Abstract
Despite some common risk factors for AF being more prevalent amongst blacks, African-Americans are increasingly being reported with lower prevalence and incidence of atrial fibrillation (AF) compared to Caucasians or whites. Contemporary studies have not provided a complete explanation for this apparent AF paradox in African Americans. Although many traditional and novel risk factors for AF have been identified, the role of ethnic-specific risk factors has not been examined. Whereas hypertension has been the most common risk factor associated with AF, coronary artery disease (CAD) also plays an important role in AF pathophysiology in whites. Thereby, elucidating the role of ethnic-specific risk factors for AF may provide important insight into why African Americans are protected from AF or why whites are more prone to develop the arrhythmia. The link between AF susceptibility and genetic processes has only been recently uncovered. Polymorphisms in renin-angiotensin system genes have been characterized as predisposing to AF under certain environmental conditions. A number of ion channel genes, signaling molecules and several genetic loci have been linked with AF. Thereby, studies investigating genetic variants contributing to the differential AF risk in individuals of African American versus European ancestry may also provide important insight into the etiology of the AF paradox in blacks.
Introduction
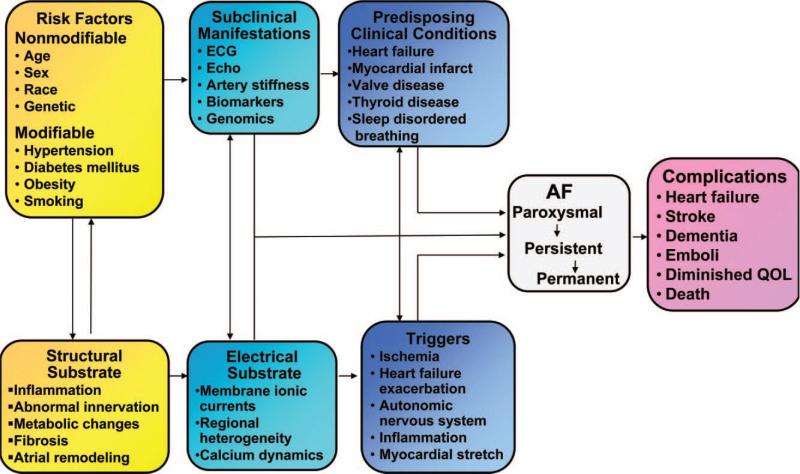
Atrial fibrillation (AF) is the most commonly encountered sustained arrhythmia among ambulatory and hospitalized patients. It is a significant cause of morbidity and mortality which consumes a substantial amount of healthcare spending, and with increasing prevalence projected to more than double by 2050.1,2 AF is often a progressive and self-perpetuating disease with complex mechanisms. It has been determined that abnormally rapid impulses from within the pulmonary veins can trigger initiation and maintenance of AF where there is a conducive and vulnerable substrate.3 The substrate for AF typically result from the effects of both electrical and structural remodeling that can arise from chronic hypertension, cardiac ischemia or infarction, autonomic disorder and genetic mutations amongst many others. AF induces further electrophysiologic changes in the atria by affecting calcium homeostasis leading to shortening of atrial effective refractory period as well as structural remodeling with atrial fibrosis, stretch, and dilatation. The ultimate consequence of these changes is a perpetuation of the arrhythmia described by the dictum “AF begets AF” (Figure 1).4
Figure 1.
Conceptual model of atrial fibrillation (AF) pathophysiology and pathogenesis. [Adapted from 65].
The Framingham heart study (FHS) identified demographic and clinical risk factors for AF in a population-based cohort of predominantly Caucasians. Congestive heart failure (CHF) and rheumatic heart disease were reported as the most powerful predictors and precursors of AF.5 Subsequently, risk factors were updated to include age, sex, diabetes, hypertension, myocardial infarction, in addition to CHF and rheumatic heart disease.6 The epidemiology of AF has changed over the past half-century with rheumatic valvular heart disease becoming almost extinct in the western world, while new risk factors such as sleep apnea,7 obesity,8 and metabolic syndrome have emerged.9, 10 Recently, chronic kidney disease (CKD) has been associated with increased risk of AF,11, 12 and doubles the risk of mortality among hemodialysis patients.13
The disproportionate rates of AF between blacks and whites are increasingly being recognized.14-18 Some risk factors for AF particularly constituents of metabolic syndrome such as hypertension, diabetes, BMI (body mass index), waist circumference, and obesity have been reported as more prevalent in blacks.19 Recent approaches have incorporated these risk factors into the metabolic syndrome, and have shown this to increase the risk for AF,9 and by equally comparable measures in both blacks and whites.10 Nevertheless, there remains a strong body of evidence in support of overall lower rates in the incidence and prevalence of AF among blacks, and hence a perceived paradox. Although a number of recent publications continue to raise doubts,20, 21. The terms “blacks or African Americans”, “whites or Caucasians”, “race” and “ethnicity” will be used in this paper by historical standards but understood to be broad generalizations being applied to a heterogenous group of persons based chiefly on the dominant phenotypic expression. In this review, we use these terms interchangeably but with keen awareness and understanding of the complexities inherent in the definitions. The role of race and genetic background on AF remains largely under-appreciated. Here we will discuss available data that supports the existence of an AF paradox in African Americans and highlight areas where further investigation are warranted for improved understanding of this phenomenon.
The Case for Racial Differences in AF burden
Upshaw22 published the first report in 2002 of his analysis of ECGs from 2123 consecutive patients at an urban Atlanta hospital. The prevalence of AF in blacks was about 2.5% as compared with 7.8% in whites. This difference was seen through all the age subgroups divided in decades. Both blacks and whites showed similar pattern of increasing prevalence with age. A database analysis of more than half a million male veterans with the diagnosis of AF from the United States Department of Veterans Affairs (VA) system database followed up with a VA health survey conducted in 1999 also revealed a significantly higher prevalence of AF amongst whites.16 Atherosclerotic Risk In Communities (ARIC) is a prospective study registry which enrolled 15,792 participants between 1987-2004 from four communities in parts of the united states historically termed ‘the stroke belt’. These are areas with high prevalence of cardiovascular risk factors. Alonso et al17 in their publication from the ARIC study registry also reported a lower incidence of AF amongst blacks. Another pertinent presentation at the 2009 Heart Rhythm Society annual meeting showing analysis of data from the Henry Ford Health System of ECGs during the year 2007 also concluded a disparate racial prevalence of AF.18, 23 That study, based on almost 200,000 patients, showed whites with twice the prevalence of AF (2.5% vs. 1.2%) as compared with blacks, despite a higher prevalence of hypertension and diabetes amongst black patients. In a more recent study, Nazeri et al24 identified race as a risk factor for new onset post-operative AF after isolated coronary artery bypass surgery (CABG). In addition to obesity, CHF, and advanced age, the authors found that Caucasians were at higher risk for post-operative AF than persons of other races. This variation was not explained by differences in traditional risk factors for post-operative AF. Therefore, while studies have consistently found higher prevalence of hypertension and strokes in blacks,25 the lower incidence and prevalence of AF in this group remains a perplexing missing link.
The Role of Risk Factors in AF
It is noteworthy that just as all of these studies have consistently reported racial differences in prevalence of AF, careful review of the literature has also consistently found significant racial differences in the prevalence of CAD. Therefore, race could be a confounding factor and perhaps a surrogate for comparing the relative effects of hypertension and CAD on AF risk in the different races. In a racially homogenous Danish population, hypertension was found in less than 20% of AF patients as compared with CAD which was found in almost 50%.26 Another homogenous population-based study evaluated numerous variables including age, gender, history of CAD, BMI, anti-hypertensive medications, waist circumference, fasting glucose, hemoglobin A1C, fibrinogen levels and left atrial enlargement on ECG as risk factors for AF but found that male gender and a history of CAD were the most powerful predictors of new-onset AF among Koreans 27. In addition, a recent publication reporting follow up of the Systolic Hypertension in Elderly (SHEP) cohort reported a 2% incidence of AF during the seven year study duration.28 Furthermore, CHF and myocardial infarction were found to be most strongly associated with AF. These studies would suggest some influence of CAD on manifestation of AF. Dissecting the relative impact of hypertension and CAD in the etiology of AF within and between the races is essential. The African American Heart Failure trial (A-HeFT) subgroup analysis found AF to occur at higher rates in black males than black females but with worse adverse impact on survival for women than men.29 Overall, 17% of the patients in A-HeFT were reported with AF which is comparable to 19.7% reported for blacks in EPOCH cohort, which was about half the incidence rate of 38.3% for whites with heart failure.14
The FHS based on a predominantly Caucasian population could not ascertain a differential impact of race on AF, but it has further recognized the complexity and interactive effects of the known risk factors as contributing unequally to the development of AF.30 The 10-year composite risk score predictive model for AF has been developed from the Framingham cohort. Hypertension remains perhaps the most commonly encountered risk factor in the general population but it does not portend the highest risk for developing AF. The Framingham risk score is yet to include the impact of race and genetic factors on modulating AF risk.
AF Paradox in African Americans
Some previous reports have postulated that the apparent AF paradox in African Americans may in part be due to methodological anomalies such as differential AF under-ascertainment between different racial and ethnic groups; a reflection of unequal access to healthcare and thereby population bias; or that disease subtypes such as paroxysmal AF may occur more often in blacks, and finally inadequately sensitive methods for AF diagnosis.21 Although we recognize some of these elements have applied historically to creating the manifest disparity in cardiovascular care for minorities, we believe there is sufficient and convincing data from numerous well-designed large epidemiological studies to counter this being contributory to the lower rates of AF amongst African Americans. The lower rates amongst blacks have now been consistently established from prospective and retrospective cohorts, amongst ambulatory and hospitalized patients with heart failure, in CKD and hemodialysis patients, among post CABG cohorts, and within prospective cohort studies weighted heavily even for over-selection of African American subjects.17
The issue of sensitivity of methods used for diagnosing AF, and particularly P-wave signal averaged electrocardiogram (P-SAECG) is deserving of a brief discussion. The clinical and predictive utility of this technology for paroxysmal AF was first reported almost two decades ago in cohorts of Japanese and Polish subjects respectively.31, 32 These early reports found abnormally increased baseline P-SAECG parameters in patients with paroxysmal AF (measured during sinus rhythm) as compared with control patients without history of the arrhythmia. Subsequently, it was found to be more sensitive than standard 12-lead ECG in showing abnormalities in patients at early stages of hypertension. We previously reported prolonged and incremental measurable changes in P-wave duration and P-wave voltage integral in patients with stage 1 and stage 2 hypertension but without any notable abnormality on standard 12 lead ECG.33 This study showed similar values between black and white normotensives but noted a significantly disproportional increase in P-SAECG measurements amongst hypertensive blacks as compared to whites. Although AF was not identified in either of our study groups, the recent report by Soliman et al,21 supported our previous finding of increased abnormal P-SAECG values in black hypertensive patients. However, in an attempt to link abnormal SAECG findings to a higher burden of stroke in blacks, the investigators postulated that this may represent a surrogate-marker for AF and hence stroke risk in blacks. This is despite the reported lower incidence of AF for blacks within their study population. Nevertheless, they suggested there may be a higher burden of subclinical or paroxysmal AF amongst blacks. However, there is little evidence in the literature to support the idea that blacks experience more episodes of paroxysmal AF than whites. Racial differences in cardiac adaptation and remodeling in response to hypertension has been reported.34 A recent pooled analysis from 3 cohort studies reported lower prevalence of AF among African-Americans compared with Caucasians, and also found whites had a left atrial diameter that was on average 2-mm larger than African Americans.35 This was suggested as a potential contributory substrate for the observed higher rates of AF in whites. Additionally, there is poor correlation between electrical and structural parameters in black patients such as ECG criteria for left ventricular hypertrophy (LVH) variably correlating with echocardiographic measurements and determinants.36 Hence, caution must be exercised before drawing such conclusions even when they appear reasonably logical. The validation of new modalities such as P-SAECG is necessary in African Americans subjects to reliably apply such generalizations.
It is now apparent that the manifestation of AF is not only complex but also highly likely the culmination of numerous risk factors. Hypertension is disproportionately prevalent among blacks, and strongly contributes to the risk of developing AF. However, other factors such as lack of CAD may also influence the development of AF in African Americans.
One potential explanation for the AF paradox in African Americans may relate to survival bias, i.e., a form of ascertainment bias due to selective exclusion of individuals who suffer mortality related to AF.37 However, it is important to note that most of the studies reporting the AF paradox in blacks adjusted for age and divided subjects into age groups by decades showing a consistent difference in incidence rates between whites and blacks. Furthermore, racial differences in AF point not only to differences in prevalence but also in incidence rates. Although survival bias is one possible explanation for the racial differences in AF, epidemiological studies would tend to argue against this as a major cause for the AF paradox in African Americans.
Role of Genetic Variants in AF
In the past decade there has been increasing data supporting a genetic predisposition to AF. Investigators at the FHS have observed that the odds of developing AF were 1.8 times higher for individuals with at least one parent diagnosed with AF compared to those without such a parental history.38 The odds ratio (OR) increased further (3.2) if one parent was affected before 75 years of age. Familial aggregation of AF is particularly prominent in individuals with idiopathic or so-called lone AF, i.e., early-onset AF without structural heart disease, for which as many as 30% of probands have a first-degree relative with the arrhythmia.39-41 The familial aggregation and high heritability of AF in these studies are consistent with common genetic mechanisms for the arrhythmia.
A Mendelian pattern of inheritance has been reported in AF kindreds, where most often the proband presents with lone AF. Mutations in genes encoding cardiac ion channels, gap junctions, atrial natriuretic peptide (ANP) and nucleoporins (NUP155) have been reported in isolated cases and small kindreds.42 Although isolated African American kindreds with familial monogenic AF have been described,43 there is a dearth of information on the role of common genetic variants in black populations. Most patients with AF have one or more identifiable risk factors, but many or even most patients with these same risk factors do not develop AF. Over the last decade, many case-control and cohort studies have been performed in subjects with AF, leading to the identification of variants associated with the disease. These studies have typically tested a small number of variants and have been directed at candidate genes previously believed to be involved in AF.
We will highlight common polymorphisms in two important pathways in the pathogenesis of AF. Over the last several years there has also been increasing evidence that activation of the renin-angiotensin-aldosterone system (RAAS) may be an important risk factor for the development of AF. There are retrospective and prospective data suggesting that angiotensin-converting-enzyme (ACE) inhibitor therapy and angiotensin receptor blockers may be associated with a lower incidence of AF in whites. In addition, a case control study of 250 Taiwanese subjects with AF and 250 controls identified polymorphisms in this pathway as risk factors for AF.44 Added support for the increasingly important role of RAAS activation in the pathophysiology of AF comes from a recent study which demonstrated a pharmacogenetic interaction between the ACE I/D polymorphism and efficacy of antiarrhythmic drug therapy in patients with lone AF.45 Most association studies have identified common genetic polymorphisms that increase the risk of developing AF. In contrast, Shreieck and colleagues46 reported that the C825T polymorphism in the coding region of the G-protein β3-subunit (GNB3) gene was associated with a 54% decrease in the adjusted risk for AF. This polymorphism has been functionally characterized and has been shown to modulate atrial inward rectifier potassium current.47 Although this study was performed in whites, it demonstrates the possibility that there may be common polymorphisms in signaling pathways that may reduce the likelihood of developing AF.
The recent advances in genotyping technology haves allowed investigators to assay hundreds of thousands of single nucleotide polymorphisms (SNPs) spread over the entire human genome. In the first genome wide association study (GWAS) of AF, a strong association was discovered between AF and two SNPs on chromosome 4q25.48 About 35% of individuals of European descent have at least one of the DNA sequence variations and the risk of AF increases by 1.72 and 1.39 per copy. The stronger variant is carried by 75% of the Chinese population, in whom it increases the risk of AF by 1.42 per copy. Although the mechanism for this observed association remains unknown, both variants are adjacent to the PITX2 gene, which is critical for cardiac development. In knockout mice, pitx2c has been implicated in the formation of the extension of left atrial myocardium into the pulmonary veins.49 Since abnormal automaticity in this region is now implicated as a common driver for many forms of AF, PITX2 is an obvious candidate gene.50 Recently, a novel genetic locus for typical AF observed in the community was described;51, 52 SNP rs2106261 on chromosome 16q22 was associated with AF with an OR of 1.25 (P = 1.8×10-15) in Caucasian populations.51 However, the role of this and other common variants associated with AF in African Americans remains to be determined.
AF Genetics in African Americans
African Americans are at a low risk for developing AF, despite a higher prevalence of many of the traditional risk factors known to increase the likelihood of AF. Whether environmental or genetic, it would therefore appear that there are ethnic-specific or novel factors that either protect African Americans from AF or make whites especially prone to AF. The etiology of this paradox remains undetermined. One recent study tested the hypothesis that European ancestry is an independent risk factor for AF. Marcus et al.53 showed that European ancestry predicted risk of incident AF and strongly supporting a genetic basis for the observed AF paradox in African Americans.
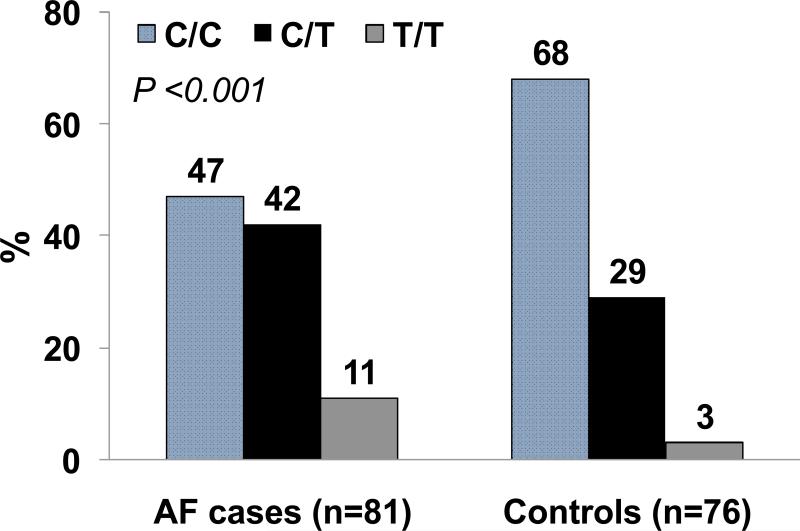
The Vanderbilt-Meharry AF Registry seeks to investigate the genetic basis for AF in African Americans and hopes to fulfill the this knowledge gap by targeted recruitment of African Americans not only for the identification of common AF genetic variants but also to examine whether response to drug therapy is genetically-mediated. We have shown that the response to antiarrhythmic therapy is modulated by the ACE I/D polymorphism in a Caucasian population 45 but its role in modulating response to pharmacologic therapy in African Americans is unknown. In a preliminary analysis of 81 African-Americans with AF enrolled in the Vanderbilt-Meharry AF Registry, and 76 controls, we demonstrated that the common 4q25 variant (rs2200733) was associated with AF with an OR of 2.29 (95% CI: 1.17 - 4.5; P<0.001) (Figure 2). The frequency of the minor allele was 32.1% in African Americans with AF and 17.1% in the control group. Although these results suggest that the non-coding SNP rs2200733 is strongly associated with AF in African Americans, this need to be replicated in larger black cohorts.
Figure 2.
Genotype frequencies for the chromosome 4q25 variant (rs2200733C/T) in African-Americans with atrial fibrillation (AF) and controls.
Future Directions
According to a published study from the Centers for Disease Control AF appears yet to be under-diagnosed in the general population based on analysis of death certificates from 1980-98.54 There is a trend of increasing notations of AF diagnosis on death certificates of blacks, whites, and Hispanics with the highest increases reported among whites. Additionally, racial disparities in awareness and treatment of AF have been reported.55
Cardiac devices such as implantable loop recorders, pacemakers and implantable cardioverter defibrillators (ICDs) are increasing being implanted in patients with heart disease. These patients are often elderly with multiple risk factors for AF. These devices may provide more supportive evidence for racial differences in AF burden in blacks and whites.
Obstructive sleep apnea (OSA) is emerging as a prominent risk factor for AF, and autonomic nervous system (ANS) changes have been shown to contribute to AF burden. 7, 56 This has been reported as the mechanism mediating increased AF that is associated with obesity-sleep apnea syndrome,57, 58 additionally to increased left atrial enlargement associated with obesity.8 Another study has reported racial differences in ANS function.59 An improved understanding of the mechanisms by which the ANS promotes AF may provide important insight into solving the complexity of the racial disparity in AF occurrence.
The manifestations of AF are likely complex and dependent on interplay between known (and unknown) risk factors that contribute to both the substrate and trigger. Whether environmental or genetic, it would appear that there are novel factors either protecting African Americans from AF or making whites especially prone to AF that remain undetermined. Large clinical trials of African Americans with AF may identify some of these novel factors. Larger left atrial diameter in Caucasians has recently been reported.35 The identification of structural elements responsible for the reported difference in atrial dimension could guide future targets and therapies for AF. The genetics of AF continues to evolve,60 with increasing focus on gender and ethnic differences in proteomic markers,61 and continued major advances in the area of pharmacogenomics.62, 63 Future studies on AF genetics should include enrollment of African Americans to identify common and rare genetic variants and signaling pathways that are important in the pathogenesis of the disease. An improved understanding of the molecular basis of AF may provide insight into the observed racial differences in development of AF.
The Jackson Heart Study was started in 2000, and has enrolled a cohort of 6500 African Americans inhabitants of Jackson, Mississippi for a prospective, longitudinal population-based study similar to the FHS.64 The study is unique in its charge to investigate cardiovascular diseases among African Americans. One of its goals is to provide insight into reasons for racial disparity in cardiovascular diseases, and to investigate inherited (genetic) factors that affect hypertension, stroke, diabetes, and heart diseases. This study should offer a unique opportunity to obtain definitive answers on novel and race-specific risk factors for AF among African Americans.
Conclusions
Caucasian race or European ancestry is associated with an increased risk of developing AF. The observed lower prevalence of AF among African Americans despite having a higher burden of risk factors is incompletely understood. The manifestations of AF are complex and likely dependent on the relative and unequal impact of numerous risk factors that modulate not only the substrate but also the triggers for AF. Furthermore, recent studies also strongly suggest that genetic factors may play an important role in the pathophysiology of AF in African Americans. Although CAD did not significantly evolve among the FHS population as an independent risk factor for AF, it is reported with lower prevalence amongst blacks. As such, it is therefore possible that CAD contributes to the differential prevalence and incidence of AF observed between Caucasian and African Americans. Exploring race-specific and novel risk factors for AF as well as determining the underlying genetic basis for the disease may provide important insight into not only the etiology and pathogenesis of AF but also go some way towards explaining the racial differences in AF prevalence between the two races.
Methods
This work was supported in part by NIH grants HL75266 and HL65962 and an AHA Established Investigator award (0940116N). The authors are solely responsible for the design and conduct of this study, all study analyses and drafting and editing of the paper.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Allessie MA, Boyden PA, Camm AJ, Kleber AG, Lab MJ, Legato MJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–77. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 7.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr., Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, et al. Metabolic Syndrome and Risk of Development of Atrial Fibrillation. The Niigata Preventive Medicine Study. Circulation. 2008;117:1255–60. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 159:850–6. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 5:173–81. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, et al. Association of Chronic Kidney Disease With Atrial Fibrillation Among Adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 4:26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The Increasing Prevalence of Atrial Fibrillation among Hemodialysis Patients. J Am Soc Nephrol. 2011;22:349–57. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429–35. doi: 10.1016/j.jacc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Michael Smith J, Soneson EA, Woods SE, Engel AM, Hiratzka LF. Coronary artery bypass graft surgery outcomes among African-Americans and Caucasian patients. Int J Surg. 2006;4:212–6. doi: 10.1016/j.ijsu.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237–45. doi: 10.1016/s0027-9684(15)31212-8. [DOI] [PubMed] [Google Scholar]
- 17.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–7. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang K. African Americans have markedly lower prevalence of atrial fibrillation despite higher prevalence of risk factors. Heart Rhythm. 2009;6(5S):S46. [Google Scholar]
- 19.Smith SC, Jr., Clark LT, Cooper RS, Daniels SR, Kumanyika SK, Ofili E, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111:e134–9. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- 20.Prineas RJ, Soliman EZ, Howard G, Howard VJ, Cushman M, Zhang ZM, et al. The sensitivity of the method used to detect atrial fibrillation in population studies affects group-specific prevalence estimates: ethnic and regional distribution of atrial fibrillation in the REGARDS study. J Epidemiol. 2009;19:177–81. doi: 10.2188/jea.JE20081032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soliman EZ, Alonso A, Goff DC., Jr. Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–56. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 22.Upshaw CB., Jr. Reduced prevalence of atrial fibrillation in black patients compared with white patients attending an urban hospital: an electrocardiographic study. J Natl Med Assoc. 2002;94:204–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Lahiri MK, Fang K, Lamerato L, Khan AM, Schuger CD. Effect of race on the frequency of postoperative atrial fibrillation following coronary artery bypass grafting. Am J Cardiol. 2011;107:383–6. doi: 10.1016/j.amjcard.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Nazeri A, Razavi M, Elayda MA, Lee VV, Massumi A, Wilson JM. Race/ethnicity and the incidence of new-onset atrial fibrillation after isolated coronary artery bypass surgery. Heart Rhythm. 7:1458–63. doi: 10.1016/j.hrthm.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 26.Frost L, Engholm G, Moller H. Husted. Decrease in mortality in patients with a hospital diagnosis of atrial fibrillation in Denmark during the period 1980-1993. Eur Heart J. 1999;20:1592–9. doi: 10.1053/euhj.1999.1713. [DOI] [PubMed] [Google Scholar]
- 27.Kim AM, On YK, Sung J, Kim JH, Song YB, Lee WS. Risk factors for predicting new-onset atrial fibrillation in persons who received health screening tests. Korean Circ J. 2007;37:609–15. [Google Scholar]
- 28.Vagaonescu TD, Wilson AC, Kostis JB. Atrial fibrillation and isolated systolic hypertension: the systolic hypertension in the elderly program and systolic hypertension in the elderly program-extension study. Hypertension. 2008;51:1552–6. doi: 10.1161/HYPERTENSIONAHA.108.110775. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JE, Hellkamp AS, Mark DB, Anderson J, Poole JE, Lee KL, et al. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J. 2008;155:501–6. doi: 10.1016/j.ahj.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr., et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–45. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukunami M, Yamada T, Ohmori M, Kumagai K, Umemoto K, Sakai A, et al. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram. Circulation. 1991;83:162–9. doi: 10.1161/01.cir.83.1.162. [DOI] [PubMed] [Google Scholar]
- 32.Opolski G, Stanislawska J, Slomka K, Kraska T. Value of the atrial signal-averaged electrocardiogram in identifying patients with paroxysmal atrial fibrillation. Int J Cardiol. 1991;30:315–9. doi: 10.1016/0167-5273(91)90009-e. [DOI] [PubMed] [Google Scholar]
- 33.Madu EC, Baugh DS, Gbadebo TD, Dhala A, Cardoso S. Effect of ethnicity and hypertension on atrial conduction: evaluation with high-resolution P-wave signal averaging. Clin Cardiol. 2001;24:597–602. doi: 10.1002/clc.4960240906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olutade BO, Gbadebo TD, Porter VD, Wilkening B, Hall WD. Racial differences in ambulatory blood pressure and echocardiographic left ventricular geometry. Am J Med Sci. 1998;315:101–9. doi: 10.1097/00000441-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, et al. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375, e1–7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DK, Marantz PR, Devereux RB, Kligfield P, Alderman MH. Left ventricular hypertrophy in black and white hypertensives. Standard electrocardiographic criteria overestimate racial differences in prevalence. JAMA. 1992;267(24):3294–9. [PubMed] [Google Scholar]
- 37.Anderson CD, Nalls MA, Biffi A, Rost NS, Greenberg SM, Singleton AB, et al. The Effect of Survival Bias on Case-Control Genetic Association Studies of Highly Lethal Diseases. Circ Cardiovasc Genet. 2011 Feb 3; doi: 10.1161/CIRCGENETICS.110.957928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CS, Parise H, D'Agostino RB, Sr., Lloyd-Jones DM, Vasan RS, Wang TJ, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–5. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 39.Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–92. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 40.Ellinor PT, Yoerger DM, Ruskin JN, Macrae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–84. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 41.Marcus GM, Smith LM, Vittinghoff E, Tseng ZH, Badhwar N, Lee BK, et al. A first-degree family history in lone atrial fibrillation patients. Heart Rhythm. 2008;5:826–30. doi: 10.1016/j.hrthm.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darbar D. Genetics of atrial fibrillation: Rare mutations, common polymorphisms, and clinical relevance. Heart Rhythm. 2008;5:483–6. doi: 10.1016/j.hrthm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, et al. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–35. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai CT, Lai LP, Lin JL, Chiang FT, Hwang JJ, Ritchie MD, et al. Renin-angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109:1640–6. doi: 10.1161/01.CIR.0000124487.36586.26. [DOI] [PubMed] [Google Scholar]
- 45.Darbar D, Motsinger AA, Ritchie MD, Gainer JV, Roden DM. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm. 2007;4:743–9. doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreieck J, Dostal S, von Beckerath N, Wacker A, Flory M, Weyerbrock S, et al. C825T polymorphism of the G-protein beta3 subunit gene and atrial fibrillation: association of the TT genotype with a reduced risk for atrial fibrillation. Am Heart J. 2004;148:545–50. doi: 10.1016/j.ahj.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Dobrev D, Wettwer E, Himmel HM, Kortner A, Kuhlisch E, Schuler S, et al. G-Protein beta(3)-subunit 825T allele is associated with enhanced human atrial inward rectifier potassium currents. Circulation. 2000;102:692–7. doi: 10.1161/01.cir.102.6.692. [DOI] [PubMed] [Google Scholar]
- 48.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;488:353–7. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 49.Mommersteeg MT, Brown NA, Prall OW, de Gier-de Vries C, Harvey RP, Moorman AF, et al. Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–9. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Klysik E, Sood S, Johnson RL, Wehrens XH, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci U S A. 107:9753–8. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–81. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;8:876–8. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, et al. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–15. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980-1998. Am J Epidemiol. 2002;155:819–26. doi: 10.1093/aje/155.9.819. [DOI] [PubMed] [Google Scholar]
- 55.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, et al. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–7. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scherlag BJ, Po S. The intrinsic cardiac nervous system and atrial fibrillation. Curr Opin Cardiol. 2006;21:51–4. doi: 10.1097/01.hco.0000198980.40390.e4. [DOI] [PubMed] [Google Scholar]
- 57.Asirvatham SJ, Kapa S. Sleep apnea and atrial fibrillation: the autonomic link. J Am Coll Cardiol. 2009;54:2084–6. doi: 10.1016/j.jacc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Ghias M, Scherlag BJ, Lu Z, Niu G, Moers A, Jackman WM, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54:2075–83. doi: 10.1016/j.jacc.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 59.Sloan RP, Huang MH, McCreath H, Sidney S, Liu K, Dale Williams O, et al. Cardiac autonomic control and the effects of age, race, and sex: the CARDIA study. Auton Neurosci. 2008;139:78–85. doi: 10.1016/j.autneu.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabeh MK, Macrae CA. The genetics of atrial fibrillation. Curr Opin Cardiol. 2010 Mar 10; doi: 10.1097/HCO.0b013e3283385734. [DOI] [PubMed] [Google Scholar]
- 61.Kullo IJ, Cooper LT. Early identification of cardiovascular risk using genomics and proteomics. Nat Rev Cardiol. 2009;7:309–17. doi: 10.1038/nrcardio.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roden DM. Cardiovascular pharmacogenomics. Circulation. 2003;108:3071–4. doi: 10.1161/01.CIR.0000110626.24310.18. [DOI] [PubMed] [Google Scholar]
- 63.Roden DM, Altman RB, Benowitz NL, Flockhart DA, Giacomini KM, Johnson JA, et al. Pharmacogenomics: Challenges and Opportunities. Ann Intern Med. 2006;145:749–757. doi: 10.7326/0003-4819-145-10-200611210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitka M. New heart study a legacy for the future. JAMA. 2000;283(1):38, 41, 44. [PubMed] [Google Scholar]
- 65.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]