Abstract
Recent reports suggest the participation of the aryl hydrocarbon receptor (AhR) in the induction mechanism of the NF-κB signaling pathway. In the current study we challenged C57BL/6 wild-type (WT) and AhR deficient (AhR−/−) mice with bacterial lipopolysaccharide (LPS) to investigate the role of the AhR in expression profiles of LPS and NF-κB target genes. Further, we analyzed the effect of LPS on the DNA binding activity of NF-κB, C/EBP and AP-1 transcription factors in liver and lung from WT and AhR−/− mice. The results show that the LPS-induced expression of several target genes was impaired in AhR−/− mice compared to WT mice. Depending on the target gene, the target tissue as well as the time of treatment, the deficiency of AhR may cause an inhibition or increase of the LPS-induced gene expression. The binding activity of NF-κB, C/EBP and AP-1 transcription factors was also affected in a time- and tissue-dependent manner. The current study shows that the AhR is implemented in LPS-induced inflammatory gene expression in vivo even in the absence of exogenous ligands of the AhR. The main implication of this finding is that the AhR functions in Toll-like receptor (TLR) and NF-κB signaling after activation by a classical stimulus, such as LPS.
Keywords: AhR, LPS, Inflammation, TCDD
1. Introduction
A recent study by Kimura et al. [1] reported that AhR-deficient mice are more sensitive to LPS-induced lethal shock than WT mice, suggesting a critical function of the AhR in the acute inflammatory response mediated by LPS and the TLR. The TLR signals perform a crucial role in innate immune responses to pathogens. Stimulation of TLR4 by LPS is well known to activate the NF-κB pathway [2]. Furthermore, the interaction of AhR with NF-κB signaling pathways has been reported in several studies demonstrating the interaction of AhR and NF-κB members RelA and RelB [3,4]. Recently, we found that the AhR interacts with NF-κB RelB and binds to a previously unidentified element RelBAhRE on the promoter of interleukin (IL)-8 and other chemokines [5,6].
The AhR may also negatively regulate inflammatory responses mediated by LPS in macrophages through inhibition of the promoter activity of IL-6 [1]. On the other hand, DiNatale et al. [7] from Gary Perdew’s laboratory demonstrated that ligand-activated AhR is involved in priming the IL-6 promoter by binding to non-consensus dioxin response elements located upstream of the IL-6 start site causing a synergistic induction of IL-6 following co-treatment treatment with IL-1β and the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).
TCDD exposure is also known to increase LPS-induced toxicity in mice by enhancing serum TNFα levels, and treatment of mice with anti-TNF antibody reduces TCDD-mediated mortality [8]. The authors concluded that an increased TNFα production may be responsible for endotoxin hypersensitivity in TCDD-treated mice. In addition, triple-knockout mice (lacking two receptors for TNF and one receptor for IL1R1) are resistant to TCDD-induced hepatocellular damage [9].
The AhR is generally accepted as a ligand-dependent transcription factor, which can be activated by polycyclic aromatic hydrocarbons such as dioxins. After binding, the activated AhR mediates responses to these chemicals, but questions remain about the endogenous function of the AhR. Recent reports and longstanding literature suggest that the AhR not only contributes to immune disorders [10], but also plays a role in regulating immune responses, such as T cell differentiation and function of dendritic cells [11–14]. One main question is how this protein engages in the function of the immune system and the inflammatory response. In the current study, we investigate the role of the AhR in the classical inflammatory response mediated through activation of TLR and NF-κB by LPS.
2. Materials and methods
2.1. Animals and treatment
Female C57BL/6J and AhR−/− mice were housed and treated at Oregon State University, Corvallis by Nancy Kerkvliet and Linda Steppan. AhR−/− mice were originally generated by Christopher Bradfield at the University of Wisconsin [15]. Mice were housed in a selective pathogen-free facility and maintained on a 12:12 h light/dark cycle and had free access to water and food according to the guidelines set by the University of Oregon. LPS was administered via a single intraperitoneal (i.p.) injection with 250 μg/kg LPS from Escherichia coli 055:B5 (Sigma, St. Louis, MO). After 3 and 6 h, four to six animals from each group control and LPS-treated were killed and their organs were excised, quickly frozen in liquid nitrogen and stored at −80 °C for analysis.
2.2. RNA isolation and quantitative real-time RT-PCR
The preparation of total RNA and synthesis of cDNA were conducted as described previously [14]. Quantitative RT-PCR was then performed with the LightCycler (Roche, Indianapolis, IN) or StepOnePlus Real-Time PCR System using the Fast SYBR Green Master Mix (Applied Biosystems Inc.) according to the manufacturer’s protocol. The data were normalized to the housekeeping gene β-actin. Primer sequences are shown in Table S1.
2.3. Western blotting
Frozen mouse liver or lung tissue samples were ground in liquid nitrogen with mortar and pestle. Then the tissue powders were added to 1 ml RIPA buffer and homogenized using the Tissuelyser. After 30–60 min incubation on ice, tissue lysate was centrifuged at 14,000g for 10 min at 4 °C. The supernatant was retained because whole cell protein extract and protein concentration was determined with Bradford regent (Bio-Rad, Hercules, CA). A representative sample of each group was used for Western blot. The antibodies against actin (sc-1616), C/EBP-β (sc-150), CYP1A1 (sc-20772), IDO-1 (sc-25809) and RelB (sc-226) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), while the purified rabbit anti-IDO2 antibody was kindly provided by Dr. Richard Metz from NewLink Genetics (Wynnewood, PA).
2.4. Electrophoretic mobility shift assay (EMSA)
Nuclear protein samples were extracted from frozen mouse lung and liver tissues using methods adopted from a previous report [16]. Oligonucleotide probes containing consensus binding sequences of C/EBP (5′-TGCAGATTGCGCAATCTGCA-3′), NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) or AP-1 (5′-TTCCGGCTGAGTCATCAAGCG-3′), were synthesized and end-labeled using γ-[32P]-ATP (MP Biomedicals, Solon, OH) and T4 polynucleotide kinase (EPICENTRE Biotechnologies, Madison, WI). EMSA experiments were conducted as previously described [5].
2.5. Statistics
Data are expressed as means ± SD. The comparison between two experimental groups was made using Student’s t test for unpaired data. For multiple comparisons, one-way ANOVA with Bonferroni test was used. p Value of less than 0.05 was considered statistically significant.
3. Results and discussion
3.1. LPS-induced expression of target genes is modified in the absence of AhR
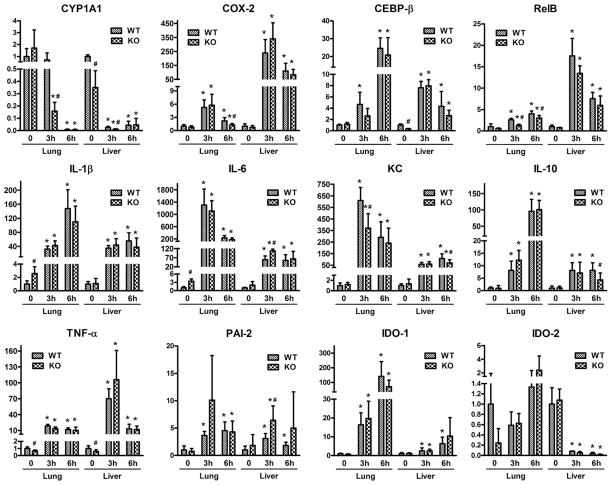
The expression of inflammatory marker genes, which are induced by LPS and also affected by AhR activation, was investigated in WT and AhR−/− mice (Fig. 1). To better illustrate the effect of LPS on mRNA expression, the constitutive expression of each individual gene in WT mice for both lung and liver was set as 1.0 (Fig. 1). Differences in the constitutive mRNA expression levels of the selected genes between lung and liver in WT mice are summarized in Supplementary Fig. S1. Most of the target genes included in this study was expressed significantly higher in lung compared to liver with the exception of C/EBPβ and IL-6, which showed similar mRNA expression levels in both tissues (Fig. S1). As previously reported, the expression of KC and IDO-2 were greater in the liver compared to lung of WT mice [6,14].
Fig. 1.
Expression of LPS target genes in the lung and liver of wild-type (WT) and AHR−/− (KO) mice. Total RNA was extracted from the lung and liver of four to six mice per group after 3 and 6 h of LPS challenge and analyzed via real-time PCR for the expression of CYP1A1, COX-2, CEBPβ, RelB, IL-1β, IL-6, KC, IL-10, TNF-α, PAI-2, IDO-1 and IDO-2. The mRNA expression levels were normalized to β-actin. *Significantly different compared to corresponding tissue from control mice (p < 0.05); #significantly different from WT mice control or treated (p < 0.05).
As shown in Fig. 1, the constitutive Cyp1A1 mRNA expression in the liver of AhR−/− mice was significantly lower compared to WT mice (0 h). After 3 h of LPS challenge, Cyp1a1 expression decreased dramatically in lung of AhR−/− mice but not in lung of WT mice. After 6 h of LPS treatment, the significant decrease of CYP1A1 mRNA level in liver and lung was comparable between WT and AhR−/− mice. The LPS-mediated decrease of CYP1A1 suggests an AhR-independent mechanism, which is in contrast to the antagonistic action of LPS on TCDD-induced CYP1A1 expression in hepatocytes [17].
Similar to CYP1A1, the constitutive expression of COX-2 in lung was about 50-fold above the expression in liver of WT mice (Fig. S1). The expression of COX-2 was clearly induced (more than 100-fold) by LPS in liver but was only fivefold increased in lung after 3 h LPS (Fig. 1). A greater induction of COX-2 was found after 3 h of LPS treatment in both tissues compared to the 6 h time point, which underlines the function of the COX-2 gene as an immediate early gene in inflammation [18]. After 6 h of LPS treatment, the expression of COX-2 was significantly lower in lung samples from AhR−/− mice compared to WT mice, which indicates a role of the AhR in the transcriptional activation of COX-2, confirming our previous studies in primary rat hepatocytes [19].
LPS is known to activate transcription factors such as CEBPβ and NF-κB member RelB, which are important regulators of the inflammatory response [20,21]. CEBPβ and RelB mRNA was increased the most after 6 h of LPS treatment in lung tissue, while in the liver, the greatest induction occurred with 3 h of LPS challenge. For CEBPβ, the LPS-induced fold change was greater in lung tissue than in liver, and we observed a lower constitutive expression in control liver samples from AhR−/− mice compared to liver from WT mice (Fig. 1). On the other hand, the induction of RelB expression by LPS was more pronounced in liver than in lung samples. Moreover, the RelB expression tended to be lower in AhR−/− than in WT mice, although the differences were only statistically significant in the lung of LPS-treated animals (Fig. 1). An enhanced degradation of RelB has been shown after LPS aerosol exposure in the lungs of WT and especially AHR−/− mice [22].
Among the selected cytokines, IL-1β and IL-6 demonstrated a similar mRNA expression pattern in the liver, and the constitutive expression was significantly higher in the control lung samples from AhR−/− mice compared to the WT counterparts. However, this difference disappeared with LPS treatment. IL-6 expression in the liver of AhR−/− mice was significantly increased compared to WT mice with 3 h of LPS-treatment (Fig. 1). Interestingly, the LPS-induced transcription of IL-6 was higher in lung than in liver after both 3 and 6 h of LPS treatment, whereas LPS-induced expression of IL-1β was significantly higher in lung only at 6 h after treatment with LPS. In lung, the expression of IL-1β increased while that of IL-6 decreased in liver from 3 to 6 h time point.
Cytokines KC and IL-10 can be induced after activation of AhR by TCDD [6,23] and were significantly induced by LPS in lung and liver (Fig. 1). The constitutive mRNA expression of KC and IL-10 was comparable in WT and AhR−/− mice, but induction of KC and IL-10 in liver of LPS treated AhR−/− mice was significantly lower after 6 h of LPS treatment compared to WT mice.
In contrast to IL-1β and IL-6, induction of TNF-α was more pronounced by LPS in liver than in lung after 3 h of LPS treatment. Furthermore, the TNF-α mRNA expression was significantly lower in control samples of both lung and liver tissues from AhR−/− mice than those from WT, suggesting the participation of AhR in constitutive expression of this gene. However, the difference between WT and AhR−/− mice disappeared after LPS treatment.
Plasminogen activator inhibitor-2 (PAI-2) has been previously identified as a target of AhR regulation [24]. A significant lower level of PAI-2 was reported from a study analyzing bone marrow-derived macrophages from AhR−/− mice compared to WT mice [25]. Results from the current study do not reveal a significant difference in PAI-2 mRNA expression levels between AhR−/− and WT mice in either lung or liver before LPS treatment (Fig. 1). However, with LPS treatment, PAI-2 expression increased in both tissues, and the expression of PAI-2 was significantly increased in liver of AhR−/− mice compared to WT mice 3 h after LPS injection. The results indicate that the AhR plays a role in the LPS-mediated induction of PAI-2, which agrees with a recent report showing that the AhR directly regulates the expression of PAI-2 through a mechanism involving NF-κB but not the normal partner protein AhR nuclear translocator [25].
IDO-1 and IDO-2 are enzymes involved in tryptophan metabolism, which might generate potential natural ligands for AhR, as shown for kynurenine [26,27]. IDO is an immunosuppressive enzyme that catabolizes tryptophan into kynurenine and other metabolites [28] in dendritic cells (DC). IDO expressing DC have been shown to regulate the differentiation of naïve T cells [29]. Recently, we found that TCDD induces IDO-1 and IDO-2 in a human DC model as well as in the lung and liver of BL6 mice in an AhR-dependent manner [14]. An AhR-dependent expression of IDO has been confirmed in Langerhans cells and bone marrow-derived DC from mice [13,30]. The results of the current study show no significant change of constitutive IDO-1 mRNA expression between WT and AhR−/− mice, but IDO-1 was induced to a greater extent by LPS in lung compared to liver. One the other hand, constitutive IDO-2 mRNA expression is lower in the lungs of AhR−/− mice compared to the lungs of WT mice, although the effect is not statistically significant (Fig. 1). In contrast to IDO-1, the mRNA expression of IDO-2 was only slightly elevated by LPS at 6 h in lung. Interestingly, we observed that the mRNA level of IDO-2 drastically drops in the liver of both WT and AhR−/− mice after 3 and 6 h of LPS challenge.
3.2. LPS decreases CYP1A1 and IDO-2 and the AhR regulates C/EBP and RelB
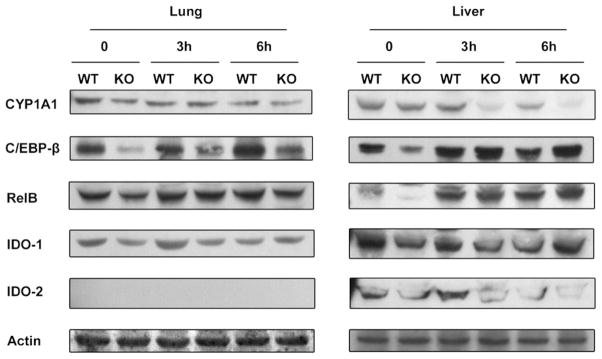
To confirm the mRNA expression profiles of selected genes with their protein levels, we performed Western blot analysis. As shown in Fig. 2, the constitutive expression of CYP1A1 was lower in the AhR−/− mice compared to WT in both lung and liver tissues. LPS treatment led to a clear decrease of CYP1A1 protein in the liver of WT and AhR−/− mice. The degradation of CYP1A1 protein was very significant after 3 h of LPS challenge and accelerated in the liver of AhR−/− mice compared to WT mice (Fig. 2), which confirms the enhanced decrease of CYP1A1 RNA expression after 3 h LPS treatment in AhR−/− mice (Fig. 1). The more rapidly decrease of CYP1A1 mRNA and protein in liver of AhR−/− could result from a lower constitutive level of CYP1A1 in AhR−/− mice. The suppressive effect of LPS on the protein level of CYP1A1 in liver of AhR−/− mice at 3 and 6 h correlates well with results from the CYP1A1 mRNA analysis whereas in liver of WT mice the protein level of CYP1A1 was not decreased after 3 h of LPS challenge (Fig. 1).
Fig. 2.
Protein levels of CYP1A1, CEBPβ, RelB, IDO-1, and IDO-2 after LPS treatment. Protein levels of selected AhR and LPS target genes in the lung and liver tissue of wild-type (WT) and AhR−/− (KO) mice were detected using Western blot analysis. Whole tissue protein from the lung and liver tissue of WT and KO mice was used for analysis. A Western blot of representative samples from each group is shown. The expression level of β-actin was used as a loading control.
The constitutive protein amount of transcription factor C/EBPβ was clearly decreased in the liver and lung of AhR−/− mice compared to WT mice (Fig. 2), which agrees with the lower mRNA expression of C/EBPβ in the liver of WT mice (Fig. 1). These observations suggest an additional role of the AhR in controlling the constitutive expression of C/EBPβ. Upon LPS treatment, the C/EBPβ protein level increased to a lesser extent in the lung of WT mice compared to AhR−/− mice, supporting the role of AhR in the regulation and activation of C/EBPβ. The results confirm our previous study showing the transcriptional activation of C/EBPβ through an activated AhR [31]. An increased C/EBPβ protein level was found in liver tissue from both WT and AhR−/− mice after LPS treatment.
We observed a distinct lower constitutive protein level of RelB in the liver of AhR−/− compared to WT mice, although the difference receded with LPS treatment (Fig. 2). In correlation with its mRNA expression, RelB protein level was increased by LPS treatment to a lesser extent in the lung compared with that in the liver.
Unlike its mRNA profile, IDO-1 protein expression was not increased by LPS treatment in the lung or liver of WT or AhR−/− mice. The decreased level of IDO-2 protein after 6 h of LPS treatment in the liver of WT and AhR−/− mice is coincident with its mRNA expression pattern (Fig. 1). The protein expression of IDO-2 in the lung was not detectable by Western blot.
3.3. AhR modifies DNA-binding activity of transcription factors mediating inflammatory responses by LPS
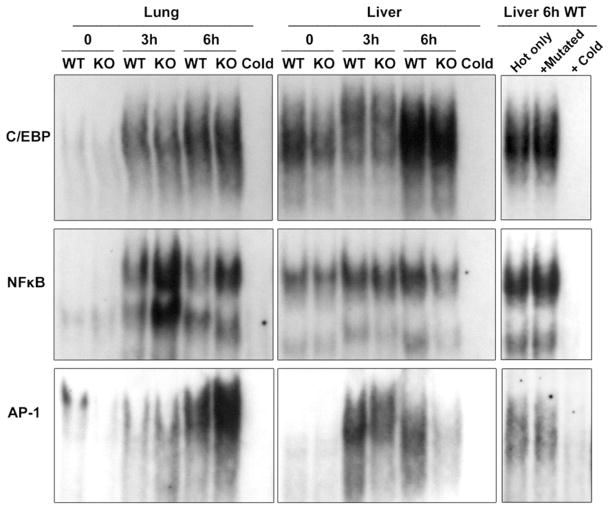
Using EMSA, we analyzed the DNA-binding activity of C/EBP, NF-κB, and AP-1 in liver and lung tissue. As shown in Fig. 3, the binding of nuclear proteins to the C/EBP probe was clearly enhanced in the lung tissue after LPS treatment. The nuclear proteins from AhR−/− lung and liver showed lower affinities to the C/EBP consensus probe than the WT counterparts at both 0 and 3 h of LPS treatment (Fig. 3). The DNA binding activity of C/EBP further increased after 6 h LPS treatment in the lung and liver but without a significant difference between AhR−/− and WT samples. The lower binding activity of C/EBP in the AhR−/− samples, with or without 3 h LPS treatment, indicate that the C/EBP pathway is less activated in the liver of AhR−/− mice than in the WT mice, and its activation by LPS is partially AhR-dependent.
Fig. 3.
Increased DNA-binding activity of NF-κB, AP-1, and C/EBP in lung and liver tissue after LPS treatment. EMSA with nuclear proteins isolated from the liver and lung after 3 and 6 h of LPS challenge was performed. Nuclear protein extracts from wild-type (WT) and AhR−/− (KO) mice were incubated with oligonucleotides containing the consensus site of C/EBP, NF-κB, or AP-1. A representative EMSA of the three samples is shown. A 100-fold excess of the unlabeled specific (Cold) or mutated (Mutated) oligonucleotides was added to confirm specificity.
DNA binding activity to a NF-κB consensus probe demonstrated different patterns in the liver and the lung. In lung, the elevated binding activity induced by LPS was very clear after 3 h of LPS treatment. The AhR−/− samples from the lung showed higher binding activity to the NF-κB element than the WT counterpart after 3 and 6 h of LPS treatment. The results agree with a study performed by Thatcher et al. [22] showing an increased NF-κB activity in the lung of AhR−/− mice. In contrast, the liver samples exhibited only slightly enhanced DNA binding activity by LPS, and the AhR−/− samples showed lower binding activities than the corresponding WT samples (Fig. 3).
AP-1 is known to be activated by LPS and other inflammatory stimuli. In lung tissue at 0 h, WT mice showed higher AP-1 binding affinity than AhR−/− mice (Fig. 3). However, LPS treatment reversed this tendency by increasing AP-1 binding of nuclear proteins from AhR−/− lung compared to WT lung tissue, indicating a stronger activation of the AP-1 pathway in the lungs of AhR−/− mice. On the other hand, liver samples had a different AP-1 binding pattern compared to the lung. As expected, LPS treatment significantly enhanced AP-1 DNA binding activity, especially after 3 h of LPS challenge. Nuclear proteins from the liver of WT mice showed higher AP-1 binding activity than those from AhR−/− mice, especially after 6 h of LPS challenge. The results show that LPS significantly activated DNA-binding of NF-κB, C/EBP, as well as AP-1 in the lung and liver of WT and AhR−/− mice in a time-dependent manner. However, the lack of AhR facilitates an enhanced LPS-induced binding activity of the transcription factors in the lung but decreases their binding activity in the liver. One reason for the tissue-specific effects of the skewed DNA binding activity of NF-κB, C/EBP and AP-1 might be differences in the phenotype of AhR−/− mice. The reported decreased liver size, subtle hepatic portal fibrosis, and decreased constitutive expression of certain drug-metabolizing enzymes, such as CYP1A1 (Figs. 1 and 2) and CYP1A2 in AhR−/− mice may contribute to the observed different response after LPS challenge.
In conclusion, we demonstrated that the AhR modifies the expression of LPS- and TLR4-regulated pro-inflammatory genes in a time- and tissue-dependent manner. The observed changes in DNA binding activity of transcription factors, which are critically involved in the activation process of pro-inflammatory genes, such as C/EBP, NF-κB, and AP-1, might be responsible for the altered LPS-induced gene expression. The current study further suggests an additional role of the AhR in controlling the constitutive expression of genes such as RelB and C/EBPβ.
Supplementary Material
Highlights.
AhR interacts with TLR4 and NF-κB signaling.
LPS-mediated inflammatory response involves AhR.
AhR-independent degradation of CYP1A1 by LPS.
LPS down-regulates expression of IDO-2 in vivo.
Acknowledgments
The authors like to thank Nancy Kerkvliet and Linda Steppan from Oregon State University, Corvallis for providing tissue samples from control and LPS treated mice. The work was supported by the American Heart Association Western AffiliateGrant 0765056Y, NIE-HS Grant R21ES15846-01A2 and Research Grant FAS0703859 from the Susan G. Komen Foundation for the Cure. We would also like to thank Dr. Suzette Smiley-Jewell for her technical help with the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2011.06.018.
References
- 1.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009;77:734–745. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian Y. Ah receptor and NF-kappaB interplay on the stage of epigenome. Biochem Pharmacol. 2009;77:670–680. doi: 10.1016/j.bcp.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007;363:722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiNatale BC, Schroeder JC, Francey LJ, Kusnadi A, Perdew GH. Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J Biol Chem. 2010;285:24388–24397. doi: 10.1074/jbc.M110.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor MJ, Lucier GW, Mahler JF, Thompson M, Lockhart AC, Clark GC. Inhibition of acute TCDD toxicity by treatment with anti-tumor necrosis factor antibody or dexamethasone. Toxicol Appl Pharmacol. 1992;117:126–132. doi: 10.1016/0041-008x(92)90227-j. [DOI] [PubMed] [Google Scholar]
- 9.Pande K, Moran SM, Bradfield CA. Aspects of dioxin toxicity are mediated by interleukin 1-like cytokines. Mol Pharmacol. 2005;67:1393–1398. doi: 10.1124/mol.105.010983. [DOI] [PubMed] [Google Scholar]
- 10.Marshall NB, Kerkvliet NI. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 12.Jin GB, Moore AJ, Head JL, Neumiller JJ, Lawrence BP. Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection. Toxicol Sci. 2010;116:514–522. doi: 10.1093/toxsci/kfq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol. 2010;246:18–28. doi: 10.1016/j.taap.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunger MK, Moran SM, Glover E, Thomae TL, Lahvis GP, Lin BC, Bradfield CA. Resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity and abnormal liver development in mice carrying a mutation in the nuclear localization sequence of the aryl hydrocarbon receptor. J Biol Chem. 2003;278:17767–17774. doi: 10.1074/jbc.M209594200. [DOI] [PubMed] [Google Scholar]
- 16.Romieu-Mourez R, Kim DW, Shin SM, Demicco EG, Landesman-Bollag E, Seldin DC, Cardiff RD, Sonenshein GE. Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol Cell Biol. 2003;23:5738–5754. doi: 10.1128/MCB.23.16.5738-5754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–39644. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- 18.Pilbeam CC, Kawaguchi H, Hakeda Y, Voznesensky O, Alander CB, Raisz LG. Differential regulation of inducible and constitutive prostaglandin endoperoxide synthase in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268:25643–25649. [PubMed] [Google Scholar]
- 19.Vogel C, Boerboom AM, Baechle C, El-Bahay C, Kahl R, Degen GH, Abel J. Regulation of prostaglandin endoperoxide H synthase-2 induction by dioxin in rat hepatocytes: possible c-Src-mediated pathway. Carcinogenesis. 2000;21:2267–2274. doi: 10.1093/carcin/21.12.2267. [DOI] [PubMed] [Google Scholar]
- 20.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 21.Bradley MN, Zhou L, Smale ST. C/EBPbeta regulation in lipopolysaccharide-stimulated macrophages. Mol Cell Biol. 2003;23:4841–4858. doi: 10.1128/MCB.23.14.4841-4858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutter TR, Guzman K, Dold KM, Greenlee WF. Targets for dioxin: genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science. 1991;254:415–418. doi: 10.1126/science.1925598. [DOI] [PubMed] [Google Scholar]
- 25.Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, Gonzalez FJ, Ikuta T, Kawajiri K, Fujii-Kuriyama Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 2009;29:6391–6400. doi: 10.1128/MCB.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayaishi O, Hirata F, Fujiwara M, Senoh S, Tokuyama T. Indoleamine 2,3-dioxygenase. Note II. Biological function. Acta Vitaminol Enzymol. 1975;29:291–293. [PubMed] [Google Scholar]
- 29.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 31.Vogel CF, Sciullo E, Park S, Liedtke C, Trautwein C, Matsumura F. Dioxin increases C/EBPbeta transcription by activating cAMP/protein kinase A. J Biol Chem. 2004;279:8886–8894. doi: 10.1074/jbc.M310190200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.