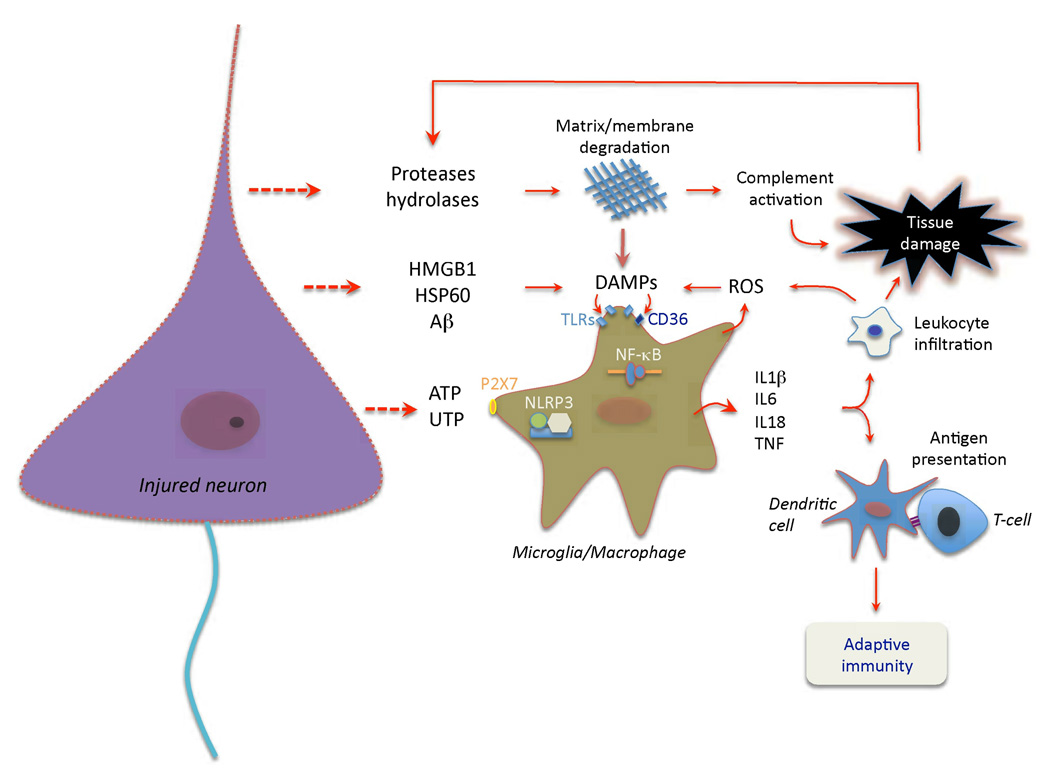
As the ischemic cascade progresses (Box 1), cell death leads to a new phase of the inflammatory response (Fig. 2). Dying and dead cells release “danger signals” that activate the immune system21. Some of these signals, like the nucleotides ATP and UTP, are released by cells under stress when the cell membrane is still intact and set the stage for the subsequent immune response22.
Figure 2. Cell death and activation of pattern recognition receptors set the stage adaptive immunity.
Release of nucleotides (ATP, UTP) from injured cells, including neurons, activates purinergic receptors on microglia and macrophages, and leads to production of pro-inflammatory cytokines25. While most of these cytokines are transcriptionally induced, IL-1β and IL-18 are processed from their pro-peptides by the activity of interleukin-1 converting enzyme (ICE; caspase 1). ICE is embedded in a multi-protein complex (NLRP3 or inflammasome147) and is activated by microglial P2×7 receptors148. Ischemic cell death leads to the formation of danger associated molecular patterns (DAMPs) molecules, which activate TLRs, especially TLR2 and 421. DAMPs released by ischemia include HMGB1, an intracellular DNA binding protein released after cellular injury, HSP60, and β-amyloid (Aβ)149, among others30. TLRs, in concert with scavenger receptors such as CD36, upregulate pro-inflammatory gene expression through the transcription factor NF-κB30,150. DAMPs also derive from matrix breakdown by lytic enzymes released from dead cells and by the action of reactive oxygen species (ROS) on lipids. The cytokine production and complement activation resulting from these events leads to increased leukocyte infiltration and enhances tissue damage, which, in turn, produces more DAMPs. Antigens unveiled by tissue damage are presented to T cells, setting the stage for adaptive immunity.
ATP and neurotransmitters
Extracellular ATP increases within minutes after ischemia as a result of neuronal and glial depolarization or escape through damaged plasma membranes23–25. ATP is also released by vascular cells and blood cells, and may promote intravascular coagulation and platelet aggregation26. High parenchymal ATP levels activate P2×7 receptors in microglia leading to release of pro-inflammatory mediators (Fig. 2). Activated microglia develop many characteristics of macrophages including ameboid morphology, migratory capacity, phagocytosis and MHC class II-restricted antigen presentation (Box 3). At the same time, neurotransmitters release after I/R may counteract the emerging inflammatory response. Microglia express a wide variety of neurotransmitter receptors, including AMPA, kainate, adrenergic, GABAB opioid, and cannabinoid receptors27. With some exceptions27 (Fig. 1), activation of these receptors downregulates microglial cytokine, ROS and NO production27, and suppresses the secretory response in mast cells28,29. Therefore, ATP represents an early neuronal danger signal, promoting the inflammatory response of resident immune cells, whereas neurotransmitter release may oppose these changes and counteract inflammation.
Box 3: The spectrum of lymphocyte subtypes.
Lymphocytes are key cells in innate and adaptive immune responses. B-lymphocytes (B-cells) are responsible for humoral immune responses characterized by the production of antibodies that attack and neutralize specific antigens51. T-lymphocytes (T cells) are involved in cellular immunity, in which extraneous antigens are suppressed by a cytotoxic cellular response. T cells express the glycoprotein CD4 or CD8 on their surface, a feature that determines their function and fate (see below).
Antigen presentation
Central to adaptive immunity is antigen presentation, which is performed by APC, mainly DC and macrophage51. APC patrol the environment and when they recognize a foreign antigen, a protein for example, they degrade it into peptides, some of which are then assembled with MHC class II molecules and displayed on the APC surface51. The MHC-class II-antigen complex is recognized by the T-cell receptor (TCR) of CD4+ T cells, and this interaction in the presence of appropriate co-stimulatory molecules on APC (B7-1, B7-2) and T cells (CD28) results in T-cell activation122. TCRs include either α–β chains or γ–δ chains, immunoglobulin-like proteins consisting of variable and fixed regions51. After antigen presentation, CD4+ T cells undergo clonal expansion in lymphoid organs, a process promoted by autocrine production of IL-251.
Helper T cells
With notable exceptions, CD4+ T cells become helper T cells (Th), so called because they do not have cytotoxic function, but act as “helpers” by coordinating and modulating immune responses123. Th cells include effector and regulatory cells123. Depending on the molecular signals present in their milieu, different subpopulations of effector Th cells can develop, each characterized by a specific pattern of cytokine production. Th type 1 (Th1) effector cells secrete IFNγ and TNF and their development is promoted by IL-12 through the transcription factor t-bet123. Th1 cells stimulate innate and T-cell induced immune responses leading to cytotoxicity. Th2 cells secrete IL-4, IL-5, IL-9, IL-10, IL-13 and promote humoral immunity, mucosal immunity, and responses directed against extracellular pathogens123. IL-4 promotes Th2 cell development, which is regulated by the transcription factor GATA-3123. Th17 cells secrete IL-17 and their development is driven by TGFβ and IL-6, and involves the transcription factor RORγt123. Th17 cells participate in autoimmunity104, but have not been implicated in cerebral ischemic damage41. Regulatory T cells (Treg or suppressor T cells) are either present naturally, or can develop from other Th subtypes in the presence of TGFβ. Treg development requires the transcription factor FoxP3123. Unlike other Th cells, Treg induce immunosuppression by producing IL-10 and TGFβ123. Therefore, Treg are critical for the maintenance of the homeostasis of the immune system by counterbalancing the destructive effects of excessive inflammation and may play a protective role in cerebral ischemia (see text).
Cytotoxic T cells
In contrast to CD4+ T cells, CD8+ T cells are cytotoxic. The TCR of CD8 expressing T cells binds the antigen presented with MHC class I molecules and co-stimulatory molecules. Unlike MHC class II, MHC class I molecules are present in every cell. After clonal expansion, activated CD8+ T cells patrol the internal environment in search of somatic cells expressing the antigens against which they were sensitized. Cytotoxic T cells kill somatic cells by permeabilizing the membrane with perforin and inducing apoptosis via granzyme-induced caspase activation or the Fas ligand pathway (Box 4). Lymphocytic cells with cytotoxic function also include natural killer (NK) cells and natural killer T cells (NKT). NK cells lack a TCR and, as such, do not require antigen presentation for their activation and cytotoxicity, which is triggered by interferons or cytokines124. NKT cells have a simplified TCR that recognizes glycolipids presented by cells also expressing the MHC class I-like glycoprotein CD1124. NKT cells exert their cytotoxic effect by releasing large amounts of IFNγ, IL-2, and TNF.
γδT cells
These are a subset of effector lymphocyte with a TCR comprising γδ chains and particularly enriched in mucous membranes125. Like NKT, these cells recognize non-peptide antigens and react to danger signals produced by stressed cells125. γδT cells can exert different functions depending on the context in which they operate, ranging from cytolysis, to antigen presentation, immunoregulation, and production of growth factors126. In cerebral ischemia, γδT cells have been implicated in both cytotoxicity and protective immunomodulation (see text).
Cell death and pattern recognition receptors in the post-ischemic brain
A different signaling landscape emerges after cells begin to die. A wide variety of molecular signals are released from the intracellular compartment or are generated by the action of lytic enzymes escaped from dead cells on matrix proteins (Fig. 2). These so-called danger associated molecular pattern molecules (DAMPs) activate pattern recognition receptors, including toll like receptors (TLRs) and scavenger receptors, widely expressed on microglia, perivascular macrophages, and brain EC30. DAMPs and purines act in concert to induce the expression of proinflammatory molecules in infiltrating leukocytes (Table 1) and to prime dendritic cells (DC) for antigen presentation (Boxes 2 and 3) (Fig. 2). Considering the high vascular density of the brain, inflammatory mediators released from parenchymal cells are likely to feed back on the vascular and perivascular compartments to reinforce and amplify the expression of cytokines, chemokines and adhesion molecules that drive the infiltration of blood borne cells into the ischemic tissue (see previous section). After neuronal death, loss of cell-cell interaction between neurons and microglia also promotes inflammatory signaling (Fig. 1). In the normal state, microglia are kept quiescent by contact with neurons. For example, CD200, a surface protein expressed in neurons, interacts with its receptor CD200R on microglia enforcing a resting phenotype31. Disruption of this interaction due to post-ischemic loss of CD20032 may promote microglial activation (Fig. 1). Similarly, CX3CL1 (fractalkine), a cell surface bound chemokine constitutively expressed by neurons, suppresses microglial activation through its microglial receptor CX3CR1. Thus, after neuronal injury loss of CX3CL1 results in enhanced microglial activation in several inflammatory disease models33. In addition, increasing concentrations of extracellular glutamate activate metabotropic glutamate receptors on microglia leading to a pro-inflammatory phenotype34 (Fig. 1). Therefore, as neuronal death develops in the ischemic core and spreads to the penumbra the loss of the immunosuppressive effect afforded by neurotransmitter release and neuron-microglia interaction may also foster post-ischemic inflammation.
Collectively, these observations suggest that the inflammatory response after I/R starts at the vascular level, driven by non-transcriptional events triggered by hypoxia, shear stress and ROS production. As ischemia damages the brain tissue, danger signals are released first from cells under stress and then from necrotic cells. Concomitant with the loss of immunosuppressive mechanisms, these signals activate purinergic receptors and pattern recognition receptors, which induce an inflammatory response in resident brain cells and infiltrating leukocytes.
Does a classical adaptive immune response contribute to ischemic brain injury?
Danger signals released from damaged cells also promote the presentation of tissue antigens that were previously hidden by the BBB or that develop as a result of the breakdown of cell membranes21 (Fig. 2). Antigen presentation leads to the development of cellular and humoral immunity directed against the antigens (Box 3). This adaptive immune response has the potential of inducing autoimmunity against the organ in which the cell death occurred, as described in the heart (Dressler’s syndrome), eye (sympathetic ophthalmia) and pancreas (diabetes)21. Furthermore, the damaging effect of adaptive immunity is well established in MS and in models of autoimmune demyelination5. The next section will review the evidence for a pathogenic role of adaptive immunity in stroke.
Stroke and adaptive immunity
Antibodies against CNS antigens develop after ischemic stroke, suggesting a humoral immune response to the injury, and circulating T cells become sensitized against CNS antigens, such as myelin basic protein (MBP) and related peptides (Table 2). APC are reduced in the periphery and increased in the ischemic brain both in rodent and human stroke35–38. The accumulation of APC coincides with the peak of lymphocytic infiltration and is associated with expression of MHC class II molecules and the co-stimulatory molecule CD8035,37,38, findings suggestive of antigen presentation (Box 3). Support for involvement of adaptive immunity also comes from studies on the role of lymphocytes in models of focal cerebral ischemia. Ischemia leads to infiltration of the major lymphocytes subtypes into the ischemic brain37 (Box 3). Increasing evidence suggests that lymphocytes contribute to ischemic injury (Table 2) (Fig. 3). Lymphocyte-deficient mice are protected from ischemic damage39,40. The protection has been attributed to T cells, because B-cell-deficient mice or lymphocyte-deficient mice reconstituted with B-cells are still protected from injury (Table 2). γδT cells also have been shown to contribute to the injury by releasing the proinflammatory cytokine IL-1741 (Fig. 3). In contrast, Treg are protective in the late stage of cerebral ischemia, an effect evident only if the injury is small42,43. Additional evidence in favor of the involvement of cell-based adaptive mechanisms in stroke damage was provided by studies in which animals were tolerized against myelin-derived peptides (Table 2). Repeated mucosal administration of myelin antigens (tolerization) prior to arterial occlusion protects rodents from ischemic brain injury44. Although tolerization is antigen specific, its beneficial effects are not restricted to immune responses directed at the inducing antigen, but are more widespread, a phenomenon termed bystander suppression44. The protection can be induced in naïve mice by adoptive transfer of splenocytes or CD4+ T cells from tolerized animals45,46, suggesting the involvement of cellular immune mechanisms. Examination of T-cell function indicates that activation of tolerized T cells by the antigen unveiled by the stroke induces a Th2 cytokine response (Box 3)45,47,48. The effect has been attributed to IL-4 and IL-10 production that favors the formation of TGFβ secreting Treg cells45,46,48. Other studies have found that administration of recombinant T-cell receptor ligand (RTL), consisting of α1 and β1 domains of MHC class II complex bound to a myelin peptide antigen (MOG 35–55), reduces stroke volume in focal ischemia49. The protective effect is associated with reduction of infiltrating inflammatory cells and may result from suppression of autoreactive T cells targeted against myelin49. Although RTLs may not bind T cells, but APC and platelets50, the findings suggest a role of cellular immunity in the mechanisms of ischemic brain injury.
Table 2.
Selected evidence for and against the involvement of adaptive immunity in ischemic brain injury
| A. In favor of adaptive immunity causing tissue damage | ||
|---|---|---|
| Evidence | Findings | References |
| CNS antigens and associated humoral response are present after stroke |
MBP, NSE, S100beta, GFAP, NMDA receptor, neurofilament |
134–138 |
| T-cell response to CNS antigens after stroke |
Lymphocyte sensitization to CNS antigens | 139 |
| APC increase in the human and rodent brain after stroke |
DC and macrophages found in perivascular space and brain parenchyma after stroke |
35–38,118 |
| γδT cells and Treg are involved in experimental stroke |
γδT cells contribute to brain damage through IL-17; Treg are protective in the late phase of cerebral ischemia |
41,42 |
| T cells sensitized against CNS antigens mediate damage in stroke |
RTL targeted to myelin-specific T cells reduces ischemic brain injury |
49 |
| Tolerization to CNS antigens is protective in experimental stroke |
Mucosal administration of MBP or MOG reduces damage in focal ischemia |
44 |
| B. Against adaptive immunity causing damage | ||
|---|---|---|
| Evidence | Findings | References |
| Temporal dissociation between adaptive response and tissue damage |
T cells mediated damage occurs early (<24hrs) after stroke, not consistent with antigen presentation and clonal expansion |
39,52 |
| T-cell mediated ischemic damage is antigen independent |
T-cell reactive against CNS or non-CNS antigens are equally damaging |
40 |
| Absence of co-stimulatory molecules necessary for antigen presentation does not effect stroke outcome |
Mice lacking CD28 or B7 are not protected from focal ischemia |
40 |
| Unlike other models of autoimmunity, both CD4+ and CD8+ T cells are involved in the injury |
CD4+ and CD8+ null mice are equally protected |
41,52 |
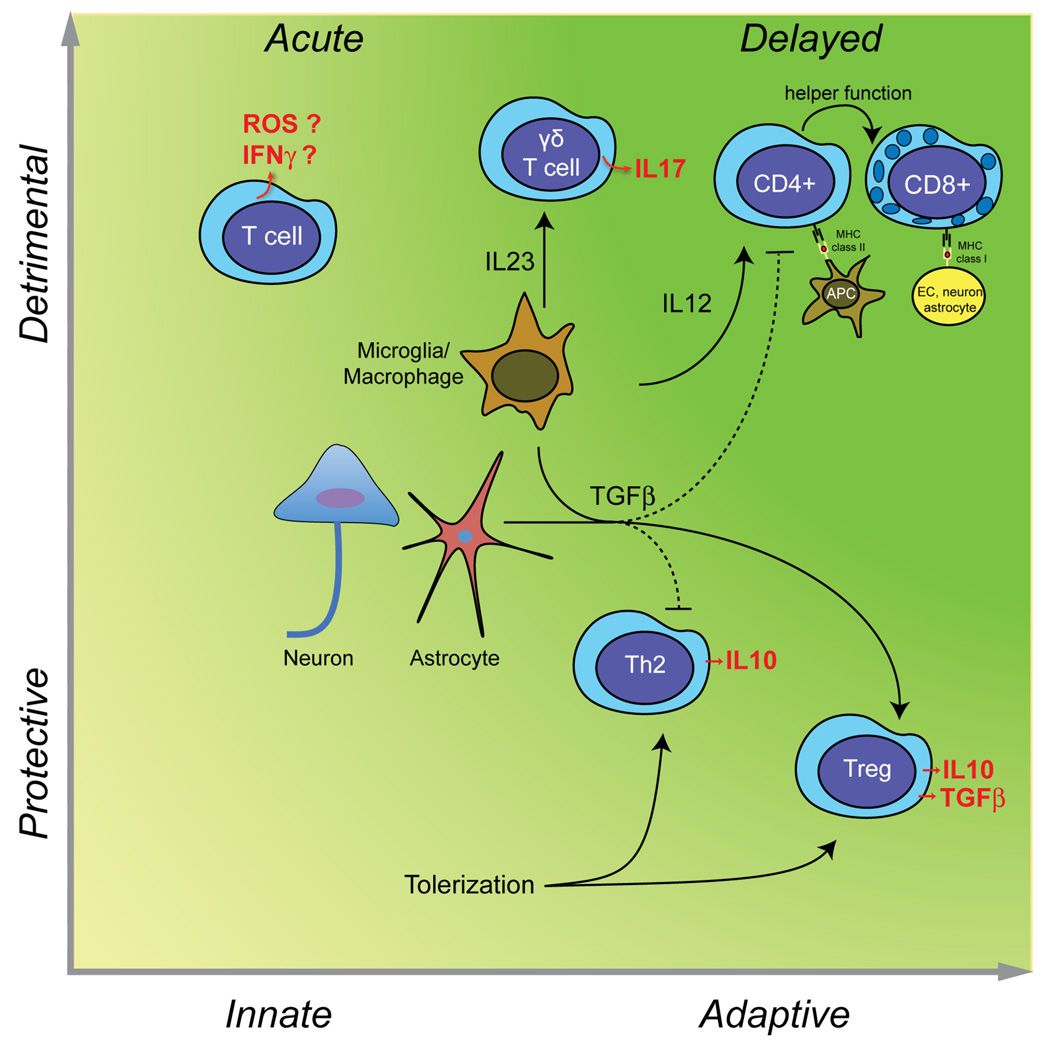
Figure 3. Deleterious and beneficial roles of T cells in stroke.
In the acute phase of cerebral ischemia, unprimed T cells contribute to tissue damage in an antigen independent manner (innate immunity), possibly through IFNγ151 and ROS152 (left upper quadrant). γδT cells, activated by IL-23 released from microglia/macrophages, produce the cytotoxic cytokine IL-17 and contributes to acute ischemic brain injury41. However, T cells can also be protective. TGFβ produced by neurons, glia, or macroglia/macrophages promotes the development of Treg cells secreting the protective cytokine IL-10 and inhibits Th1 and Th2 responses. Treg cells are protective in models of cerebral ischemia42. Induction of mucosal tolerance with CNS antigens produces an adaptive response, which leads to the establishment of autoreactive Th2 cells producing IL-1048 and Treg cells producing IL-10 and TGFβ107,141 is highly protective in experimental stroke (right lower quadrant). As discussed in the text, there is no evidence that adaptive immunity contributes to acute ischemic brain injury. However, weeks and months after stroke, autoreactive CD4+ and CD8+ T cells targeting CNS antigens could develop (right upper quadrant). The resulting cell death could play a role in the delayed brain damage and atrophy that occurs after stroke83.
Despite the evidence supporting an autoimmune response against the post-ischemic brain, there are inconsistencies with the hypothesis that classical adaptive immunity contributes ischemic brain injury. The temporal profile of the involvement T cells in brain damage is not consistent with established concepts of adaptive immunity51 (Fig. 3). Thus, the protective effect observed in lymphocyte deficient mice or afforded by blocking postischemic trafficking of T cells into the ischemic brain occurs 24–48 hrs after ischemia40, whereas adaptive responses require an interval of 7–10 days from antigen presentation to the clonal expansion of autoreactive T cells and immune attack on the target organ51 (Box 3). Furthermore, reconstitution of lymphocyte deficient mice with T cells targeting non-CNS antigens worsens ischemic damage, and mice lacking co-stimulatory molecules essential for antigen-specific T-cell response are not protected from ischemia40. It is also surprising that unlike autoimmune responses in other organs, where there is a prevalence for either T-helper or T-effector cell participation, both CD4+ and CD8+ T cells are involved in ischemic injury52. Collectively, these observations argue against an autoimmune attack on the brain resulting from presentation of CNS antigen released by the stroke.
The lymphocyte puzzle
If lymphocytes do not mediate an autoimmune attack on the brain how do they contribute to the early phase of ischemic brain injury? One possibility is that these cells participate in the cerebrovascular dysfunction occurring after ischemia or have prothrombotic actions leading to microvascular occlusions52. These effects would be deleterious by preventing reperfusion and by compromising collateral flow after I/R. However, lack of lymphocytes does not improve post-ischemic cerebral blood flow at least in the acute phase53, nor does it suppress thrombus formation40. Therefore, effects of lymphocytes altering microvascular perfusion or patency seem unlikely. Another possibility is that NKT or γδT cells, T cells that have a simplified TCR and may not require antigen processing and MHC presentation, as well as NK cells (Box 3), are responsible for these early cytotoxic effects of lymphocytes. In support of this hypothesis, NKT cells are not present in Rag1−/− mice and SCID mice54 and they could contribute to explain the early neuroprotection observed in these models of lymphocyte deficiency39,40. However, CD1-deficient mice lack NKT cells and are not protected from ischemic injury at 24 hrs40, suggesting that NKT cells may not be involved in the early phase of the injury. γδT cells have been implicated in ischemic brain injury, but their involvement seems restricted to the late phase of cerebral ischemia (4 days)40,41. Considering the limited number of studies available, the involvement of NK, NKT and γδT cells, lymphocyte subtypes that act in a fashion akin to innate immunity, needs further exploration.
Collectively, these data suggest that, although an antigen specific immune response may develop following stroke, evidence that autoreactive T cells attack brain antigens exposed by ischemic damage against which they were sensitized is lacking. Lymphocytes do play a role in the development and progression of the injury, but the mechanism of their powerful effect does not conform to the tenets of classical autoimmunity. The role of NK, NKT and γδT cells, which could contribute to the acute phase of the injury, needs further exploration. Similarly, considering the evidence for humoral immune responses in stroke (Table 2), the contribution of B-cells to the damage needs a more in depth assessment.
Resolution of inflammation and tissue repair
Post-ischemic inflammation is a self-limiting process that eventually subsides and prepares the terrain for the structural and functional reorganization of the injured brain. The factors governing resolution of inflammation and the reestablishment of tissue homeostasis are still poorly understood, particularly in brain. Increasing evidence suggest that resolution of inflammation is not a passive process due to exhaustion of the signaling, but is orchestrated by the interplay of a large number of mediators which actively suppress the inflammatory response55. Major steps in process include removal of dead cells, development of an anti-inflammatory milieu, and generation of pro-survival factors fostering tissue reconstruction and repair55,56.
Clearing dead cells
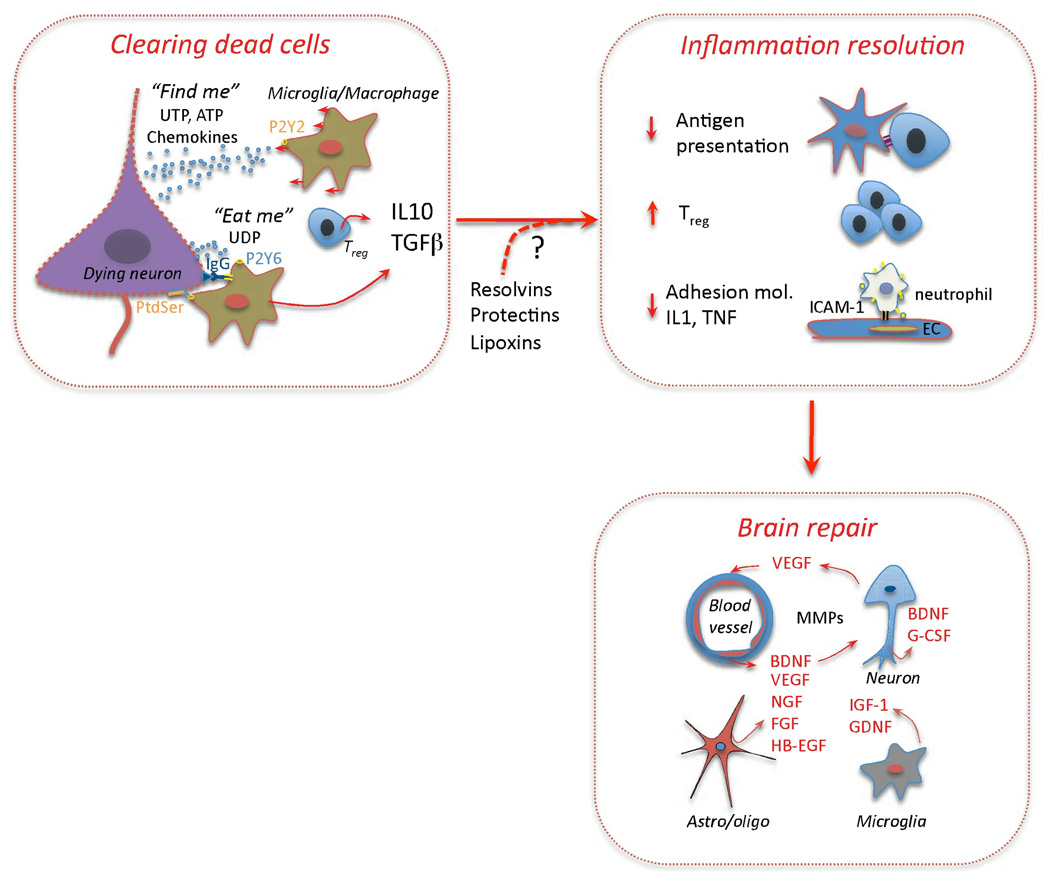
Microglia and infiltrating macrophages constitute the predominant phagocytes removing dead cells and tissue debris after stroke57,58, a process orchestrated by “find me” and “eat me” signals. “Find-me signals”, including purines released from injured cells and chemokines, attract microglia and macrophages to the site of injury59,60 (Fig. 4). These phagocytic cells are then presented with “eat-me signals” associated with dying or dead cells (Fig. 4).
Figure 4. Resolution of inflammation and tissue repair.
Clearing of dead cells and suppression of inflammation are key events in brain repair. “Find-me signals” (UTP, ATP) attract microglia and macrophages through P2Y2 receptors. “Eat-me signals” include UDP, which act on P2Y6 receptors to stimulate microglial phagocytosis153, and phosphatidylserine (PtdSer), which is translocated to the outer leaflet of the plasma membrane of apoptotic cells154. PtdSer binding proteins involved in the clearance of dead cells include MGF-E8 on microglia155 and TIM4 on macrophages154. Immunoglobulins directed against CNS antigens, which appear after stroke (Table 2), may also promote phagocytosis by engaging Fc receptors on phagocytic cells. Phagocytosis promotes secretion of IL-10 and TGFβ56, which, in turn, suppress antigen presentation, promote Treg formation, inhibit expression of adhesion molecules in EC and production of proinflammatory cytokines61,156. TGFβ and IL-10 are also neuroprotective157,158 and may facilitate brain repair processes. In addition, arachidonic and omega-3 fatty acids metabolites lipoxins, resolvins, and protectins, which play an active role in the resolution of inflammation in other organs55, could also contribute to suppress post-ischemic inflammation. Growth factors and MMPs produced by EC, neurons, astrocytes, oligodendrocytes and microglia are critical molecules driving tissue reorganization and repair63,159.
TGFβ, IL-10 and the anti-inflammatory milieu
TGFβ and IL-10 are pleiotropic immunoregulatory cytokines that play a crucial role in the development of the anti-inflammatory milieu associated with tissue repair (Fig. 4). The production of these cytokines is promoted by phagocytosis and occurs in concert with the removal of dead cells56. TGFβ is upregulated after ischemia primarily in microglia and macrophages and, in addition to its neuroprotective properties, has also profound effects on immune cells. Although well known for its proinflammatory effects, TGFβ can suppress inflammation by inhibiting Th1 and Th2 responses and promoting Treg cell development61. Similarly, the immunoregulatory cytokine IL-10, produced by different cells including Treg cells42, has both neuroprotective and anti-inflammatory activities (Fig. 4). Therefore, post-ischemic production of TGFβ and IL-10 can facilitate tissue repair by promoting the resolution of inflammation and exerting direct cytoprotective effects on surviving cells in the ischemic territory.
Growth factors
Post-ischemic production of growth factors helps to establish an environment that is favorable to neuronal sprouting, neurogenesis, angiogenesis, gliogenesis and matrix reorganization62–64. Inflammatory cells, as well as neurons and astrocytes65,66, are capable of producing a vast array of growth factors (Table 1). For example, microglia are required for the full expression of IGF-167, a critical factor in post-ischemic neuronal sprouting66, whereas reactive astrocytes are required for functional recovery after stroke68. Vascular endothelial growth factor (VEGF), a key growth factor in post-ischemic angiogenesis, is produced by reactive astrocytes69, and its action may require neutrophil MMPs, suggesting a link between inflammatory cells and angiogenesis70. However, VEGF administration early after ischemia or in excessive doses may enhance the damage71,72. The role that inflammatory signaling in brain recovery has also been highlighted by studies in which the transcriptome of sprouting neurons was defined indicating involvement of MHC I class molecules and complement subunits66.
The evidence presented above indicates that cells of the immune system serve a fundamental role in all the phases of post-ischemic brain recovery. But, the limited data available provide only a glimpse into the complex sequence of events that reestablish the structural and functional homeostasis of the brain after stroke. Additional investigations on recently identified mediators instrumental to inflammation resolution and tissue repair, such as lipoxins, resolvins, protectins, progranulins, and cyclopentenone prostaglandins (Table 1) (Fig. 4)55,56, are needed to fully elucidate the role of the immune system in brain repair after stroke.
Stroke and systemic immunity
Concomitant with the inflammatory response involving the brain, immunological changes are also observed in the blood, bone marrow, spleen and other lymphoid organs3,73. Genome profiling of peripheral blood in stroke patients has demonstrated characteristic patterns of inflammatory gene expression that can help determine the cause of ischemic stroke74, reflecting the specificity of the systemic inflammatory response to brain injury. In rodent models as in patients with ischemic stroke, white blood cell count and expression of cytokines and inflammatory markers are increased within hours after ischemia75–78. Such acute phase response is followed by a dramatic immunodepression, especially in patients with large strokes, characterized by lymphopenia, reduced functional activity of monocytes, upregulation of anti-inflammatory cytokines, lymphocyte apoptosis and splenic atrophy78,79. These immunological changes are associated with an increased tendency to respiratory and urinary tract infections, which are responsible for considerable morbidity and mortality in stroke patients3,4. Infections tend to occur in patients with larger stroke and with low CD4 lymphocyte counts and elevated levels of IL-10 and IL-6, reflecting immunodepression80. How these systemic immune changes are mediated is not completely understood, but there is evidence that sympathetic activation and the attendant release of stress steroids and catecholamines are involved3,4. Thus, cortisol and catecholamines are elevated in stroke patients most susceptible to infection81, and steroid antagonists and the β-adrenergic receptor antagonist propanolol counteract post-stroke lymphocyte apoptosis and infection propensity in rodent models82.
Bright and dark sides of post-stroke immunosuppression
What is the biological significance of post-stroke immunodepression? One possibility is that the lymphopenia and immunosuppression limit the development of autoreactive T cells targeted to CNS antigens and dampen a potential autoimmune attack on the brain3,4. As discussed in the previous section the relative rapidity of the development of ischemic brain injury is not consistent with the temporal profile of an adaptive response against the brain. However, it is conceivable that sensitization to CNS antigens plays role in the long-term outcome of the stroke (Fig. 3). About 30% of stroke survivors have dementia, often associated with brain atrophy83. The bases for the cerebral atrophy are not entirely clear, but immunological mechanisms triggered by the stroke cannot be discounted. Pathological studies have shown an inflammatory infiltrate that persists for years after the stroke. For example, mononuclear cells, perivascular cuffing and macrophages are present, respectively, in 42, 44, and 75% of brains with stroke in a large series1 and T cells and DC are observed in “old” strokes38. However, these focal inflammatory changes have not been linked to post-stroke immunosuppression or dementia, and their pathogenic significance remains uncertain.
On the other hand, post-stroke immunosuppression is deleterious in that it increases the incidence of infections, a major determinant of poor neurological outcome, morbidity and mortality3,4. Acute infection could also negatively affect stroke outcome by upregulating co-stimulatory molecules and promoting antigen presentation21. This possibility is supported by studies in which bacterial lipopolysaccharide (LPS) administered at the time of reperfusion to simulate post-stroke infection, worsens the outcome of experimental stroke84 and increases post-ischemic brain atrophy assessed 1 month after stroke85,86. The effect is associated with increased expression of B7.1, a co-stimulatory molecule needed for efficient antigen presentation (Box 3), as well as T-cell sensitization against CNS antigens and a Th1 cytokine response85,86. Therefore, post-stroke immunosuppression is detrimental by increasing the incidence of systemic infections and, possibly, by promoting antigen presentation and autoimmunity against the brain, which may play a role in the long-term sequelae of the stroke. At the same time, immunosuppression could be beneficial by attenuating such delayed autoimmune response.
Bench to bedside: trials, tribulations and therapeutic opportunities
Ischemic stroke remains an enormous therapeutic challenge. Currently, thrombolysis with tissue plasminogen activator (tPA) is the only effective therapy, but due to the narrow therapeutic window of less than 4.5 hrs and safety concerns, fewer than 5% of stroke patients receive this treatment87. Among the potential therapeutic approaches targeting the ischemic cascade (Box 1), preclinical studied in rodent models suggest that suppression of inflammation offers unique advantages. First, these treatments have an extended therapeutic window and are effective when administered up to 12–24 hrs after stroke88,89. Therefore, they could be used in patients who fail the time window for thrombolysis. Second, because suppression of inflammation is also beneficial in models of cerebral hemorrhage90, concerns about worsening brain injury in patients in whom hemorrhagic stroke has not been excluded would be minimized and early treatment by emergency medical teams would become feasible. Finally, considering that the inflammation particularly deleterious in ischemia associated with reperfusion91,92, suppression of inflammation would be a fitting complement to reperfusion therapy using thrombolytics or intravascular clot removal. Although these considerations are based on animal models, which may not recapitulate in full the human disease (Box 1), inflammation is a critical pathogenic component of human stroke and remains an attractive target for therapeutic intervention.
Anti-inflammatory agents
Blocking antibodies directed against adhesion molecules (ICAM-1, MAC-1), or recombinant neutrophil inhibitory factor have not been effective in clinical trials93. In the case of the ICAM-1 trial, the negative outcome has been attributed to deleterious immunoactivation resulting from administration of a mouse antibody to humans, as reproduced in an experimental study in which murine antibodies to rat ICAM-1 were administered to rats94. Although there also might be other reasons for these failures93, a likely contributing factor is that post-ischemic inflammation acts through multiple redundant pathways that cannot be effectively suppressed by blocking a single cytokine or adhesion molecule, as attempted in these clinical trials. Thus, neutralizing upstream mediators of the signaling cascade or blocking multiple inflammatory pathways would be more effective (Table 3). For example, blocking upstream components of inflammatory signaling, such as complement, TLR or scavenger receptors is highly protective in experimental models (Supplemental Table 1). Furthermore, minocycline, an agent with multiple neuroprotective actions including broad anti-inflammatory properties, has shown promise in clinical trials95 (Table 3). Another strategy, described in the next section, is to develop approaches in which the immune system is directed to suppress the deleterious effects of inflammation while enhancing its protective potential.
Table 3.
Examples of therapeutic approaches targeting multiple inflammatory pathways
| Intervention | Target | Potential downside |
Development stage |
References |
|---|---|---|---|---|
| Inhibition of TLRs, complement, or scavenger receptors |
Upstream events in inflammatory signaling |
Worsening of post- stroke infections |
Preclinical | See suppl. table 1 |
| Minocycline | Multiple, including early inflammatory signaling |
Generally safe, but neurotoxic in some settings |
Clinical (Phase III trial planned) |
95,140 |
| T-cell receptor ligand |
T cells | Mechanism unknown |
Preclinical | 49 |
| T-cell based approaches |
Suppression of γδT cells |
Feasibility unclear | Preclinical | 41,42 |
| Upregulation of Treg | ||||
| Tolerization | Promotes Th2 responses |
Prevention only | Preclinical | 44,106,141 |
| Deleterious humoral response |
||||
| Remote preconditioning |
Multiple, including inflammation |
Prevention, but being tested also in acute stroke |
Clinical (Phase I trial in subarachnoid hemorrhage completed; ischemic stroke trials ongoing) |
142,143 |
Immunomodulation and T-cell based approaches
Ischemic tolerance provides an example of protective immunomodulation. Ischemic tolerance or preconditioning is a phenomenon in which a sub-lethal injurious stimulus protects an organ against a subsequent lethal stimulus. For example, a short non-damaging ischemic insult to the brain (local preconditioning) or other organs (remote preconditioning) protects the brain from a subsequent damaging ischemic insult2,96. Similarly, administration of low doses of LPS protects the brain from ischemic damage26. Although ischemic tolerance is well know to protect the brain by simultaneously suppressing multiple pathways in the ischemic cascade2, modulation of the post-ischemic immune response has emerged as one of its key effector mechanisms30,97. In the tolerance induced by LPS, post-ischemic TLR4 signaling is redirected towards production of IFNβ, which, in turn, reprograms the immune system to suppress the production of pro-inflammatory cytokines and the infiltration of inflammatory cells98. On the other hand, NF-κB-dependent inflammatory mediators, such as IL-1, TNF, iNOS-derived NO and ROS, are also required for the full expression of the tolerance99–102, indicating that the protection does not rely just on the suppression of deleterious inflammatory mediators, but on a fine balance between pro- and anti-inflammatory signaling. One of the challenges, therefore, is to learn how to modulate the immune system to replicate the beneficial inflammatory milieu induced by preconditioning. Tolerization may provide the opportunity to achieve this goal. Induction of immune tolerance through mucosal exposure to myelin antigens or E-selectin promotes a protective Th2 response, which acts through multiple pathways to suppress the deleterious effects of inflammation47. Due to the need to establish tolerization prior to injury, this approach, like preconditioning, would be more appropriate for stroke prevention in high-risk patients than acute stroke treatment. Another strategy is based on administration of RTL (Table 3). As discussed in the section on adaptive immunity, RTL suppresses the infiltration of inflammatory cells and provides neuroprotection even if administered after the onset of cerebral ischemia49. Similarly, administration of the immunomodulatory co-polymer Poly-YE ameliorates neurological function without reducing injury volume, an effect attributed to increased production of growth factors and hippocampal neurogenesis103. The full translational potential of treatments based on immunomodulation has not been established. For example, therapeutic window, efficacy in females and in aging, protection in the presence of cardiovascular risk factors and efficacy in higher order species need further exploration. Nevertheless, its powerful protective effects justify additional investigations in this direction.
The recent identification of IL-17 secreting T cells as a critical effector of the tissue damage in autoimmune diseases (Th17 cells)104 and cerebral ischemia (γδT cells)41 raises the possibility that counteracting IL-17 could be beneficial in cerebral ischemia as in experimental allergic encephalomyelitis104. Boosting the protective roles of Treg could also be beneficial42, although a destructive role of these cells has also been proposed105. These approaches would be desirable because they target the delayed phase of the injury and are anticipated to have a particularly wide therapeutic window. However, as noted above the role of lymphocytes in ischemic injury is poorly understood and the full implications of suppressing the action of specific T and B-cell populations remain to be defined.
Fighting inflammation: a double-edged sword?
As discussed earlier in this review, immune cells and inflammation play an important role in tissue repair and reorganization. These beneficial effects have to be considered in developing therapeutic approaches based on restraining post-ischemic inflammation. The concern is that counteracting the inflammatory response to ischemic injury may ameliorate the tissue damage in the acute phase, but it may compromise repair mechanisms and worsen the long-term outcome of the injury. Due to the paucity of experimental studies in the recovery phase, there is no definitive experimental evidence that anti-inflammatory treatments interfere with repair processes in the post-ischemic brain. However, pro-survival effects of immune cells stemming from growth factor production, neurogenesis and neuroplasticity are well-established (Table 2)105. The essential role of inflammation in tissue repair highlights the difficulties with approaches based on full-blown suppression of inflammation. Furthermore, in light of stroke-induced immunosuppression, the infectious complications of therapies suppressing inflammation also need to be taken into account (Table 3). Therapies based on immunomodulation, in which the overall immune response is deviated from a Th1 to a Th2-type response, also have a dark side. In models of MS, tolerization with myelin antigens induces a protective Th2 response in the acute phase, but, in the long term, such Th2 response promotes B-cell differentiation and leads to a humoral attack against myelin which worsens the neurological outcome106. A similar worsening in the chronic phase has also been reported in tolerization applied to models of cerebral ischemia107. Therefore, the delayed effects of humoral immunity could counteract the short-term benefit of suppression of cellular immunity. A more complete understanding of the immunology of stroke would enable the development of targeted approaches to selectively suppress the deleterious effects of inflammation.
Conclusions
Immunity and inflammation are an integral part of the pathogenic processes triggered by I/R. Inflammatory signaling is responsible for early molecular events triggered by the arterial occlusion and culminating in the invasion of the brain by blood-borne leukocytes. Although its ultimate goal is to reestablish homeostasis, inflammation inflicts considerable damage to the metastable penumbral tissue. Adaptive immunity is deeply involved in the central and peripheral events triggered by cerebral ischemia, but evidence that a classical autoimmune response against brain antigens unveiled by tissue damage contributes to the acute phase of the damage is lacking. Lymphocytes invade the ischemic brain and contribute to tissue damage, but the rapidity of their deleterious effect is not consistent with an adaptive immune response targeted to the brain. Remarkably, selected lymphocyte subpopulations are protective, acting to dampen the cytotoxic effects of other inflammatory cells and promoting tissue recovery. The mechanistic bases for this dichotomous response of lymphocytes remains to be elucidated (Fig. 3). Although the participation of inflammation and immunity in tissue recovery is well established in other organs, very little is known about these processes in the brain. Even less clear are the long-term effects of the adaptive immune response associated with stroke, and their role in the sequelae of ischemic damage, such as brain atrophy and dementia. Similarly, it is unclear if the immune system develops a memory of the antigen exposure and if an autoimmune response develops following subsequent exposure to the same antigen, as it may occur in cases of recurrent strokes. Although much has been learned on the interplay between the peripheral immunosuppression and the central immune activation associated with stroke, the impact that these processes have on short and long-term tissue outcome remains poorly understood. The realization that the immune system and inflammation are central to the pathophysiology of stroke has raised the prospect of new therapeutic approaches to counteract ischemic injury. However, our understanding of the crosstalk between the immune system and the ischemic brain is still rudimentary and, as suggested by failed clinical trials, not adequate to guide therapeutic interventions. Modulation of adaptive immunity may afford the opportunity to deviate the post-ischemic immune response away from tissue damage and towards protection, an approach very effective in stroke models. However, immunomodulation can also have deleterious effects that need to be considered. Nevertheless, the remarkable impact that modulation of the immune system has on stroke damage and repair, justifies the aggressive pursuit of basic and clinical investigations seeking to unravel the fundamental processes governing the interaction of the ischemic brain with the immune system.
Box 4: How does inflammation kill?
ROS, NO, the complement system, apoptosis inducing receptors, and the perforin pathway represent the major effectors of cell death in inflammation.
ROS and NO
In the setting of inflammation, O2− is produced mainly by NADPH oxidase, an enzyme expressed in virtually all inflammatory cells (Box 2 and 3). At the same time, large amounts of NO are produced by de novo expression of iNOS89. NO reacts preferentially with O2− to form the cytotoxic molecule peroxynitrite. H2O2 is derived from O2− dismutation and gives rise to the highly toxic hydroxyl radicals via the Haber-Weiss reaction, facilitated by the increased availability of free iron in ischemia127. These toxic molecules alter cellular proteins, lipids, and ribonucleic acids leading to cell dysfunction or death and have been implicated in the tissue damage produced by post-ischemic inflammation. For example, suppression of NADPH oxidase or iNOS activity protects the brain in the late phase of cerebral ischemia (Supplemental Table 1).
The complement system
The complement system is a proteolytic cascade composed of different subunits (C1 through C9) that leads to cell lysis via the formation of a pore like structure assembled from C9 termed the membrane attack complex (MAC). Complement also enhances phagocytosis (opsonization), or acts as a chemotactic-activating stimulus for inflammatory cells. The classical pathway for complement activation involves the interaction of the C1q subunits with immune complexes or IgM to activate the cascade. The lectin pathway utilizes mannose and N-acetylglucosamine-binding lectin to activate subunits C2 and C3, while the alternative pathway amplifies the C3 activity generated by the other pathways to form the MAC. After ischemia, complement is activated and its components are either upregulated in glia and neurons, or enter the brain through BBB breakdown128. The involvement of complement in ischemic damage is indicated by the fact that deletion of endogenous complement inhibitors increases brain injury, whereas their upregulation or administration is protective (Supplemental Table 1).
Apoptosis inducing receptors (Fas and TRAIL)
Fas (CD95), a member of the TNF-receptor superfamily, is expressed on neurons and glia after ischemia129, while its ligand FasL (CD95L) is present in neurons, microglia, cytotoxic T cells (CTL), γδT cells and NK cells130. Fas ligation results in formation of the death inducing signaling complex (DISC) and subsequent activation of caspase-8 and of the pro-apoptotic factor Bid. Similarly, the TNF-related apoptosis-inducing ligand (TRAIL) is expressed de novo after ischemia in astrocytes and microglia131, and induces apoptosis by engaging its receptors on neurons and glia. This pathway is involved in ischemic cell death because TRAIL inhibition or Fas mutation ameliorates ischemic injury131,132.
Perforin/Granzyme
This pathway is utilized by CTL, NKT, and NK cells and usually requires molecular recognition associated with MHC class I or class I-like molecules. After engagement of accessory surface receptors, e.g., ICAM-1, degranulation occurs releasing the protease granzyme, perforin - homologous to the complement subunit C9 - and the proteoglycan serglycin. This complex is internalized by the target cell, and granzyme triggers apoptosis by activating caspase-3 and Bid. The involvement of this pathway in ischemic brain injury is suggested by the observations that genetic deletion of perforin is protective133.
Supplementary Material
References
- 1.Mena H, Cadavid D, Rushing EJ. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol. 2004;108:524–530. doi: 10.1007/s00401-004-0918-z. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meisel C, Schwab J, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 4.Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. 2009;158:1174–1183. doi: 10.1016/j.neuroscience.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol. 2009;65:239–248. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- 6.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinsky DJ, et al. Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. The Journal of clinical investigation. 1996;97:493–500. doi: 10.1172/JCI118440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 11.Hyman MC, et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. The Journal of clinical investigation. 2009;119:1136–1149. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12:193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atochin DN, et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. The Journal of clinical investigation. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa M, Zhang JH, Nanda A, Granger DN. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339–1347. doi: 10.2741/1330. [DOI] [PubMed] [Google Scholar]
- 15.Yemisci M, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 16.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 17.Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- 19.Konsman JP, Drukarch B, Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (Lond) 2007;112:1–25. doi: 10.1042/CS20060043. [DOI] [PubMed] [Google Scholar]
- 20.Sairanen TR, Lindsberg PJ, Brenner M, Siren AL. Global forebrain ischemia results in differential cellular expression of interleukin-1beta (IL-1beta) and its receptor at mRNA and protein level. J Cereb Blood Flow Metab. 1997;17:1107–1120. doi: 10.1097/00004647-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Melani A, et al. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Schock SC, et al. Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res. 2007;1168:129–138. doi: 10.1016/j.brainres.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 25.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 26.Bune LT, Thaning P, Johansson PI, Bochsen L, Rosenmeier JB. Effects of nucleotides and nucleosides on coagulation. Blood Coagul Fibrinolysis. 2010;21:436–441. doi: 10.1097/MBC.0b013e328338db27. [DOI] [PubMed] [Google Scholar]
- 27.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Peachell P. Regulation of mast cells by β-agonists. Clin Rev Allergy Immunol. 2006;31:131–142. doi: 10.1385/CRIAI:31:2:131. [DOI] [PubMed] [Google Scholar]
- 29.Samson MT, et al. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. J Immunol. 2003;170:4953–4962. doi: 10.4049/jimmunol.170.10.4953. [DOI] [PubMed] [Google Scholar]
- 30.Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoek RM, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto H, et al. Expression of CD200 by macrophage-like cells in ischemic core of rat brain after transient middle cerebral artery occlusion. Neurosci Lett. 2007;418:44–48. doi: 10.1016/j.neulet.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 34.Chapman GA, et al. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20:RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felger JC, et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun. 2010;24:724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostulas N, et al. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke. 2002;33:1129–1134. doi: 10.1161/hs0402.105379. [DOI] [PubMed] [Google Scholar]
- 37.Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz A, et al. Transient decrease in circulating dendritic cell precursors after acute stroke: potential recruitment into the brain. Clin Sci. 2010;118:147–157. doi: 10.1042/CS20090154. [DOI] [PubMed] [Google Scholar]
- 39.Hurn PD, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinschnitz C, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 41.Shichita T, et al. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 42.Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 43.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4(+)FoxP3(+) regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2010 doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker KJ, et al. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frenkel D, et al. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. Journal of immunology (Baltimore, Md : 1950) 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- 46.Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- 47.Becker KJ. Sensitization and tolerization to brain antigens in stroke. Neuroscience. 2009;158:1090–1097. doi: 10.1016/j.neuroscience.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frenkel D, et al. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Subramanian S, et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itakura A, et al. Characterization of human platelet binding of recombinant T cell receptor ligand. Journal of neuroinflammation. 2010;7:75. doi: 10.1186/1742-2094-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbas Ak. Basic Immunology Updated Edition: Functions and Disorders of the Immune System. Saunders; 2010. [Google Scholar]
- 52.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 53.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2010;88:107–116. doi: 10.1038/icb.2009.94. [DOI] [PubMed] [Google Scholar]
- 55.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 57.Schilling M, et al. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Denes A, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- 59.Rappert A, et al. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci. 2004;24:8500–8509. doi: 10.1523/JNEUROSCI.2451-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 61.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenberg DA, Jin K. Growth factors and stroke. NeuroRx. 2006;3:458–465. doi: 10.1016/j.nurx.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmichael ST. Translating the frontiers of brain repair to treatments: starting not to break the rules. Neurobiol Dis. 2010;37:237–242. doi: 10.1016/j.nbd.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 65.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li S, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayakawa K, et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:871–882. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang ZG, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Hao Q, et al. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 71.Manoonkitiwongsa PS, Schultz RL, McCreery DB, Whitter EF, Lyden PD. Neuroprotection of ischemic brain by vascular endothelial growth factor is critically dependent on proper dosage and may be compromised by angiogenesis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:693–702. doi: 10.1097/01.WCB.0000126236.54306.21. [DOI] [PubMed] [Google Scholar]
- 72.Zhang ZG, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. The Journal of clinical investigation. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denes A, et al. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jickling GC, et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann Neurol. 2010;68:681–692. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Offner H, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 76.Emsley HCA, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. doi: 10.1016/s0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 77.Smith CJ, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haeusler KG, et al. Immune responses after acute ischemic stroke or myocardial infarction. International journal of cardiology. 2010 doi: 10.1016/j.ijcard.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 79.Gendron A, et al. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in Wistar rats. Brain Res. 2002;955:85–97. doi: 10.1016/s0006-8993(02)03368-1. [DOI] [PubMed] [Google Scholar]
- 80.Vogelgesang A, et al. Functional status of peripheral blood T-cells in ischemic stroke patients. PLoS ONE. 2010;5:e8718. doi: 10.1371/journal.pone.0008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 82.Prass K, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 84.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zierath D, et al. CNS immune responses following experimental stroke. Neurocrit Care. 2010;12:274–284. doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fonarow GC, et al. Timeliness of Tissue-Type Plasminogen Activator Therapy in Acute Ischemic Stroke: Patient Characteristics, Hospital Factors, and Outcomes Associated With Door-to-Needle Times Within 60 Minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 88.Gesuete R, et al. Recombinant C1 inhibitor in brain ischemic injury. Ann Neurol. 2009;66:332–342. doi: 10.1002/ana.21740. [DOI] [PubMed] [Google Scholar]
- 89.Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J. Physiol. 1995;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- 90.Lee S-T, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain : a journal of neurology. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 91.Prestigiacomo CJ, et al. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- 92.Stowe AM, et al. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis. 2009;35:82–90. doi: 10.1016/j.nbd.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.del Zoppo GJ. Lessons from stroke trials using anti-inflammatory approaches that have failed. In: Dirnagl U, Elger B, editors. Neuroinflammation in stroke. Berlin - Heidelberg: Springer-Verlagh; 2004. pp. 155–184. [DOI] [PubMed] [Google Scholar]
- 94.Furuya K, et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32:2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- 95.Fagan SC, et al. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke; a journal of cerebral circulation. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jensen HA, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123:714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- 97.Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling--a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- 98.Marsh B, et al. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-κB is a key event in brain tolerance. J Neurosci. 2001;21:4668–4677. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kunz A, et al. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohtsuki T, Ruetzler CA, Tasaki K, Hallenbeck JM. Interleukin-1 mediates induction of tolerance to global ischemia in gerbil hippocampal CA1 neurons. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:1137–1142. doi: 10.1097/00004647-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Pradillo JM, et al. TNFR1 upregulation mediates tolerance after brain ischemic preconditioning. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:193–203. doi: 10.1038/sj.jcbfm.9600019. [DOI] [PubMed] [Google Scholar]
- 103.Ziv Y, et al. A novel immune-based therapy for stroke induces neuroprotection and supports neurogenesis. Stroke. 2007;38:774–782. doi: 10.1161/01.STR.0000255784.27298.23. [DOI] [PubMed] [Google Scholar]
- 104.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 105.Schwartz M, London A, Shechter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience. 2009;158:1133–1142. doi: 10.1016/j.neuroscience.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 106.Genain CP, et al. Late complications of immune deviation therapy in a nonhuman primate. Science (New York, NY) 1996;274:2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- 107.Gee JM, et al. Long term immunologic consequences of experimental stroke and mucosal tolerance. Exp Transl Stroke Med. 2009;1:3. doi: 10.1186/2040-7378-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lipton P. Ischemic Cell Death in Brain Neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 109.Bacigaluppi M, Comi G, Hermann DM. Animal models of ischemic stroke. Part one: modeling risk factors. Open Neurol J. 2010;4:26–33. doi: 10.2174/1874205X01004020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- 111.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 113.Bechmann I, et al. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 114.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 116.Tanaka R, et al. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 117.Geissmann F, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reichmann G, Schroeter M, Jander S, Fischer HG. Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol. 2002;129:125–132. doi: 10.1016/s0165-5728(02)00184-4. [DOI] [PubMed] [Google Scholar]
- 119.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 121.Kreisel D, et al. Mouse vascular endothelium activates CD8+ T lymphocytes in a B7-dependent fashion. J Immunol. 2002;169:6154–6161. doi: 10.4049/jimmunol.169.11.6154. [DOI] [PubMed] [Google Scholar]
- 122.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 123.Wan YY. Multi-tasking of helper T cells. Immunology. 2010;130:166–171. doi: 10.1111/j.1365-2567.2010.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 125.Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 126.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 127.Selim MH, Ratan RR. The role of iron neurotoxicity in ischemic stroke. Ageing Res Rev. 2004;3:345–353. doi: 10.1016/j.arr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 128.Yanamadala V, Friedlander RM. Complement in neuroprotection and neurodegeneration. Trends Mol Med. 2010;16:69–76. doi: 10.1016/j.molmed.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosenbaum DM, et al. Fas (CD95/APO-1) plays a role in the pathophysiology of focal cerebral ischemia. J Neurosci Res. 2000;61:686–692. doi: 10.1002/1097-4547(20000915)61:6<686::AID-JNR12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 130.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cui M, et al. Blocking TRAIL-DR5 signaling with soluble DR5 reduces delayed neuronal damage after transient global cerebral ischemia. Neurobiol Dis. 2010;39:138–147. doi: 10.1016/j.nbd.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 132.Martin-Villalba A, et al. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci. 1999;19:3809–3817. doi: 10.1523/JNEUROSCI.19-10-03809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liesz A, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain : a journal of neurology. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- 134.Jauch EC, et al. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 135.Bornstein NM, et al. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- 136.Dambinova SA, et al. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- 137.Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670–2677. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- 138.Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- 139.Rocklin RE, Sheremata WA, Feldman RG, Kies MW, David JR. The Guillain-Barré syndrome and multiple sclerosis. In vitro cellular responses to nervous-tissue antigens. N Engl J Med. 1971;284:803–808. doi: 10.1056/NEJM197104152841501. [DOI] [PubMed] [Google Scholar]
- 140.Gordon PH, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet neurology. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 141.Chen Y, et al. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci U S A. 2003;100:15107–15112. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet neurology. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote Ischemic Limb Preconditioning After Subarachnoid Hemorrhage: A Phase Ib Study of Safety and Feasibility. Stroke; a journal of cerebral circulation. 2011 doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. Journal Neuroimmunol. 1999;98:77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 145.Schilling M, Strecker JK, Ringelstein EB, Kiefer R, Schabitz WR. Turn-over of meningeal and perivascular macrophages in the brain of MCP-1-, CCR-2- or double knockout mice. Exp Neurol. 2009;219:583–585. doi: 10.1016/j.expneurol.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 146.Terao S, et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 148.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 149.Tesco G, et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kunz A, et al. Nuclear factor-κB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J Neurosci. 2008;28:1649–1658. doi: 10.1523/JNEUROSCI.5205-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromolecular Med. 2005;7:229–242. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- 152.Brait VH, et al. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koizumi S, et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 155.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 156.Strle K, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 157.Buisson A, et al. Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor β1. FASEB J. 1998;12:1683–1691. [PubMed] [Google Scholar]
- 158.Grilli M, et al. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- 159.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. Febs J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.