Abstract
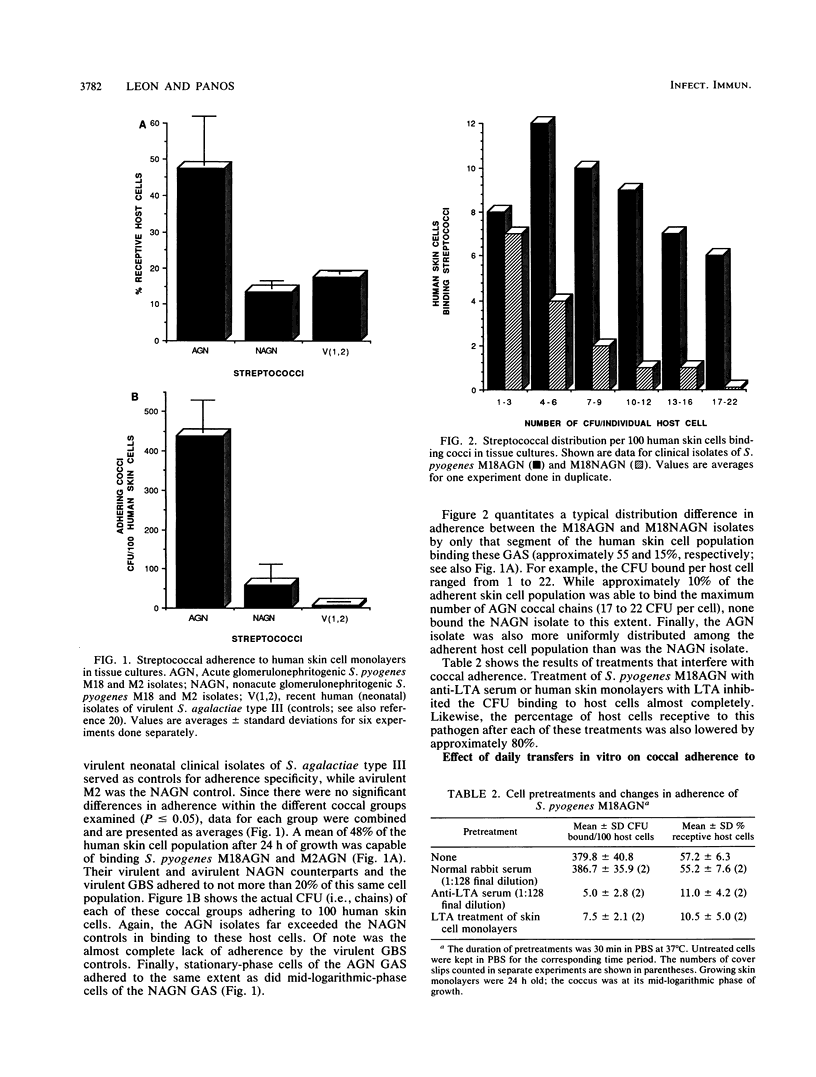
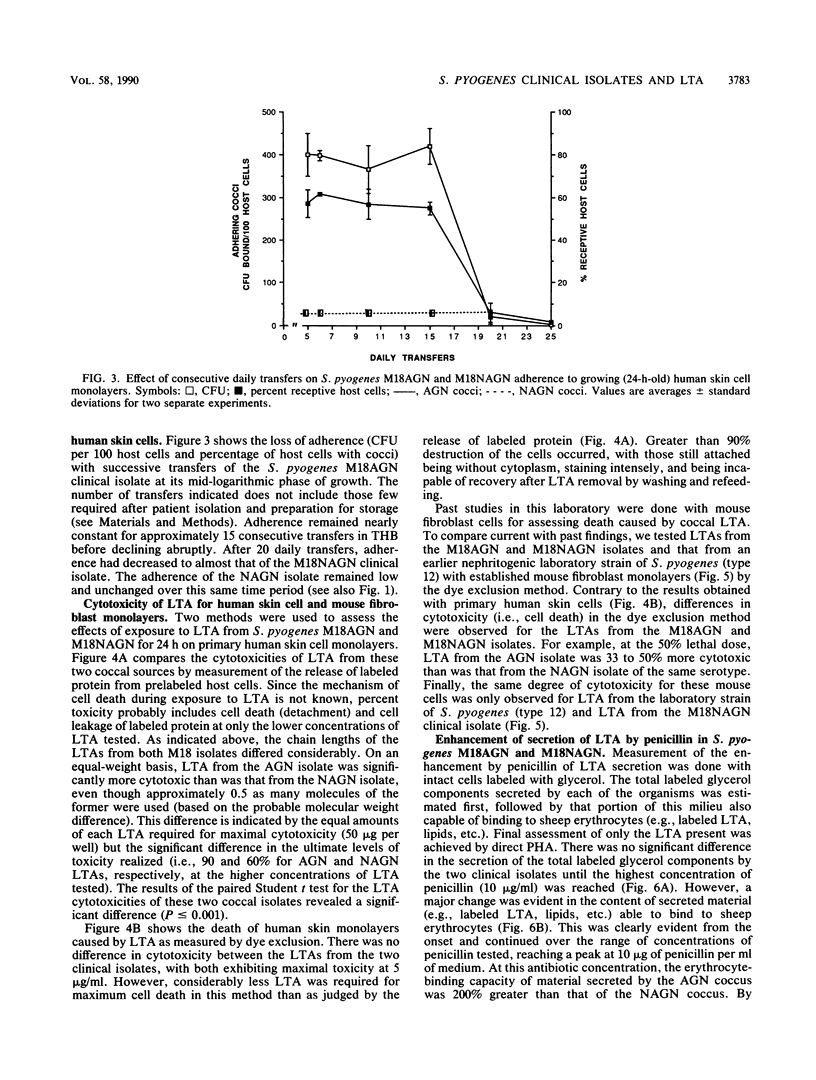
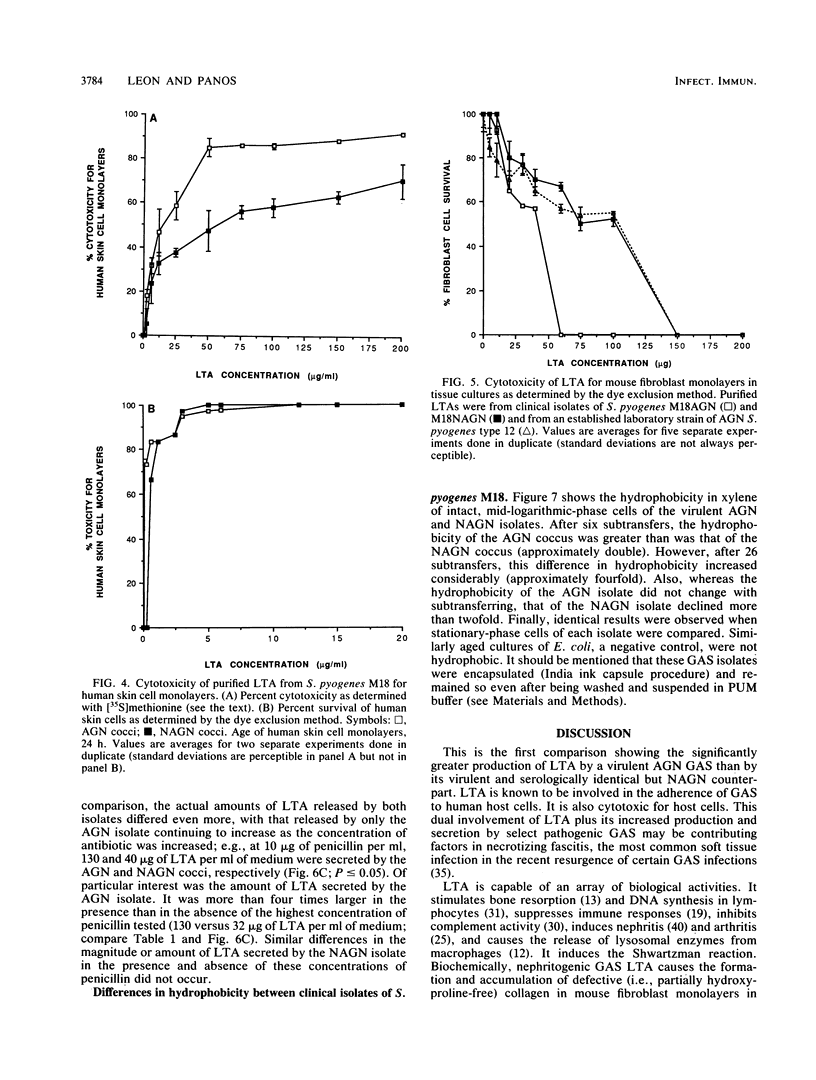
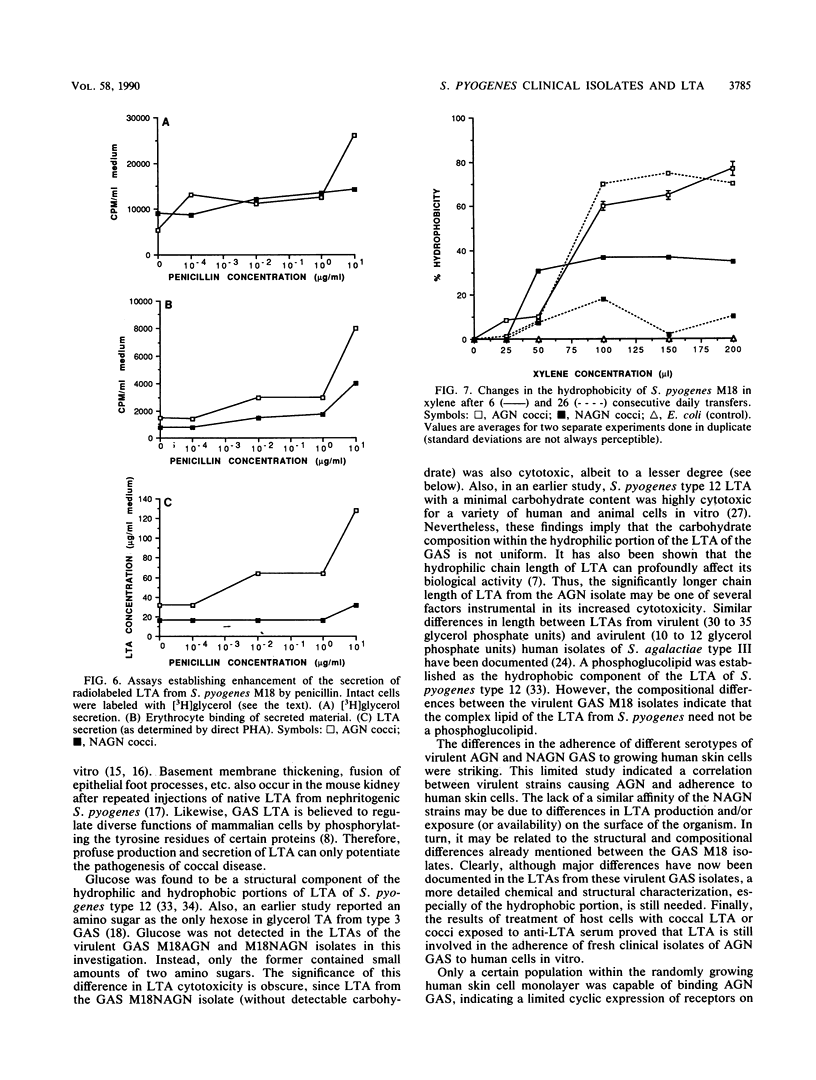
Minimally subcultured clinical isolates of virulent nephritogenic and nonnephritogenic Streptococcus pyogenes of the same serotype showed major differences in lipoteichoic acid (LTA) production, secretion, and structure. These were related to changes in coccal adherence to and destruction of growing human skin cell monolayers in vitro. A possible relationship between cellular LTA content and group A streptococcal surface hydrophobicity was also investigated. Nephritogenic S. pyogenes M18 produced twice as much total (i.e., cellular and secretory) LTA as did the virulent, serologically identical, but nonnephritogenic isolate. Also, the LTAs from these organisms differed markedly. The polyglycerol phosphate chain of LTA from the nephritogenic isolate was longer (1.6 times) than was that from the nonnephritogenic isolate. Likewise, both LTAs indicated the presence of alanine and the absence of glucose. Amino sugars were found in LTA from only nephritogenic S. pyogenes. Teichoic acid, as a cellular component or secretory product, was not detected. The adherence of two different nephritogenic group A streptococcal serotypes (M18 and M2) exceeded that of the serologically identical but nonnephritogenic isolates (by about five times), indicating a correlation between virulent strains causing acute glomerulonephritis and adherence to human skin cell monolayers. Likewise, LTA from nephritogenic S. pyogenes M18 was more cytotoxic (1.5 times) than was that from the nonnephritogenic isolate for human skin cells, as determined by protein release. This difference was not perceptible by the more sensitive dye exclusion method (i.e., requiring less LTA), which emphasizes changes in host cell morphology and death. Also, the secretion of LTA by only virulent nephritogenic S. pyogenes M18 was exacerbated by penicillin (a maximum of four times). Finally, while the adherence of nephritogenic S. pyogenes M18 decreased markedly after continued subculturing in vitro, the surface hydrophobicity did not.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Courtney H. S., Simpson W. A., Beachey E. H. Relationship of critical micelle concentrations of bacterial lipoteichoic acids to biological activities. Infect Immun. 1986 Feb;51(2):414–418. doi: 10.1128/iai.51.2.414-418.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVuono J., Panos C. Effect of L-form Streptococcus pyogenes and of lipoteichoic acid on human cells in tissue culture. Infect Immun. 1978 Oct;22(1):255–265. doi: 10.1128/iai.22.1.255-265.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly C. L., Dale J. B., Courtney H. S., Beachey E. H. Tyrosine phosphorylation of a 94-kDa protein of human fibroblasts stimulated by streptococcal lipoteichoic acid. J Biol Chem. 1985 Oct 25;260(24):13342–13346. [PubMed] [Google Scholar]
- Gaworzewska E., Colman G. Changes in the pattern of infection caused by Streptococcus pyogenes. Epidemiol Infect. 1988 Apr;100(2):257–269. doi: 10.1017/s095026880006739x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt J. C., Jr, Panos C. Teichoic acids of Streptococcus agalactiae: chemistry, cytotoxicity, and effect on bacterial adherence to human cells in tissue culture. Infect Immun. 1984 Feb;43(2):670–677. doi: 10.1128/iai.43.2.670-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Tomasz A. Penicillin-resistant and penicillin-tolerant mutants of group A Streptococci. Antimicrob Agents Chemother. 1982 Jul;22(1):128–136. doi: 10.1128/aac.22.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop P. J., O'Grady R. L., Knox K. W., Wicken A. J. Stimulation of lysosomal enzyme release from macrophages by lipoteichoic acid. J Periodontal Res. 1980 Sep;15(5):492–501. doi: 10.1111/j.1600-0765.1980.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Hausmann E., Lüderitz O., Knox K., Weinfeld N. Structural requirements for bone resorption by endotoxin and lipoteichoic acid. J Dent Res. 1975 Jun;54(SPEC):B94–B99. doi: 10.1177/00220345750540023401. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon O., Panos C. An electron microscope study of kidney basement membrane changes in the mouse by lipoteichoic acid from Streptococcus pyogenes. Can J Microbiol. 1987 Aug;33(8):709–717. doi: 10.1139/m87-124. [DOI] [PubMed] [Google Scholar]
- Leon O., Panos C. Cytotoxicity and inhibition of normal collagen synthesis in mouse fibroblasts by lipoteichoic acid from Streptococcus pyogenes type 12. Infect Immun. 1983 May;40(2):785–794. doi: 10.1128/iai.40.2.785-794.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon O., Panos C. Effect of streptococcal lipoteichoic acid on prolyl hydroxylase activity as related to collagen formation in mouse fibroblast monolayers. Infect Immun. 1985 Dec;50(3):745–752. doi: 10.1128/iai.50.3.745-752.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno T., Slade H. D. Composition and properties of a group A streptococcal teichoic acid. J Bacteriol. 1970 Jun;102(3):747–752. doi: 10.1128/jb.102.3.747-752.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. A., Urban J., Jackson R. W. Effects of a streptococcal lipoteichoic acid on host responses in mice. Infect Immun. 1976 May;13(5):1408–1417. doi: 10.1128/iai.13.5.1408-1417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Leon O., Panos C. Adherence of Streptococcus agalactiae to synchronously growing human cell monolayers without lipoteichoic acid involvement. Infect Immun. 1988 Feb;56(2):505–512. doi: 10.1128/iai.56.2.505-512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ne'eman N., Ginsburg I. Red cell-sensitizing antigen of group A streptococci. II. Immunological and immunopathological properties. Isr J Med Sci. 1972 Nov;8(11):1807–1816. [PubMed] [Google Scholar]
- Nealon T. J., Beachey E. H., Courtney H. S., Simpson W. A. Release of fibronectin-lipoteichoic acid complexes from group A streptococci with penicillin. Infect Immun. 1986 Feb;51(2):529–535. doi: 10.1128/iai.51.2.529-535.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Association of elevated levels of cellular lipoteichoic acids of group B streptococci with human neonatal disease. Infect Immun. 1983 Mar;39(3):1243–1251. doi: 10.1128/iai.39.3.1243-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Kinetic and chemical analyses of the biologic significance of lipoteichoic acids in mediating adherence of serotype III group B streptococci. Infect Immun. 1985 Oct;50(1):107–115. doi: 10.1128/iai.50.1.107-115.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect Immun. 1984 Feb;43(2):523–530. doi: 10.1128/iai.43.2.523-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., Pilowsky K., Zilm P. S. The effect of growth rate on the adhesion of the oral bacteria Streptococcus mutans and Streptococcus milleri. Arch Oral Biol. 1984;29(2):147–150. doi: 10.1016/0003-9969(84)90119-5. [DOI] [PubMed] [Google Scholar]
- Silvestri L. J., Knox K. W., Wicken A. J., Hoffmann E. M. Inhibition of complement-mediated lysis of sheep erythrocytes by cell-free preparations from Streptococcus mutans BHT. J Immunol. 1979 Jan;122(1):54–60. [PubMed] [Google Scholar]
- Simpson W. A., Dale J. B., Beachey E. H. Cytotoxicity of the glycolipid region of streptococcal lipoteichoic acid for cultures of human heart cells. J Lab Clin Med. 1982 Jan;99(1):118–126. [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Beachey E. H. Binding of streptococcal lipoteichoic acid to the fatty acid binding sites on serum albumin. J Biol Chem. 1980 Jul 10;255(13):6092–6097. [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Membrane lipoteichoic acid of Streptococcus pyogenes and its stabilized L-form and the effect of two antibiotics upon its cellular content. J Bacteriol. 1976 Aug;127(2):855–862. doi: 10.1128/jb.127.2.855-862.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. L., Tanner M. H., Winship J., Swarts R., Ries K. M., Schlievert P. M., Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989 Jul 6;321(1):1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- Tomlinson K., Leon O., Panos C. Morphological changes and pathology of mouse glomeruli infected with a streptococcal L-form or exposed to lipoteichoic acid. Infect Immun. 1983 Dec;42(3):1144–1151. doi: 10.1128/iai.42.3.1144-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treser G., Semar M., McVicar M., Franklin M., Ty A., Sagel I., Lange K. Antigenic streptococcal components in acute glomerulonephritis. Science. 1969 Feb 14;163(3868):676–677. doi: 10.1126/science.163.3868.676. [DOI] [PubMed] [Google Scholar]
- Veasy L. G., Wiedmeier S. E., Orsmond G. S., Ruttenberg H. D., Boucek M. M., Roth S. J., Tait V. F., Thompson J. A., Daly J. A., Kaplan E. L. Resurgence of acute rheumatic fever in the intermountain area of the United States. N Engl J Med. 1987 Feb 19;316(8):421–427. doi: 10.1056/NEJM198702193160801. [DOI] [PubMed] [Google Scholar]
- Wald E. R., Dashefsky B., Feidt C., Chiponis D., Byers C. Acute rheumatic fever in western Pennsylvania and the tristate area. Pediatrics. 1987 Sep;80(3):371–374. [PubMed] [Google Scholar]
- Waltersdorff R. L., Fiedel B. A., Jackson R. W. Induction of nephrocalcinosis in rabbit kidneys after long-term exposure to a streptococcal teichoic acid. Infect Immun. 1977 Sep;17(3):665–667. doi: 10.1128/iai.17.3.665-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



