Abstract
Importance of the field
P21-activated kinases (PAKs) are involved in multiple signal transduction pathways in mammalian cells. PAKs, and PAK1 in particular, play a role in such disorders as cancer, mental retardation and allergy. Cell motility, survival and proliferation, the organization and function of cytoskeleton and extracellular matrix, transcription and translation are among the processes affected by PAK1.
Areas covered in this review
We discuss the mechanisms that control PAK1 activity; its involvement in physiological and pathophysiological processes; the benefits and the drawbacks of the current tools to regulate PAK1 activity; the evidences that point to PAK1 as a therapeutic target; and the likely directions of future research.
What the reader will gain
The reader will gain a better knowledge and understanding of the areas covered in this review.
Take-home message
PAK1 is a promising therapeutic target in cancer and allergen-induced disorders. Its suitability as a target in vascular, neurological and infectious diseases remains ambiguous. Further advancement of this field requires progress on such issues as the development of specific and clinically acceptable inhibitors, the choice between targeting one or multiple PAK isoforms, elucidation of the individual roles of PAK1 targets and the mechanisms that may circumvent inhibition of PAK1.
Keywords: angiogenesis, cancer, p21-activated kinases, Rho GTPases, signal transduction, allergy
1. Introduction
In the early-1990s, Manser and co-workers made the seminal observation that specific downstream effects of a group of Rho GTPases (P21) in rat brain cytosol is mediated through a p21-activated kinase (PAK)[1]. In a gel-overlay assay, these researchers identified 3 proteins of 68, 65 and 62 kDa in a specific screen designed for identifying the binding partners of Rho-GTPases. From subsequent studies, these three proteins were identified to be the members of group I PAK family; PAK1, PAK2 and PAK3 that are activated by GTP bound, but not GDP bound, Rac and cdc42[2-4]. With this discovery, a new area of research on serine-threonine kinase PAK was originated, that later found to be important for many physiological function and as a major underlying cause of many pathological conditions. The structure, substrate specificity and functional role of group I PAKs have been evolutionarily conserved right from the protozoan to the yeast and mammals5. PAKs are important for a variety of cellular functions such as cytoskeletal remodeling, focal adhesion assembly, cell migration[6], survival[7], mitosis[8] as well as transcriptional regulation and protein synthesis involving ERK and NFκB pathways[9, 10]. Currently, PAKs are among the best characterized downstream effectors of the Rho family GTPases Rac and cdc42 in the regulation of lamellipodia and filopodia formation, respectively[6, 11].Furthermore, deregulation of PAK activity has been linked to a variety of cancers[12]. In addition to Rac and cdc42, many other signaling pathways have also been implicated in the regulation of PAK activity. These include activating and inactivating phosphorylations of PAK, inactivation by phosphatases, direct activation through protein-protein interactions, and inactivation via interaction with inhibiting proteins (see below).
In addition to the group I PAKs, recent studies have uncovered a new family of kinases, whose kinase domains have similarities to that of group I PAKs. These group II PAKs include isoforms PAK4, PAK5 (also known as PAK7) and PAK6[13]. Although termed as PAKs, these proteins display some important differences from group I PAKs[14]. The most prominent difference is the lack of activation of group II enzymes by a Rho-GTPase mediated mechanism[14]. Despite the similarities in the kinase domains, structural and biochemical differences between the two groups of PAKs suggest a different intra-cellular substrate specificity and cellular function for these two groups[13]. However, most of the few currently known substrates of group II PAKs such as Raf, BAD, LIMK, GEF1, Par-1 and estrogen receptor are also phosphorylated by group I PAKs[14-16]. A recent study using position scanning peptide library approach suggested that group I PAKs phosphorylate substrates that have consensus sequence RRRRRSWYFS, whereas the group II PAKs phosphorylates RRRRRSWASP[17]. It is important to note that despite the high degree of similarity group I PAKs may perform distinct functions even when present in the same cell[18, 19]. Furthermore, individual PAKs may be represented by different splice variants (the information is accessible at www.uniprot.org), which may possess distinct biological properties. For example, in human PAK1B, an alternatively spliced variant of PAK1, the canonical sequence of amino acids 518-545 (HQFLKIAKPLSSLTPLIAAAKEATKNNH) is replaced by VRKLRFQVFSNFSMIAASIPEDCQAPLQPHSTDCCS.
PAK1 is a participant in a complex network of molecular interactions[9, 15, 20-22], as is evidenced by a large list of its regulators, co-factors and downstream targets (see Tables 1 and 2 for examples). It is important to note that depicting any of the individual interactions as inhibitory or stimulatory is an oversimplification: at least in some cases, the end result is a qualitative change in the behavior of the protein, such as caused by intracellular re-localization. In the current review, we will focus on normal and pathological functions of PAK1, its structural and functional similarities with other isoforms, the modes of its natural and artificial regulation, as well the prospects of therapeutic targeting of this enzyme.
Table 1.
Examples of interacting partners of PAK1
| FUNCTION | INTERACTING PARTNER |
Activation (+) /Inhibition (−) |
REF |
|---|---|---|---|
| CYROSKELETAL PROTEINS |
Paxillin | N/A | 83, 180 |
| Akt | +/− | 126 | |
| KINASES | CdK5 | − | 181 |
| Cdk2 | − | 182 | |
| Heregulin-β1 | + | 183 | |
| PDK1 | + | 50 | |
| PI3-K | + | 184 | |
| PHOSPHATASES | PP2A | − | 66 |
| POPX1 | − | 67 | |
| POPX2 | − | 67 | |
| ADAPTER PROTEINS | Grb-2 | + | 61 |
| Nck | + | 62 | |
| GTP-ases | cdc42 | + | 1 |
| CHP | + | 53, 185 | |
| Rac1 | + | 2 | |
| Rac2 | + | 39 | |
| Rac3 | + | 186 | |
| TC10 | + | 54 | |
| Wrch-1 | + | 55 | |
| GEFs | α-PIX | + | 40 |
| β-PIX | − | 41 | |
| OTHERS | CIB1 | + | 187 |
| Gαβ | − | 188 | |
| SphingolipidS | + | 46 | |
| RILPL2 | + | 189 | |
| CRIPak | − | 190 | |
| hPIP1 | − | 191 | |
| LC8 | N/A | 192 | |
| GIT1/PKL | N/A | 193, 194 | |
| FMR1 | + | 145 | |
| Huntingtin | N/A | 146 | |
| HIV-1/Nef | + | 153 | |
Table 2.
Examples of substrates of PAK1
| FUNCTION | CELL TYPE/ANIMAL | SUBSTRATE | PHOSPHORYLATION SITE | REF |
|---|---|---|---|---|
| APOPTOSIS | MCF-7 | DLC1 | S88 | 81, 195 |
| MCF-7 | FKHR | S256 | 195 | |
| 293T, Rat-1, BALB3T3 | BAD | S111 | 125 | |
| PROLIFERATION | HeLa, NIH-3T3 variant S2-6, Cos-7, 293T | C-Raf1 | S338, S339 | 10 |
| HeLa, NIH-3T3 variant S2-6, 293, REF52 | MEK1 | S298 | 196 | |
| HeLa | Plk1 | S49 | 81 | |
| HeLa, MCF-7, ZR75, Ishikawa, MDA-MB-231 | ERα | S305 | 97 | |
| MCF-7 | Histone H3 | S10 | 198 | |
| NIH3T3, RT4 Schwann cells | Merlin | S518 | 199 | |
| NIH3T3, Cos-7 | AuroraA | T288, S342 | 200 | |
| 293 | B-Raf | S638, S446 | 201 | |
| 293 | MEKK1 | S67 | 202 | |
| CYTOSKELETAL ASSEMBLY/CELL MOTILITY |
Baby hamster kidney-21, HeLa | MLCK | S439, S991 | 72 |
| Cos-1, HeLa, Jurkat T cells | GEF-H1 | S885 | 203 | |
| Cos-7, NIH3T3 | GIT1 | S517 | 200 | |
| Cos-7 | Vimentin | S25, S38, S50, S56, S65, S72 | 204 | |
| HEp-2 | Op18/Stathmin | S16 | 205 | |
| NIH3T3, 293 | NET1 | S152, S153 | 206 | |
| MCF-7 | P41-ARC | T21 | 76 | |
| Smooth muscle | Caldesmon | S657,S687 | 207 | |
| - | CPI17 | T38 | 208 | |
| Smooth muscle | Desmin | - | 209 | |
| MCF-7 | Filamin A | S2152 | 210 | |
| 293 | LIM kinase | T508 | 211 | |
| - | PP1-MBS | T641 | 208 | |
| 293 | α-PIX | S488 | 17 | |
| 293 | β-PIX | S340,S525 | 212 | |
| 293T, HeLa | Rho-GDI | S101, S174 | 213 | |
| MCF-7 | TCoB | S65, S128 | 214 | |
| A431 cells | CtBP1 | S158 | 215 | |
| Breast cancer cells | SNAIl | S246 | 216 | |
| Cardiac muscle cells | TroponinI | S149 | 210 | |
| Endothelial Cells | Paxillin | S273 | 180 | |
| OTHERS | K562 leukemia cells | PGM | T466 | 217 |
| Human neutrophils | P47 phox | S303, S304,S320,S338 | 32 | |
| MCF-7, ZR75, MCF12A | ESE1 | S207 | 18 | |
| Mouse mammary epithelial cells | STAT5a | S779 | 219 | |
| PC12 | Synapsin I | S603 | 220 | |
| Swiss 3T3 | P67 phox | T233 | 221 | |
| 293 | Gαz | S16 | 43 | |
| 293T | PGAM-B | S23, S118 | 222 | |
| 293 | SHARP | S3486, T3568 | 214 | |
| MCF-7 | ILK | T173, S246 | 79 | |
2. Tissue distribution of PAKs
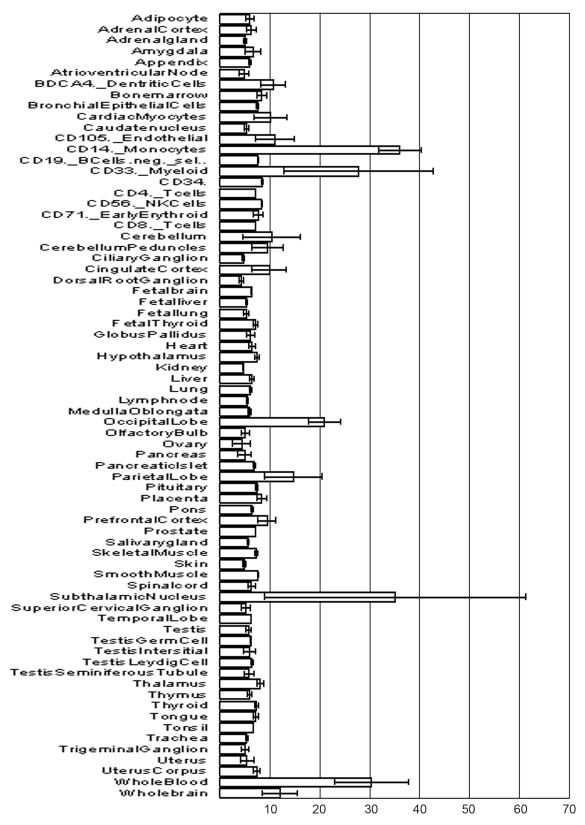
Tissue distribution of a potential therapeutic target is an important parameter that may offer clues about the likely sites affected by such a therapy. Tissue distribution of individual PAK isoforms has been studied using a variety of RNA- and protein-based detection techniques. A serial analysis of gene expression (SAGE) indicates that mammalian brain is a unique organ that expresses significant levels of mRNAs for all the 6 different isoforms of PAK abundantly[15]. This may indicate the importance of PAK isoforms in neurological function[23]. Microarray analyses of mRNA expression[24] (accessible at www.BioGPS.gnf.org) show that, among the various isoforms, group I PAKs are expressed in most of the tissues (Figure 1). PAK1 and PAK2 proteins1 as well as PAK1 mRNA[25] are reported to be highly expressed in brain. While heart tissue expresses high levels of PAK1 protein[26], high levels of PAK1 mRNA levels has been reported in neutrophils[27]. Presence of abundant PAK3 mRNA has been indicated in brain[25] and the pituitary[28]. PAK4, the best studied member among the group II PAKs[29, 30] has been reported to be highly expressed in tissues such as prostate, testis, lung, heart, brain and liver. While PAK4 knockout mice are embryonically lethal[31], PAK5 knockout[32] and PAK5/PAK6 double knockout mice[33] are viable. The lack of gross phenotypic defects in PAK5 and PAK6 knockout animals is consistent with the SAGE data, which suggests that these isoforms have the most restricted expression pattern of all the PAKs. While PAK5 RNA is abundant in brain[34], PAK6 is expressed in testis, prostate, brain, kidney and placenta[13, 35].
Figure 1. Relative expression of PAK-1 mRNA in human tissue.
Oligonucleotide microarray hybridization data has been obtained from www.biogps.com. Relative signal intensity values are depicted in arbitrary units with ranges.
It is important to note that the reports on tissue distribution of various PAK isoforms are not always perfectly concordant. Barring possible artifacts in individual studies, the discrepancies could be attributed to imperfect correlation between the protein and RNA levels, to inability of some techniques to discriminate between the individual isoforms or splice variants of such isoforms, and to a different degree of detail in analyzing a “tissue” or an “organ” (e.g. total brain vs separate brain structures; whole blood vs distinct sets of blood cells). Of note, the lack of correlation between the mRNA and protein levels may be a sign of translational regulation by miRNAs, as has been already documented for PAK1[36], and warrants further investigation.
3. Structure and mechanism of action of group I PAKs
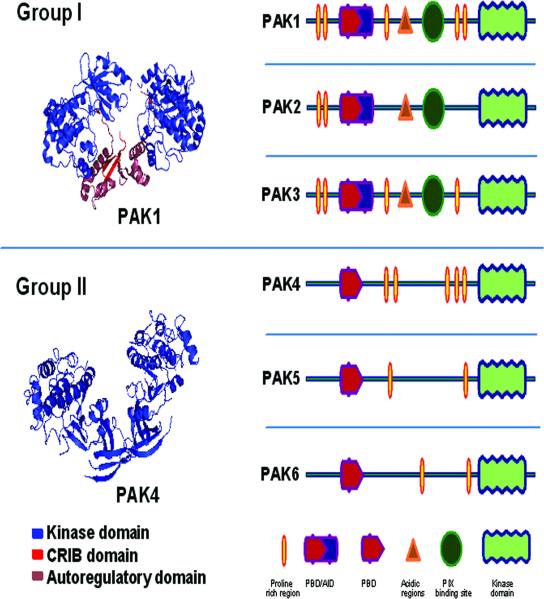
PAKs in general have similar structural organization (Figure 2), including an N-terminal regulatory domain and a C-terminal catalytic domain. The conserved N-terminal non-catalytic domain contains a p21 binding domain (PBD) (amino acids 67-113 in human PAK1), which binds the GTP bound members of the Rac and cdc42 family[37-39]. One characteristic feature of group I PAKs is that its regulatory domain consists of an auto-inhibitory domain (AID) (amino acids 83-149 in human PAK1) that overlaps partially with the PBD[38]. Additionally, the proline-rich region in the regulatory domain that bind to adapter proteins Nck and Grb2 as well as guanine exchange nucleotide factor PIX/COOL are also highly conserved among group I PAKs[40, 41]. A region of amino acid diversity between the proline-rich domains such as T212 in human PAK1 and D212 in human PAK2 confers selective specificity to the different PAK isoforms for its caspase cleavage[42]. Catalytic domain of human group I PAKs have highly conserved regions in amino acids 255-559 in PAK1, 235-509 in PAK2 and 254-258 in PAK3. In between the N-terminal domain and catalytic domain, there is an acidic region rich in Glu/E and Asp/D whose functional significance is yet to be determined[43]. In contrast to group I PAKs[38], group II PAKs do not possess an auto-inhibitory domain, proline-rich regions to interact with Nck and Grb2, and the acidic regions (Figure 2)[14] (Information is available at www.pymol.org and http://www.sgc.ox.ac.uk/structures/KIN.html). Many recent reports indicate that regulation of PAK expression and activation has implications for various physiological and pathological processes. Up-regulation of PAK1 expression has been reported in many types of cancers (see below) and ischemic conditions such as stroke[44]. Similarly, phosphorylation and subsequent activation of PAK1 is important for a variety of cellular functions, including actin cytoskeleton remodeling, cell migration and extracellular matrix (ECM) assembly (see below). The structural[38], genetic[43] as well as biochemical[45, 46] analyses has provided necessary insights into the mechanisms of GTPase-mediated activation of group I PAKs. In the current model, PAK activity within a resting homodimer is inhibited via a trans-inhibitory switch through the interaction of its auto-inhibitory domain (AID) with the kinase domain of the dimer partner. The interaction of GTP-bound (activated) Rac1 or Cdc42 with the CRIB of PAK1 results in a structural re-arrangement that disrupts the mutual interaction and removes the trans-inhibitory switch[47, 48,49]. While GTPases bind weakly to the so called CRIB (cdc42 and Rac interactive binding) region in the regulatory domain (amino acids 75-90 in human Pak1), interaction of GTPases with the adjacent PBD region further strengthen this binding[38].
Figure 2. Schematic representation of human group I and group II PAKs indicating the structural similarities and differences.
Left upper panel show a 2.3 Ao resolution structure of human PAK1 (Group I) kinase domain from the available information on their crystal structures (retrieved from www.PyMOL.org). The asymmetric unit of the crystals contains two complexes, a auto-regulatory domain (aa 7-149) and a Kinase domain (aa 249-545) with a mutation in the kinase domain (K299R) that ablates its catalytic activity. These domains are linked into a dimer by the N-terminal segments (aa 78-88) of the auto-regulatory fragments. Right upper panel represents linear structure of Group I PAK isoforms. Group I PAKs contain a conserved overlapping PBD/CRIB/AID region. Interaction of Rac and cdc42 with the CRIB domain has been shown to release the group I PAKs from auto-inhibition. Left lower panel shows 1.6 Ao resolution structure of enzymatically active human PAK-4 catalytic domain (aa 291-591) when it is phosphorylated at the activating loop positions corresponding to Ser-474. It can be noted that the overlapping CRIB (aa 75-90) and AID (aa 83-149) regions that are present in group I PAKs is missing in PAK4. Right lower panel is a linear representation of group II PAKs. Group II PAKs lack AID, CRIB, acidic and PIX binding regions that are present in Group I PAKs.
Studies in vitro have demonstrated that upon release of AID, PAK1 undergoes autophosphorylation at Thr-423 in the catalytic domain[50]. However, studies in vivo suggest that phosphorylation of Thr-423 require an additional kinase such as PDK1[50]. It has been reported that autophosphorylation of PAK1 at Ser-144 and PAK2 at Ser-139 also plays a role in maximal activation of these kinases[1, 45, 46].
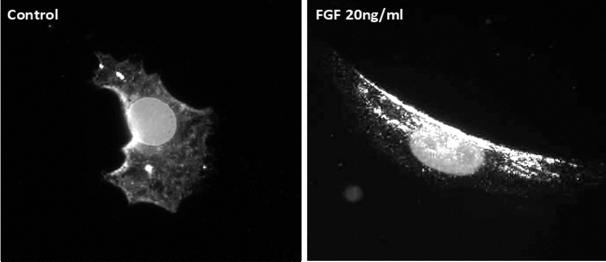
Regulation of PAK1 by extracellular stimuli typically involves redistribution of this protein within a cell, as illustrated in Figure 3. The figure illustrates that, in resting NIH 3T3 fibroblasts, PAK1 is mostly located intracellularly in the cytosol. Upon stimulation with basic fibroblast growth factor, PAK1 was observed to translocate to the cell periphery, in accordance with the proposed role for PAK1 in cytoskeletal re-modeling in fibroblasts (Figure 3). This is also in agreement with the published literature on PAK1 localization in cells[51]. Although group II PAKs can interact with the GTPases under certain circumstances, this interaction does not result in their activation, but is reported to be necessary for their translocation to a different cellular compartment[52].
Figure 3. Intracellular localization of PAK1.
Fluorescent microscopic images of NIH 3T3 fibroblasts treated with control (PBS) and fibroblast growth factor (FGF; 20ng/ml) showing intracellular distribution of PAK1. In a resting cell, PAK1 is localized mostly in the cytoplasm. In order to study the PAK1 translocation in activated cells, NIH 3T3 fibroblasts were treated with 20ng/ml of FGF and incubated for 30 minutes in a CO2 incubator at 37°C. Cells were fixed with 2% para-formaldehyde and stained with PAK1 antibody (Santa Cruz biotechnology). Upon treatment with FGF, PAK1 is seen to be translocated to the plasma membrane according to its well-characterized role in the regulation of cytoskeletal dynamics and cell motility.
Among the GTPases, isoforms of Rac such as Rac1, Rac2 and Rac3[1, 37], cdc42[1] as well as CHP/Wrch2/RhoV[53], TC10/RhoQ[54] and Wrch1/RhoU[55] are known to activate group I PAKs. Interestingly, a mutant of cdc42 (Y40C), which cannot interact with group I PAKs, retains its ability to interact with group II PAKs [29]. A short lysine-rich region in human PAK1 (amino acids 66-68) upstream of the CRIB domain confers some specificity to certain GTPases to bind to the CRIB region[37, 39]. Activating mutations (Q61L) in Rac and cdc42 that convert them into GTP-hydrolysis deficient variants increase the affinity for effector binding[47]. Subsequent studies revealed that interaction between PBD of PAK and activated GTPase may also act as an allosteric mechanism promoting its activation[38,46]. Once PAK is activated, it is not necessary to have the GTPase bound to the kinase for its prolonged activity[1].
Many recent studies have identified a family of guanine exchange nucleotide factors (GEFs) that are specifically involved in the GTPase-mediated activation of PAK. This family of GEFs termed PIX (PAK Interacting Exchangers) or COOL (Cloned Out Of a Library) interact with Rac and cdc42 and form a complex with PAK thereby resulting in its activation[40, 41]. Interaction of αPIX with PAK1 was shown to induce PAK1 activation involving Rac or cdc42[56, 57]. However, the molecular mechanism of αPIX-mediated PAK activation remains elusive. In addition to αPIX, a second member of PIX family, βPIX is involved in the modulation of PAK activity[41, 57]. However, there are conflicting reports on whether interaction of βPIX with PAK enhance or inhibit its activity[58, 59].
4. Alternative mechanisms of group I PAK activation
Although PAK is a major downstream candidate of Rho family of GTPases, additional mechanisms have been implicated in the regulation of PAK activity. Sphingolipids and their derivatives have been shown to activate PAK[60]. Amino-terminal domain of PAK has PXXP-motifs that are binding sites for SH-3 containing adapter proteins such as Nck and Grb2[61, 62]. Interaction with these proteins recruits PAK to the plasma membrane resulting in its activation[61, 62]. This apparently GTPase-independent activation of PAK1 may be due to its localization in proximity to 3-phospho-inositide dependent kinase-1 (PDK1) that phosphorylates the conserved Thr423 of the PAK1 activation loop[9, 60]. Membrane recruitment of PAK has also been shown to modulate receptor tyrosine kinase mediated responses[61, 62]. Serine-threonine kinase Akt, which enhances PAK1 activity, has been proposed as one of the kinases for Ser21 of PAK1[63], although the context of this serine is distinct from the classical Akt recognition motif. Phosphorylation of Ser21 decreases association of PAK1 with Nck and is likely to affect cell polarity and migration[63]. Plasma membrane-recruited PAK1 is also subjected to tyrosine phospohorylation in a GTPase dependent manner, as demonstrated by McManus and co-workers in a study involving transformed cells expressing constitutively active v-ErbB receptor[64]. Etk/Bmx, a Tec family member of non-receptor tyrosine kinase, has been shown to directly bind and phosphorylate PAK1[65].
Importance of multiple phosphorylations for activation of PAK also suggests a prominent role for phosphatases in the regulation of PAK activity. Serine-threonine phosphatase PP2A has been shown to interact with PAK[66]. Two additional phosphatases that are known to de-activate PAK are POPX1 and POPX2 (Partner of Pix1 and Pix2)[67]. These phosphatases interact with the Pix family of PAK-specific guanine exchange nucleotide factors and become part of a multi-protein complex that also involves PAK. These phosphatases are shown to dephosphorylate Thr423 in the activation loop of PAK1 [67].
5. PAK signaling in cytoskeletal remodeling and cell motility
Small Rho GTPases such as Rac and cdc42 have been projected as the major regulators of cytoskeletal dynamics[9]. Both these GTPases mediate their effects through PAK, and PAK utilizes its catalytic activity as well as interaction with other proteins in the regulation of GTPase dependent cytoskeletal remodeling [16, 56]. Many cytoskeletal and adaptor proteins, guanine exchange nucleotide factors, intermediate filaments, microtubules, integrins, kinases as well as phosphatases have been shown to interact with or get phosphorylated by PAK[9, 20]. Myosins, a large family of actin-based molecular motor proteins, are targets of phosphorylation by PAK in the regulation of cell spreading, motility and cell division68. Ste20 and Cla4, two PAK-related kinases in Sachharomyces cerivisiae that are highly homologous to group I PAKs, are among the first identified Rac/cdc42 effectors regulating myosin light chain (MLC) phosphorylation both in vitro and in vivo[69, 70]. Mammalian PAK phosphorylates MLC on Ser19 in neuronal cells, resulting in the stabilization of the localized actin network through formation of a GIT1/PIX/Rac/PAK complex[71]. Alternatively, PAK can also regulate activity of myosin light chain kinase (MLCK) and indirectly modulate MLC phosphorylation[72]. In addition to MLC, PAK can also phosphorylate LIM Kinase (LIMK), a serine-throenine kinase involved in the regulation of assembly of actin cytoskeleton and dis-assembly microtubules[73]. Phosphorylation of LIMK at Thr508 triggers phosphorylation of cofilin, a cytoskeletal protein that acts as an actin capping and severing protein[74]. LIMK is also involved in mediating the formation of lamellipodia, membrane ruffles, filopodia and cell motility via Rac/cdc42-PAK signaling[9]. PAK1 is necessary for the phosphorylation of actin binding protein filamin A at Ser2152, which is necessary for the cross-linking of actin networks. In turn, filamin A interact with the CRIB region of PAK stimulating its activation, thus operating a local loop activation of PAK[75]. In addition, PAK can also phosphorylate p41-Arc (Arp2/3; a 41 kD subunit actin-related 2/3 complex) at Ser21[76], cortactin, an F-actin binding protein involved in actin polymerization at Ser113[77] and caldesmon, an actin filament regulatory protein at Ser657 and Ser687[78] in the regulation of actin polymerization.
A study performed by Rakesh Kumar’s group suggests that phosphorylation of integrin linked kinase (ILK) by PAK1 at Thr 173 and Ser 246 in vitro and in vivo plays a role in nuclear export of ILK[79]. Although functional significance of this PAK1-mediated nuclear function of ILK is not very clear, this study indicates that changes in the nuclear activity of ILK are directly linked to the changes in nuclear lamins, which are critical proteins necessary for the nuclear integrity and function.
PAK is also involved in the phosphorylation of proteins that control microtubule dynamics. Stathmin, also known as oncoprotein 18 (op18), destabilizes microtubules by binding to tubulin dimers and inhibits tubulin polymerization to promote microtubule dis-assembly[80]. Phopshorylation of op18 by PAK at Ser16 inactivates this protein, resulting in stabilization of microtubules at the leading edges of migrating cells. Tubulin cofactor B (TCoB) augments the heterodimerization of α/β-tubulins and is phosphorylated by PAK at Ser65 and Ser128[81]. PAK can also phosphrylate dynein light chain 1 (DLC1), a component of cytoplasmic dynein complex, which moves along the microtubules. DLC1 is phosphorylated by PAK1 at Ser88, which affects vesicle formation and trafficking[76].
Apart from its prominent role in actin and microtubule remodeling, PAK is also known to phosphorylate a variety of other substrates in the regulation of cell motility. PAK has been reported to phosphorylate the armadillo adaptor protein, a drosophila counterpart of mammalian β-catenin, at Ser561 and Ser688, in the regulation of cadherin-mediated adhesion[82]. Paxillin, an adaptor protein abundant in integrin dependent focal and fibrillar adhesions, is phosphorylated by PAK at a serine residue[83]. A more detailed account of the signaling partners of PAK1 in the regulation of cytoskeletal assembly and cell motility is provided in Tables1 and 2 as well as in Figure 4.
Figure 4. Schematic representation of PAK1 and some of its substrates regulating various cellular functions.
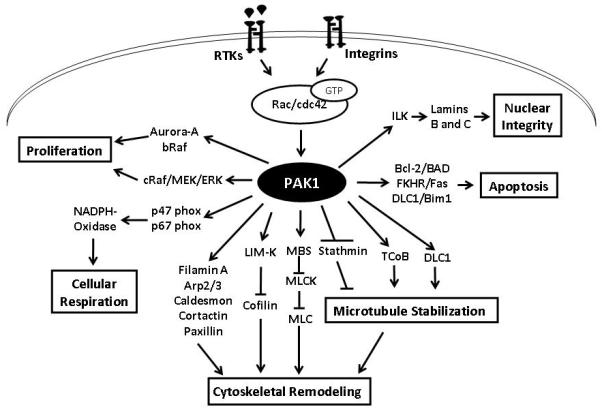
Various stimuli, such as growth factors and integrins, activates PAK1 via GTP-bound Rac and Cdc42. Enhanced expression of Lamins B and C via phosphorylation and activation of Integrin Linked Kinase (ILK) has been implicated in the regulation of nuclear integrity. PAK1 is known to protect cells from apoptosis through at least three different pathways involving FKHR, Bcl-2 and DLC1. In the regulation of cellular proliferation, PAK1 regulates activity of Raf and Aurora kinases. PAK1 is also known to regulate cellular respiration via phoshorylations of p47- and p67- phox proteins. The most studied and well-characterized function of PAK1 is in the regulation of cytoskeletal remodeling. While PAK1 regulates microtubule dynamics through inhibition of stathmin and activation of TCoB and DLC1, it also regulates actin assembly and disassembly through phosphorylations of LIM Kinase and cytoskeletal proteins such as Filamin A, Arp2/3, Caldesmon, Cortactin and Paxillin. Apart from this, PAK1 also phosphorylates myosin binding unit (MBS) that in turn, inhibits phosphorylation of myosin light chain (MLC).
6. PAK signaling in vascular biology and angiogenesis
Migration of endothelial cells and fibroblasts is essential for angiogenesis and tissue remodeling[84]. In order to migrate in a specific direction, cells must adhere and detach to the substratum (extracellular matrix proteins) in a coordinated manner. Coordination of events such as formation of filopodia, lamellipodia and focal adhesions in the leading edge and detachment of focal adhesions at the lagging edge of the cell is necessary for the directional migration. Rho family of GTPases (Rho, Rac and cdc42) is well known as regulators of directional migration in vascular cells[85]. Importance of PAK in the regulation of lamellipodia formation and migration of vascular cells is known for a long time[86]. Many mouse gene knockout studies have also revealed the importance of PAK in the regulation of cell migration by other kinases such as Rac GTPase[87] and Akt[6, 88]. Impaired cytoskeletal assembly and cell migration observed in Rac1–/– and Akt1–/– fibroblasts was rescued by over-expression of constitutively active form of PAK1 (T423E mutant)[6, 87]. In addition to fibroblasts, PAK is also important for the directional migration of endothelial cells[89]. Inactivation of Rac1 and PAK1 in vitro in endothelial cells and fibroblasts results in impaired cytoskeletal assembly, formation of lamellipodia and migration[6, 90]. In contrast, constitutively active mutants of Rac1 and PAK1 results in enhanced cell motility in these cell types.
In addition to its role in migration, Rac/Pak signaling has also been implicated in the regulation of cell survival, cell cycle and proliferation[91]. While VEGF-mediated activation of PAK in endothelial cells is primarily focused on cytoskeletal remodeling, PAK activation by bFGF is necessary for the anchorage-dependent cell survival in endothelial cells[92]. However, specific role of PAK and the mechanism by which PAK regulates endothelial cell survival has not yet been fully addressed. The first in-depth study on the role of PAK in endothelial cells was performed by Kiosses and co-workers[86]. In this study, 14 different mutants, truncated and wild-type variants of PAK were used to determine the function of PAK in endothelial cell function in vitro. Expression in endothelial cells of either an inactive mutant or the auto-inhibitory domain (PAK 83-149) of PAK1 significantly inhibited endothelial motility[93]. Interaction of PAK with Nck adaptor protein is also shown to stimulate PAK kinase activity in endothelial cells. In a study involving dominant-negative p65 PAK peptide that disrupts the interaction between PAK and Nck, endothelial migration and tube formation in 3D Matrigel environment in vitro was blunted[94]. This finding was corroborated in vivo using a chick allantoic membrane (CAM) angiogenesis assay[94]. Nck-mediated activation of PAK is also necessary for integrin activation, focal adhesion turn-over and maintenance of endothelial-barrier integrity[95].
Formation of a lumen in blood vessels is an essential step in neo-vascularization. However, due to the difficulty in developing proper in vivo models, events leading to lumen formation are poorly understood. A number of attempts were made to unveil this process using 3D matrix gel models in vitro[96]. Formation of endothelial lumens (endothelial tubes) in 3D collagen gel matrices is regulated by the coalescence of newly formed intracellular vacuoles that was dependent on integrin-mediated outside-in activation of Rac-PAK signaling[97]. These observations along with another study performed in a zebrafish model in vivo[98] suggests that Rac-PAK signaling is a key regulatory pathway mediating vascular lumen formation in vivo.
Another important event in vascular biology that is directly under the control of PAK is the regulation of endothelial-barrier function and vascular permeability. Rac-PAK signaling is expected to play a dual-role in the process since it is known to regulate both assembly and dis-assembly of VE-cadherin-based cell-cell junctions. VE-cadherin mediated activation of Rac-PAK signaling stabilizes the endothelial-barrier junctions [99]. VE-cadherin null endothelial cells exhibit impaired Rac activity and impaired localization of its guanine exchange nucleotide factor Tiam1[100]. In contrast, permeability stimulating agents such as thrombin disrupts endothelial-barrier junctions via activation of Rac-PAK signaling[101]. At the same time, VEGF mediated activation of endothelial cells disrupts endothelial-barrier via Rac-mediated internalization of VE-cadherins[102]. In response to stimuli from serum, VEGF, TNF, bFGF, histamine and thrombin, endothelial cells exhibit Rac-mediated activation of PAK via phosphorylation at Ser141 resulting in its translocation to endothelial cell-barrier junctions[103]. PAK can also directly phosphorylate VE-cadherin on a highly conserved serine residue which controls its stability at the cell-cell junctions[102]. Apart from this, atherogenic flow profiles, oxidized LDLs and pro-atherogenic cytokines stimulate PAK in endothelial cells and its translocation to barrier junctions; whereas blocking PAK activity reverses endothelial-barrier leakage in atherosclerosis prone regions[89]. Inhibition of PAK activity and function by disrupting the protein complex of PAK/Pix/GIT using a cell permeable peptide that prevents interaction between PAK and reduces vascular leakage in a mouse model of acute lung injury caused by lipopolysaccharide treatment[58]. Inactive mutations in PAK in zebrafish exhibits cerebral hemorrhage without imparting any obvious alterations in the vasculature[104]. While it is evident that Rac-PAK signaling is important for the regulation of endothelial cell and fibroblast function, extracellular matrix remodeling, vascular permeability, lumen formation as well as angiogenesis, these studies suggest that PAK may emerge as an important therapeutic target for many of the integrin, angiogenesis, matrix and vascular permeability related clinical conditions. A recent report indicating that interference with PAK function prevents smooth muscle cell proliferation and pathological vascular remodeling[105] also suggests PAK1 as a potential target in such conditions as hypertension, atherosclerosis, and vascular stenosis.
7. PAK1 in human cancer
Changes in the levels and activity of PAKs, and PAK1 in particular, are frequently described in human malignancies. A few examples listed in Table 3 demonstrate that this phenomenon is seen in tissues of various origins and using a variety of techniques. The reported abnormalities include amplification of the corresponding gene, elevated levels of the mRNA and protein, and increased accumulation of phosphorylated and, presumably, activated form of this enzyme. In addition to malignant tumors, benign schwannomas that arise in patients with neurofibromatosis type 2 display the elevated levels of phosphorylated and, presumably, active PAK1[106]. There are also intriguing observations about accumulation of phosphorylated PAK1 specifically in the nuclei of malignant cells[107], which parallel the changes observed during progression of breast cancer in a mouse model of the disease[108]. Importantly, elevated level of PAK1 was identified as an independent prognostic predictor of poor survival in ovarian cancer[107]. In breast cancer, nuclear expression of PAK1 in conjunction with phosphorylation of estrogen receptor on the PAK1 site (serine 305) predicts resistance to tamoxifen therapy, while the cytoplasmic levels of PAK1 correlate with recurrence rate and mortality[109, 110]. Similarly, in patients with gastric cancer higher levels of PAK1 were associated with advanced tumor stages, metastasis and reduced survival[111]. There are also numerous reports of elevated PAK1 activity in cell lines, although in most cases such reports cannot rule out that the changes had been selected or caused by in vitro cultivation of the cells.
Table 3.
Examples of alterations in PAK1 status in human cancers
| Cancer type | Reported events | Detection method | References |
|---|---|---|---|
| Bladder carcinoma | elevated levels of RNA and protein |
oligo-microarray; qPCR, IHC |
223 |
| Breast carcinoma | elevated levels of RNA, total and phosphorylated protein; nuclear accumulation; gene amplification; increased kinase activity |
WB; qPCR; IHC; aCGH; in vitro kinase assay. |
110, 119, 224-228 |
| Colorectal carcinoma | elevated levels of protein; gene amplification |
mCGH; aCGH; FISH; IHC. |
229, 230 |
| Gastric carcinoma | elevated levels of protein | WB | 111 |
| Glioblastoma | elevated levels of phosphorylated protein |
IHC | 231 |
| Hepatocellular carcinoma | elevated levels of RNA and protein |
qPCR,WB, IHC | 232 |
| NK and NK-like T cell lymphomas |
gene amplification | mCGH; aCGH | 233 |
| Ovarian carcinoma | elevated levels of RNA, total and phosphorylated protein; gene amplification. |
qPCR; WB; FISH | 107, 234 |
| Renal cell carcinoma | elevated levels of RNA and protein |
qPCR; WB; IHC | 235 |
| Shwannoma | elevated levels of phosphorylated protein |
WB | 106 |
qPCR – quantitative RT-PCR; IHC – immunohistochemistry; WB – Western blotting; aCGH – array comparative genomic hybridization; mCGH - metaphase comparative genomic hybridization; FISH- fluorescence in situ hybridization.
Overall, there is little doubt that the levels and activity of PAK1 are frequently increased in various cancers. The essential question is what contribution this protein makes to cancer progression and how critical this contribution is.
Cancer progression is a multistage process during which an evolving population of malignant cells has to overcome numerous hurdles of natural tumor suppressive mechanisms. For example, for a solid tumor to form and to progress to a metastatic state, the diseased cell has to proliferate despite the inhibitory effects of its neighbors and the lack of exogenous mitogens, to attract the flow of nutrients and oxygen in order to sustain the tumor growth, to escape its normal physical niche, to survive in an environment that may lack its normal survival factors, and to maintain a higher rate of metabolism and protein synthesis in order to support its more active “lifestyle”. When the involvement of some of the better known oncogenes in these processes was elucidated, PAKs came into the spotlight as potential components of the relevant molecular mechanisms. Remarkably, PAKs, and PAK1 in particular, have been linked in one way or another to all of these phenomena. Such a comprehensive involvement makes PAK1 an attractive therapeutic target.
Kinases from Akt family are frequently activated in malignant cells, where they contribute to multiple oncogenic traits[112]. One of the best known phenomena induced by activated transforming oncogenes in cells of solid tissue origin is the lack of contact inhibition[113]. In confluent cultures of cells this is typically manifested by characteristic foci of multi-layer growth. The activated form of Akt relieves rodent fibroblasts from contact inhibition and this process requires the activity of PAK1 [6]. Reduction of PAK1 activity in Rat1 cells dissociates the activation of Akt from that of the ERKs, and reduced to ability of Akt to induce focus formation and tumor growth in a xenograft model[6]. Interestingly, activation of Akt facilitates redistribution of PAK1 to the plasma membrane in a pattern similar to that assumed by cRaf in the same conditions. Such co-localization may provide a mechanistic basis for the role that PAK1 plays as a liaison between the PI3K/Akt and the Raf/MEK/ERK pathways. Importantly, the decrease in PAK1 function is well tolerated by non-transformed Rat1a cells[6].
Another common oncogenic event is the activation of the oncogenes from Ras family. Ras is known to require the function of Akt for efficient transformation. Accordingly, at least some transformed features of Ras-expressing cells are also PAK1- dependent. In particular, focus formation and another characteristic of oncogenic transformation, growth in semi-solid medium, were abolished when PAK1 function was suppressed in Ras-transformed rat fibroblasts[114].Growth enhancement by Ras involves the activation of cyclin-dependent kinases (CDKs), which is achieved by reducing the production of CDK inhibitor p27 and by enhancing the production of CDK activator cyclin D1. While both of these phenomena depend on phosphoinositol-3-kinase, the latter specifically depends on PAK1 and on PAK1 - dependent activation of ERKs[115]. Interestingly, it appears that in Ras-transformed cells, in comparison to their non-transformed counterparts, there is a heightened dependence on PAK1 for keeping the MAP kinase cascade active and, ultimately, for cell growth. It also appears that constitutive activation of Raf may be able to supplant the requirement for PAK1. These observations are very important for future clinical development of PAK inhibitors: they point both to potential utility and specificity of such an approach, as well as to a possible mechanism of resistance to such a therapy.
The molecular pathology of neurofibromatosis type 2, which arises due to the lack of merlin, a putative negative regulator of PAK1, suggests that reducing activity of the latter may have a therapeutic value in this disease[106]. Indeed, rat schwannoma cells appear sensitive to inhibition of PAK1, while inhibition of all three type I PAKs suppressed transformation of NIH3T3 cells by dominant-negative mutant of merlin[106]. Although, the conduct of the critical experiments in rodent cells and the lack of some technical controls leave the significance and relevance of this study in need of further confirmation, it is important to note that merlin-deficient human mesothelioma cell lines are also hypersensitive to the treatments aimed at PAK1.[116]
Of note, oncogenic viruses often rely on the same mechanisms that are associated with spontaneous disease. For example, Kaposi sarcoma virus, a herpesvirus with particular significance to the malignancies in AIDS patients, requires a PAK1-dependent pathway for efficient transformation of cultured cells[117], while hepatitis B virus induces PAK1-dependent relocalization of Raf-1 to mitochondria[118]. In both of these cases, the clinical relevance of these phenomena is yet to be confirmed.
If PAK1 is indeed the main component of the major oncogenic signaling pathways, one may expect that direct activation of PAK1 would recapitulate at least some features of oncogenic transformation. Indeed, overexpression of the activated mutant form of PAK1 (PAK1 T423E) in tissue culture models stimulates anchorage-independent growth in breast cancer cell lines[119], and yields hyperplasia of mammary epithelium[120] and, eventually, breast carcinomas[108] in transgenic mice with tissue-specific expression of the transgene. Certainly, overexpression of an artificially mutated protein, especially, when achieved through a promoter with a very complex hormonal control, on its own would not suffice to draw clinically-relevant conclusions. Nevertheless, these results are concordant with the observations on PAK1 in human malignancies and on the consequences of PAK1 inhibition in transformed cells (see above).
It is important to note that the ability to grow in semi-solid medium requires simultaneous enhancement of proliferative and survival signals, the latter to avert anoikis. It is uncertain whether the pro-survival signaling of Ras was affected in the experiments that observed suppression of Ras-mediated transformation by dominant-negative PAK1[114]. There are many examples of studies that describe the involvement of group I PAKs in protection from cell death, including anoikis and killing by chemotherapeutic agents [7, 121-123]. The specific mechanism of anti-apoptotic protection was originally reported to involve direct inactivation of pro-apoptotic Bcl-2-family member Bad[124]. Serines 112 and 136 of Bad were originally reported as relevant targets [124]. However, a later report, which examined multiple PAK1 and Raf-1 mutants and combinations thereof in vivo and in vitro assays, indicates that these positions are poor targets for PAK1 itself, but, instead, are targets of PAK1 – direct activity of Raf-1[125]. Interestingly, anti-apoptotic function of PAK1 is well established in cells that express very low levels of Bad[126], suggesting that additional mechanisms may be also involved in this phenomenon. In fact, at least in 3D cultures of normal mammary epithelium cells, the protective function of α6β4 integrin is mediated through Rac1 and PAK1, which in turn activate NFkB. In these cells the sensitization rendered by inactivation of PAK1 was overcome by activation of NFkB via an alternative mechanism [127]. Interestingly, an earlier report by the same group suggests that this protection is independent of ERKs and PI3K[128]. However, the prior study of anti-apoptotic function of α6β4 integrin in transformed cells has implicated PI3K/Akt pathway in this process[129]. Barring experimental artifacts, these data imply that in different cell environments different modes of PAK1 operation contribute to cell survival. This may indicate an attractive opportunity to selectively abolish protective functions of this protein in tumor, but not in most normal cells.
Another possible involvement of PAK1 in resistance to programmed cell death may be through maintaining expression of anti-apoptotic protein survinin [130]. Although the original observation was made in osteoclasts, survivin is a major pro-survival protein in cells from a variety of cancers[131], and it is worth further investigation whether its expression in those cells is PAK1-dependent as well.
In order for a cancerous cell to escape from its normal environment, the heightened mobility, as rendered by elevated activity of PAK1, has to be matched by the ability to destroy and restructure extracellular matrix[132]. Not surprisingly, matrix metalloproteinases, which normally restructure tissues during embryogenesis and inflammation, are commonly found expressed in advanced tumors, where they also contribute to the processing of certain bioactive peptides[133]. In this regard, it is noteworthy that the increase in pericellular proteolysis observed in a model of pre-malignant progression of breast cancer corresponded to and was dependent on the increase in the activity of PAK1[134].
It has been reported that inflammatory cytokines induce the expression of MMP-9 through activation and stabilization of PAK1, which activates JNK and MMP-9 expression [135]. Specific relevance of this phenomenon to cancer has yet to be demonstrated, but one may consider a scenario in which MMP-9 is contributed to the tumor by appropriately activated stroma cells[136]. It is worth noting that mutational activation of some signaling cascades (e.g. that of NFkB) known to be active in cancer cells leads to increased secretion of potentially inflammatory cytokines (e.g.[137]). It is conceivable that one may attempt to target PAK1 function in the normal cells of stroma to suppress the invasiveness and metastasis of the tumor.
Finally, it is important to note that the gene encoding PAK1 is located in relative proximity to the one encoding cyclin D1. These genes are often co-amplified and, consequently, co-expressed. Therefore, one has to be cautious in attributing the consequences of amplification of human 11q13 to either one of these genes individually. Furthermore, it is likely that the properties of a sizeable number of tumors are determined specifically by the interplay between these proteins, rather than by either one of them alone, and the issue of their functional interaction merits further investigation.
8. PAK1 in neurological and mental disorders
PAK3 in clearly involved in some neurodegenerative disorders and variants of mental retardation[138-140] (reviewed in 141) and plays a special role in synapse formation and plasticity in hippocampus[142]. However, the involvement of PAK1 in these processes is less clear-cut. For example, both PAK1 and PAK3 were reduced in the hippocampus affected by Alzheimer disease, yet only PAK3 was affected in some other areas of the diseased brain[140]. However, this reported loss of the PAKs from the cytosol appears to be accompanied by re-localization of PAKs to the membrano-cytoskeletal fractions, where they appear to be active[143]. Using staining for drebnin and reduction in dendrites as indicators, Dr. Cole’s group has observed that a dominant-negative form of PAK1 sensitizes, while the wild type form protects from some effects of beta-amyloid oligomers in cultured primary neurons[140, 143]. However, in both cases it is hard to rule out that ectopically expressed PAK1 in some of these experiments acted as a surrogate for the highly homologous PAK3.
Dominant-negative PAK1, which, potentially, inhibits other PAK isoforms as well, upon expression in mouse forebrain affected synapse morphology and consolidation of long-term memory[144], but rescued some defects of a mouse model of Fragile X syndrome[145].
In case of Huntington’s disease, PAK1 specifically co-localizes with huntingtin inclusions in the affected brain146. In tissue culture models, interference with PAK1 function modestly decrease the formation of aggregates by mutant huntingtin, while the constitutively active PAK1 enhances the aggregation[146]. Accordingly, similar activity was reported for PAK1 regulator α-PIX[147]. The matter is complicated, however, by the observation that kinase activity of PAK1 is dispensable for this phenomenon[146]. Overall, it appears that pathological changes in the brain could be associated both with elevated and reduced function of PAKs and the specific role of PAK1 in these processes may be variable as well.
9. PAK1 in infection and immunity
The PAK-dependent cellular events are significant for the biology and pathogenicity of various viruses (recently reviewed elsewhere[21, 148]). It is interesting to note that historically the nucleocapsid protein of Rous sarcoma virus was one of the first targets of the kinase later identified as PAK2, and this phosphorylation appears important for the packaging of viral RNA[149, 150]. However, we are not aware of any evidence that PAK1 is critically important for in vivo propagation of this virus. In the pathogenicity of another group of retroviruses, the lentiviruses, the roles of the two PAKs are subjects of an ongoing controversy. It is clear that lentiviral Nef protein interacts with at least one of these kinases[151, 152], but contradictory data have been reported on which one it is[153-155]. Although Nef is an important player in lentiviral pathogenesis[156], it appears that its interaction with PAK is not absolutely indispensible for proliferation of HIV-1 and development of AIDS[156-158], reducing the value of PAKs as therapeutic targets. Since PAK1 is important for macropinocytosis[159], it plays a role in infection by various viruses that depend on this process, such as adenoviruses[160] and vaccinia virus[161]. PAK1 and PAK2 have been shown to play important, but distinct roles in alphaherpesvirus infection[162].
The fundamental cellular processes affected by PAK1 are important for the function of the cells of immune system as well. As mentioned above, the activation of ERKs, as well as formation of broad stable lamellipodia in macrophages during spreading depend on the presence of PAK1 in the cells[163]. While PAK1 was reported to play a role in directional migration of several types of myeloid cells towards a chemoattractant[164], chemotaxis of PAK1-/- macrophages towards CSF-1 appears normal[163]. Although it remains to be seen whether these effects of PAK1 remain critical in the context of in vivo response to pathogens, it would be prudent to consider the immune system as a potential site of side effects of any future anti-PAK1 therapy. This may be further exacerbated by the protective role that PAK1 may play in the resistance of microvascular endothelial cells towards invading bacteria[165].
Fortunately, the effects of PAK1-deficiency on the immune system have a very encouraging up-side. As demonstrated by otherwise relatively healthy PAK1-/- mice, Pak1 is critical for disassembly of cortical F-actin upon allergen stimulation, and PAK1 deficiency prevents the release of pro-inflammatory molecules from the granules of mast cells during the IgE-associated allergic responses[166].
10. Current approaches to inhibition of PAK1
The position of PAK1 in the middle of multiple signal transduction pathways (Figure 4) makes it very likely that any intervention affecting the upstream elements of those pathways will affect PAK1 among other molecules. In these cases the actual target of intervention is clearly distinct from PAK1 and, typically, the consequences of such an intervention cannot be attributed solely to the changes in PAK1 status. Hence, a detailed discussion of such targeting strategies falls outside of the scope of the present review. Functional inhibition of PAK1 itself has been achieved experimentally using several forms of dominant-negative mutants, RNA interference and a number of chemical inhibitors with various degrees of specificity.
PAK1 participates in a multitude of protein-protein interactions, which creates opportunities to disrupt all or some of these functions by expressing various fragments of mutant forms of this kinase that could compete with the endogenous protein. PAK1 exists as a dimer prior to activation and functions within a large multi-protein complex. Therefore, if a non-functional (e.g. devoid of kinase activity) PAK1 is introduced into a cell it may successfully compete with the endogenous form for inclusion into such complexes. Conceivably, a dimer of the wild type and mutant proteins may be less capable of activation, which, presumably, involves trans-autophosphorylation[49]. Indeed, a “kinase-dead” mutant (e.g. PAK1 K299R) is an effective dominant-negative reagent, which is commonly used to document PAK-dependence of various phenomena (e.g.6) . Also, the autoinhibitory domain of PAK, when expressed without the rest of the protein, binds to all group I PAKs and is a potent suppressor of their function [167]. This protein was expressed in a tissue-specific manner to investigate the PAK-dependent processes in mouse forebrain, where it affected synaptic morphology and impaired memory consolidation, but also relieved some symptoms in the model of fragile X syndrome [144, 145].
It is important to note, that some functions of PAK1 may be carried out independently of its kinase activity. For example, it has been proposed that in such a manner PAK1 may play a role of a scaffold for Akt-PDK-1 interaction [168]. This concern could be somewhat alleviated by the use of very short fragments of PAK1. For example, a cell-permeable small peptide called WR-PAK18, which includes a PIX-binding site of PAK1 (PPVIAPRPEHTKSVYTRS) added to a transmembrane transport peptide (RRWRRWWRRWWRRWRR), selectively blocks the PAK-PIX interaction and can suppresses RAS transformation in vitro[169, 170]. Remarkably, the peptide was selective in its growth suppression, inhibiting Ras-transformed, but not the parental non-transformed cells. Similarly, addition to endothelial cells of a peptide, which combines the Nck-binding motif (KPPAPPMRNTSTM) from PAK1 and the transmembrane transport motif from HIV-1 Tat (YGRKKRRQRRRG), effectively disrupted the interaction between PAK1 and Nck and reduced motility, contractility and ability to form blood vessels [94]. The fact that the short peptides may be active in vivo[94, 140] suggests that this route could be explored for the development of therapeutic interventions. In this case, a reduction in cost and further protection from proteolysis may become welcome improvements.
The use of dominant-negative forms or fragments of PAK1 carries an inevitable question about the specificity of their effects. Since those molecules take up the place of PAK1 in complexes with other proteins, one may be reasonably concerned that the observed effects ensue from sequestration of the latter, which might affect both PAK1 - dependent and PAK1- independent functions. It is also difficult, if not impossible, to separate the functions of PAK1, -2 and -3, which share many common interactions. Furthermore, expression of the kinase inhibitory domain of PAK1 alone was reported to inhibit cell growth even when the mutations were introduced to prevent the interaction of this peptide with PAK1[171]. This casts a shadow of doubt over a large body of work conducted using this tool. Therefore, it is critical that the conclusions reached using the dominant-negative proteins be confirmed by alternative means.
An obvious complementary approach to the use of dominant-negative peptides is to suppress PAK1 via RNA interference. Successful suppression of PAK1 expression by RNAi is readily achievable [6, 107], and provides a better discrimination between the individual PAK isoforms than the use of dominant-negative proteins. It is important to note that RNA interference is associated with a well-recognized danger of artifacts and off-target effects and necessitates appropriate controls (discussed in172).
Typically of a kinase, PAK1 is sensitive to the molecules that occupy its ATP-binding site. For example, a rather promiscuous kinase inhibitor staurosporin binds PAK1 with high affinity 173. A celecoxib derivative OSU-03012, which has been investigated primarily because of its effect on PDK1, inhibits PAK1 as well [174], but cannot be considered as a specific inhibitor. Considerably more specific are the bulky ATP antagonists CEP-1347 and KT D606, which do not fit in the ATP pockets of most kinases, but inhibit both PAKs and Mixed Lineage Kinases (MLKs), which possess an unusually large ATP pocket [175]. Although the transformation-suppressive activity of these compounds has been attributed to PAK inhibition, they are much more potent against MLKs, their specificity against individual PAKs has yet to be studied in detail, and their affinity towards PAKs is too low for clinical considerations. Similarly, the large ATP pocket of PAK1 has been targeted by bulky octahedral ruthenium complexes [176], but the ability of these compounds to target other PAKs and other proteins in general still remains to be explored. For research application, the conclusions based on the use of these compounds inevitably have to be corroborated by complementary techniques to exclude the contribution of alternative targets to the phenomena in question.
An alternative route towards developing small molecule inhibitors of PAK1 follows the example of dominant-negative fragments in disrupting specific protein interactions, rather than the kinase activity per se. The proof of principle has been achieved in a high throughput screen that discovered a molecule (named IPA-3) that prevents the functional interaction between PAK1 and Cdc42 [177]. The original screening had in vitro PAK1 activity as a read-out, but the use of Cdc42 as an activator of PAK1 in the reaction mixture enabled the discovery of IPA-3. IPA-3 inhibits group I PAKs, although the effect is the strongest against PAK1 [177]. A broad analysis using Invitrogen’s Z-Lyte assay revealed that at least 9 other kinases (out of a collection of 214 purified wild type or mutant enzymes) loose 50% or more of their activity in vitro in the presence of IPA-3 [177]. Notable on this list are Akt2 (68% inhibition), GSKα (66.8%), GSKβ (53.9%). p38α (70.1%), and PLK3 (88.1%). Among the kinases that showed lower, but still substantial inhibition are BRAF (46.4%), Aurora A (41.5%), SGK-1 (46.4%) and IKKβ (30%). This estimate of the breadth off-target effects is a conservative one and has to be taken very cautiously. In fact, the expected mechanism of IPA-3 activity (prevention of PAK1-Cdc42 interaction) could not be detected in this assay, which tested purified enzymes without their activators. IPA-3 does not inhibit PAK1 molecules, which already have been activated, but appears to disrupt the effect of Cdc42 by binding covalently to the autoregulatory domain of PAK1 [178]. It is not clear how it affects the other sensitive kinases. Furthermore, efficient inhibition of PAK1 by IPA-3 required relatively high doses of the latter, which could be explained, at least in part, by inactivation of this compound by reducing molecules. Even though IPA-3 is unlikely to become a therapeutic compound, it should serve as an inspiration for the further screens targeted at the activating mechanisms, rather than the enzymatic activity of this and other kinases.
11. Expert opinion: Is PAK1 a suitable target for therapy?
As discussed above, there is growing evidence that PAKs are involved in the phenomena that are clinically significant for various cardio-vascular disorders, but the specificity of PAK1 involvement is still uncertain. Studies indicate that even closely related PAKs (e.g. PAK1 and PAK2) have non-identical sets of substrates. The issue is further complicated because of the multiple and sometimes opposing roles of PAKs in these processes and certainly merits further investigation.
The reports on the involvement of PAK1 in various diseases of the brain indicate that both up- and down-regulation of this enzyme may be associated with pathological changes. This, along with the uncertainty about the relative contribution of other isoforms, clouds the prospect of targeting PAK1 for therapeutic intervention in these conditions. Furthermore, these observations necessitate a close attention to the affects that any anti-PAK therapy targeted at other organs might have on the nervous system, including the cognitive functions and the memory. In this regard, failure of an anti-PAK1 agent to penetrate the blood-brain barrier may not be a detriment to its therapeutic utility. Similarly complicated is the question of PAK1 targeting in infections: while it may partially attenuate certain viruses, it would also negatively impact some functions of the immune system. In fact, the recent report of PAK1-deficient animals having IgE-mediated responses to allergens[166] may indicate that, at least, for such acute life-threatening conditions as anaphylaxis the benefits of suppressing PAK1 may outweigh the risks.
Overall, the main effort of PAK-oriented therapy comes from the field of oncology. In oncology, an ideal candidate for therapeutic targeting should frequently play a critical role in progression and persistence of the disease, while being less essential for the survival of the whole organism. In addition, there should be efficient strategies to target it, while the cancer cells should be unlikely to develop resistance to it, or there should be a strategy to overt or to overcome the development of such resistance. Does PAK1 satisfy these criteria?
The currently available data point to PAK1 as an important intermediate in the signaling pathways that are engaged through various mechanisms in the majority of cancers. Moreover, there is a growing body of evidence to suggest that the role of PAK1 in these cases is essential for survival and progression of the tumor. It has to be noted that these evidence were obtained either using inhibitors with imperfect specificity, of by more specific means, but in in vitro or xenograft models. A direct experiment with specific suppression of PAK1 in the context of a natural tumor is yet to be reported, and some of the earlier conclusions may have to be re-evaluated or refined using inhibition of individual PAK isoforms.
The reports on specific vulnerability of transformed cells to suppression of PAK1 and the fact that PAK1-deficient animals retain normal life span, growth, or fertility[166], suggest that a therapy targeted at PAK1 is likely to be well tolerated by a patient. However, a much more detailed evaluation of the PAK1 knockout mice is warranted before a conclusion could be made about severity of their phenotype. Also, we cannot rule out the possibility that compensatory mechanisms were engaged during the development of these animals, and the consequences of acute inhibition of PAK1 may be more sever.
RNA interference provides an efficient way of suppressing PAK1 in model systems, but the clinical applications of this technology, however promising, still await successful resolution of the issues of efficient and targeted delivery, in vivo stability and safety (reviewed elsewhere[179]). Unfortunately, short of RNA interference, currently there are no tools to target PAK1 specifically. Although a compound suitable for clinical use has not been described in the literature yet, targeting of the unusually large ATP-binding pocket and specific targeting of the interaction of PAK1 with its interactor proteins have yielded very promising results. It is likely that both approaches will be pursued further. In addition to targeting the cdc42-dependent mechanism of activation, inhibition of the functional interaction between PAK1 and Akt may emerge as another strategy for selective targeting of the cancer-specific functions of PAK1.
The issue of isoform-specificity in targeting PAKs is very complex. On the one hand, the consequences of combined knockouts of all group I PAKs in an organism have not been reported yet, and it is certain that targeting a single isoform would incur fewer side effects than targeting all of them. On the other hand, with a few notable exceptions, the bulk of the published data on PAK inhibition was generated with techniques that poorly discriminate between the isoforms. In oncology, this issue goes beyond the question of whether PAK1 is essential for growth and survival of an individual tumor: if another isoform can fully compensate for the loss of PAK1 function, a treated tumor is more likely to acquire resistance to therapy. Therefore, inhibition of more than one isoform may be required for effective treatment, increasing the risk of side effects. In general, the studies on the possible mechanisms of resistance to PAK-directed therapy are conspicuously missing today, and this gap in knowledge would have to be filled before such an intervention proceeds to clinical trials. Furthermore, delineation of specific mechanisms of PAK involvement in oncogenesis may point to common downstream steps that are required for the oncogenic function of all the isoforms. Exclusive targeting of these steps may have fewer side effects, but the same anti-cancer efficacy as targeting PAKs.
Overall, in our opinion there is sufficient evidence to implicate PAKs, and PAK1 in particular, as attractive targets for the therapy of cancer and certain other diseases. Development of compounds with improved pharmacological properties, clarification of the roles of individual PAK isoforms, and an understanding of the mechanisms that could circumvent inactivation of PAK1 are the research area of critical importance for the further clinical advancement of PAK-oriented therapy.
Acknowledgements
The authors would like to thank the anonymous reviewers for their comments and constructive criticism.
Declaration of interest ES Kandel is sponsored by the National Institute of Health grants CA098176, CA1337708 and CA116060.
PR Somanath is sponsored by American Heart Association grant 0830326N and University of Georgia Research Foundation start-up funds.
References
- 1.Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994 Jan 6;367(6458):40–6. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GA, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. Embo J. 1995 May 1;14(9):1970–8. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knaus UG, Morris S, Dong HJ, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein--coupled receptors. Science. 1995 Jul 14;269(5221):221–3. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- 4.Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995 Sep 29;270(39):22731–7. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Molli PR, Pakala SB, Nguyen TM Bui, Rayala SK, Kumar R. PAK thread from amoeba to mammals. Journal of cellular biochemistry. 2009 Jul 1;107(4):579–85. doi: 10.1002/jcb.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somanath PR, Vijai J, Kichina JV, Byzova T, Kandel ES. The role of PAK-1 in activation of MAP kinase cascade and oncogenic transformation by Akt. Oncogene. 2009 Jun 25;28(25):2365–9. doi: 10.1038/onc.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marlin JW, Eaton A, Montano GT, Chang YW, Jakobi R. Elevated p21-activated kinase 2 activity results in anchorage-independent growth and resistance to anticancer drug-induced cell death. Neoplasia. 2009 Mar;11(3):286–97. doi: 10.1593/neo.81446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maroto B, Ye MB, von Lohneysen K, Schnelzer A, Knaus UG. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene. 2008 Aug 21;27(36):4900–8. doi: 10.1038/onc.2008.131. [DOI] [PubMed] [Google Scholar]
- 9.Bokoch GM. Biology of the p21-activated kinases. Annual review of biochemistry. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 10.Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem. 2005 Nov 4;280(44):36609–15. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- 11.Somanath PR, Byzova TV. 14-3-3beta-Rac1-p21 activated kinase signaling regulates Akt1-mediated cytoskeletal organization, lamellipodia formation and fibronectin matrix assembly. J Cell Physiol. 2009 Feb;218(2):394–404. doi: 10.1002/jcp.21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nature reviews. 2006 Jun;6(6):459–71. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 13.Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. The international journal of biochemistry & cell biology. 2002 Jul;34(7):713–7. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 14.Eswaran J, Lee WH, Debreczeni JE, Filippakopoulos P, Turnbull A, Fedorov O, et al. Crystal Structures of the p21-activated kinases PAK4, PAK5, and PAK6 reveal catalytic domain plasticity of active group II PAKs. Structure. 2007 Feb;15(2):201–13. doi: 10.1016/j.str.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arias-Romero LE, Chernoff J. A tale of two Paks. Biology of the cell / under the auspices of the European Cell Biology Organization. 2008 Feb;100(2):97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 16.Eswaran J, Soundararajan M, Knapp S. Targeting group II PAKs in cancer and metastasis. Cancer metastasis reviews. 2009 Jun;28(1-2):209–17. doi: 10.1007/s10555-008-9181-4. [DOI] [PubMed] [Google Scholar]
- 17.Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, et al. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem. 2007 May 25;282(21):15667–78. doi: 10.1074/jbc.M700253200. [DOI] [PubMed] [Google Scholar]
- 18.Bright MD, Garner AP, Ridley AJ. PAK1 and PAK2 have different roles in HGF-induced morphological responses. Cellular signalling. 2009 Dec;21(12):1738–47. doi: 10.1016/j.cellsig.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008 Jun;28(12):4162–72. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer metastasis reviews. 2009 Jun;28(1-2):51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacheco A, Chernoff J. Group I p21-activated kinases: emerging roles in immune function and viral pathogenesis. The international journal of biochemistry & cell biology. 2010 Jan;42(1):13–6. doi: 10.1016/j.biocel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vadlamudi RK, Kumar R. p21-activated kinase 1: an emerging therapeutic target. Cancer Treat Res. 2004;119:77–88. doi: 10.1007/1-4020-7847-1_5. [DOI] [PubMed] [Google Scholar]
- 23.Kreis P, Barnier JV. PAK signalling in neuronal physiology. Cellular signalling. 2009 Mar;21(3):384–93. doi: 10.1016/j.cellsig.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proceedings of the National Academy of Sciences of the United States of America. 2004 Apr 20;101(16):6062–7. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burbelo PD, Kozak CA, Finegold AA, Hall A, Pirone DM. Cloning, central nervous system expression and chromosomal mapping of the mouse PAK-1 and PAK-3 genes. Gene. 1999 May 31;232(2):209–15. doi: 10.1016/s0378-1119(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 26.Clerk A, Sugden PH. Activation of p21-activated protein kinase alpha (alpha PAK) by hyperosmotic shock in neonatal ventricular myocytes. FEBS letters. 1997 Feb 10;403(1):23–5. doi: 10.1016/s0014-5793(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 27.Dharmawardhane S, Brownson D, Lennartz M, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pseudopodia, membrane ruffles, and phagocytic cups in activated human neutrophils. Journal of leukocyte biology. 1999 Sep;66(3):521–7. doi: 10.1002/jlb.66.3.521. [DOI] [PubMed] [Google Scholar]
- 28.Kageyama K, Sakihara S, Suda T. Regulation and role of p21-activated kinase 3 by corticotropin-releasing factor in mouse pituitary. Regulatory peptides. 2009 Jan 8;152(1-3):88–94. doi: 10.1016/j.regpep.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, et al. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. Embo J. 1998 Nov 16;17(22):6527–40. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002 Jan 4;277(1):550–8. doi: 10.1074/jbc.M105732200. [DOI] [PubMed] [Google Scholar]
- 31.Qu J, Li X, Novitch BG, Zheng Y, Kohn M, Xie JM, et al. PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol Cell Biol. 2003 Oct;23(20):7122–33. doi: 10.1128/MCB.23.20.7122-7133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Minden A. Targeted disruption of the gene for the PAK5 kinase in mice. Mol Cell Biol. 2003 Oct;23(20):7134–42. doi: 10.1128/MCB.23.20.7134-7142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nekrasova T, Jobes ML, Ting JH, Wagner GC, Minden A. Targeted disruption of the Pak5 and Pak6 genes in mice leads to deficits in learning and locomotion. Developmental biology. 2008 Oct 1;322(1):95–108. doi: 10.1016/j.ydbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Pandey A, Dan I, Kristiansen TZ, Watanabe NM, Voldby J, Kajikawa E, et al. Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene. 2002 May 30;21(24):3939–48. doi: 10.1038/sj.onc.1205478. [DOI] [PubMed] [Google Scholar]
- 35.Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J Biol Chem. 2001 May 4;276(18):15345–53. doi: 10.1074/jbc.M010311200. [DOI] [PubMed] [Google Scholar]
- 36.Reddy SD, Ohshiro K, Rayala SK, Kumar R. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res. 2008 Oct 15;68(20):8195–200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knaus UG, Bokoch GM. The p21Rac/Cdc42-activated kinases (PAKs) The international journal of biochemistry & cell biology. 1998 Aug;30(8):857–62. doi: 10.1016/s1357-2725(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 38.Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, et al. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000 Aug 4;102(3):387–97. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 39.Knaus UG, Wang Y, Reilly AM, Warnock D, Jackson JH. Structural requirements for PAK activation by Rac GTPases. J Biol Chem. 1998 Aug 21;273(34):21512–8. doi: 10.1074/jbc.273.34.21512. [DOI] [PubMed] [Google Scholar]
- 40.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, et al. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Molecular cell. 1998 Jan;1(2):183–92. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 41.Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998 Sep 11;273(37):23633–6. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- 42.Walter BN, Huang Z, Jakobi R, Tuazon PT, Alnemri ES, Litwack G, et al. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. J Biol Chem. 1998 Oct 30;273(44):28733–9. doi: 10.1074/jbc.273.44.28733. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Frost JA, Cobb MH, Ross EM. Reciprocal signaling between heterotrimeric G proteins and the p21-stimulated protein kinase. J Biol Chem. 1999 Oct 29;274(44):31641–7. doi: 10.1074/jbc.274.44.31641. [DOI] [PubMed] [Google Scholar]
- 44.Mitsios N, Saka M, Krupinski J, Pennucci R, Sanfeliu C, Wang Q, et al. A microarray study of gene and protein regulation in human and rat brain following middle cerebral artery occlusion. BMC neuroscience. 2007;8:93. doi: 10.1186/1471-2202-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatti A, Huang Z, Tuazon PT, Traugh JA. Multisite autophosphorylation of p21-activated protein kinase gamma-PAK as a function of activation. J Biol Chem. 1999 Mar 19;274(12):8022–8. doi: 10.1074/jbc.274.12.8022. [DOI] [PubMed] [Google Scholar]
- 46.Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem. 2001 May 18;276(20):17347–53. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- 47.Thompson G, Owen D, Chalk PA, Lowe PN. Delineation of the Cdc42/Rac-binding domain of p21-activated kinase. Biochemistry. 1998 May 26;37(21):7885–91. doi: 10.1021/bi980140+. [DOI] [PubMed] [Google Scholar]
- 48.Roig J, Traugh JA. Cytostatic p21 G protein-activated protein kinase gamma-PAK. Vitamins and hormones. 2001;62:167–98. doi: 10.1016/s0083-6729(01)62004-1. [DOI] [PubMed] [Google Scholar]
- 49.Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, et al. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. Journal of molecular biology. 2006 Aug 11;361(2):312–26. doi: 10.1016/j.jmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 50.King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, et al. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J Biol Chem. 2000 Dec 29;275(52):41201–9. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- 51.Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. The Journal of cell biology. 1997 Sep 22;138(6):1265–78. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Frost JA. Multiple Rho proteins regulate the subcellular targeting of PAK5. Biochemical and biophysical research communications. 2006 Dec 15;351(2):328–35. doi: 10.1016/j.bbrc.2006.09.172. [DOI] [PubMed] [Google Scholar]
- 53.Aronheim A, Broder YC, Cohen A, Fritsch A, Belisle B, Abo A. Chp, a homologue of the GTPase Cdc42Hs, activates the JNK pathway and is implicated in reorganizing the actin cytoskeleton. Curr Biol. 1998 Oct 8;8(20):1125–8. doi: 10.1016/s0960-9822(98)70468-3. [DOI] [PubMed] [Google Scholar]
- 54.Neudauer CL, Joberty G, Tatsis N, Macara IG. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr Biol. 1998 Oct 22;8(21):1151–60. doi: 10.1016/s0960-9822(07)00486-1. [DOI] [PubMed] [Google Scholar]
- 55.Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes & development. 2001 Jul 15;15(14):1796–807. doi: 10.1101/gad.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniels RH, Zenke FT, Bokoch GM. alphaPix stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J Biol Chem. 1999 Mar 5;274(10):6047–50. doi: 10.1074/jbc.274.10.6047. [DOI] [PubMed] [Google Scholar]
- 57.Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem. 2002 Feb 15;277(7):5644–50. doi: 10.1074/jbc.M107704200. [DOI] [PubMed] [Google Scholar]
- 58.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell. 2007 Jun;18(6):2346–55. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuda C, Kameyama K, Suzuki A, Mishima W, Yamaji S, Okamoto H, et al. Affixin activates Rac1 via betaPIX in C2C12 myoblast. FEBS letters. 2008 Apr 9;582(8):1189–96. doi: 10.1016/j.febslet.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 60.Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, et al. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998 Apr 3;273(14):8137–44. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- 61.Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996 Aug 30;271(35):20997–1000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 62.Puto LA, Pestonjamasp K, King CC, Bokoch GM. p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J Biol Chem. 2003 Mar 14;278(11):9388–93. doi: 10.1074/jbc.M208414200. [DOI] [PubMed] [Google Scholar]
- 63.Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol Cell Biol. 2003 Nov;23(22):8058–69. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McManus MJ, Boerner JL, Danielsen AJ, Wang Z, Matsumura F, Maihle NJ. An oncogenic epidermal growth factor receptor signals via a p21-activated kinase-caldesmon-myosin phosphotyrosine complex. J Biol Chem. 2000 Nov 10;275(45):35328–34. doi: 10.1074/jbc.M005399200. [DOI] [PubMed] [Google Scholar]
- 65.Bagheri-Yarmand R, Mandal M, Taludker AH, Wang RA, Vadlamudi RK, Kung HJ, et al. Etk/Bmx tyrosine kinase activates Pak1 and regulates tumorigenicity of breast cancer cells. J Biol Chem. 2001 Aug 3;276(31):29403–9. doi: 10.1074/jbc.M103129200. [DOI] [PubMed] [Google Scholar]
- 66.Westphal RS, Coffee RL, Jr., Marotta A, Pelech SL, Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem. 1999 Jan 8;274(2):687–92. doi: 10.1074/jbc.274.2.687. [DOI] [PubMed] [Google Scholar]
- 67.Koh CG, Tan EJ, Manser E, Lim L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr Biol. 2002 Feb 19;12(4):317–21. doi: 10.1016/s0960-9822(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 68.Brzeska H, Szczepanowska J, Matsumura F, Korn ED. Rac-induced increase of phosphorylation of myosin regulatory light chain in HeLa cells. Cell motility and the cytoskeleton. 2004 Jul;58(3):186–99. doi: 10.1002/cm.20009. [DOI] [PubMed] [Google Scholar]
- 69.Leberer E, Dignard D, Harcus D, Thomas DY, Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. Embo J. 1992 Dec;11(13):4815–24. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu C, Lytvyn V, Thomas DY, Leberer E. The phosphorylation site for Ste20p-like protein kinases is essential for the function of myosin-I in yeast. J Biol Chem. 1997 Dec 5;272(49):30623–6. doi: 10.1074/jbc.272.49.30623. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005 Mar 30;25(13):3379–88. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999 Mar 26;283(5410):2083–5. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 73.Bernard O. Lim kinases, regulators of actin dynamics. The international journal of biochemistry & cell biology. 2007;39(6):1071–6. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Po’uha ST, Shum MS, Goebel A, Bernard O, Kavallaris M. LIM-kinase 2, a regulator of actin dynamics, is involved in mitotic spindle integrity and sensitivity to microtubule-destabilizing drugs. Oncogene. 2009 Nov 2; doi: 10.1038/onc.2009.367. [DOI] [PubMed] [Google Scholar]
- 75.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, et al. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002 Sep;4(9):681–90. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 76.Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO reports. 2004 Feb;5(2):154–60. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008 Feb 1;121(Pt 3):369–78. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 78.Morita T, Mayanagi T, Yoshio T, Sobue K. Changes in the balance between caldesmon regulated by p21-activated kinases and the Arp2/3 complex govern podosome formation. J Biol Chem. 2007 Mar 16;282(11):8454–63. doi: 10.1074/jbc.M609983200. [DOI] [PubMed] [Google Scholar]
- 79.Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proceedings of the National Academy of Sciences of the United States of America. 2007 Apr 17;104(16):6782–7. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J Biol Chem. 2004 Feb 13;279(7):6196–203. doi: 10.1074/jbc.M307261200. [DOI] [PubMed] [Google Scholar]
- 81.Vadlamudi RK, Barnes CJ, Rayala S, Li F, Balasenthil S, Marcus S, et al. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol Cell Biol. 2005 May;25(9):3726–36. doi: 10.1128/MCB.25.9.3726-3736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menzel N, Melzer J, Waschke J, Lenz C, Wecklein H, Lochnit G, et al. The Drosophila p21-activated kinase Mbt modulates DE-cadherin-mediated cell adhesion by phosphorylation of Armadillo. The Biochemical journal. 2008 Dec 1;416(2):231–41. doi: 10.1042/BJ20080465. [DOI] [PubMed] [Google Scholar]
- 83.Hashimoto S, Tsubouchi A, Mazaki Y, Sabe H. Interaction of paxillin with p21-activated Kinase (PAK). Association of paxillin alpha with the kinase-inactive and the Cdc42-activated forms of PAK3. J Biol Chem. 2001 Feb 23;276(8):6037–45. doi: 10.1074/jbc.M005854200. [DOI] [PubMed] [Google Scholar]
- 84.Weis SM. Evaluating integrin function in models of angiogenesis and vascular permeability. Methods in enzymology. 2007;426:505–28. doi: 10.1016/S0076-6879(07)26021-5. [DOI] [PubMed] [Google Scholar]
- 85.Mammoto A, Mammoto T, Ingber DE. Rho signaling and mechanical control of vascular development. Current opinion in hematology. 2008 May;15(3):228–34. doi: 10.1097/MOH.0b013e3282fa7445. [DOI] [PubMed] [Google Scholar]
- 86.Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. The Journal of cell biology. 1999 Nov 15;147(4):831–44. doi: 10.1083/jcb.147.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo F, Debidda M, Yang L, Williams DA, Zheng Y. Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly. J Biol Chem. 2006 Jul 7;281(27):18652–9. doi: 10.1074/jbc.M603508200. [DOI] [PubMed] [Google Scholar]
- 88.Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem. 2006 Nov 24;281(47):36443–53. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
- 89.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, et al. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. The Journal of cell biology. 2007 Feb 26;176(5):719–27. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. Faseb J. 2008 Jun;22(6):1829–38. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 91.Moya EM Galan, Le Guelte A, Gavard J. PAKing up to the endothelium. Cellular signalling. 2009 Dec;21(12):1727–37. doi: 10.1016/j.cellsig.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003 Jul 4;301(5629):94–6. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- 93.Connolly JO, Simpson N, Hewlett L, Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol Biol Cell. 2002 Jul;13(7):2474–85. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, et al. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ Res. 2002 Apr 5;90(6):697–702. doi: 10.1161/01.res.0000014227.76102.5d. [DOI] [PubMed] [Google Scholar]
- 95.Stoletov KV, Gong C, Terman BI. Nck and Crk mediate distinct VEGF-induced signaling pathways that serve overlapping functions in focal adhesion turnover and integrin activation. Experimental cell research. 2004 Apr 15;295(1):258–68. doi: 10.1016/j.yexcr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 96.Nakatsu MN, Hughes CC. An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods in enzymology. 2008;443:65–82. doi: 10.1016/S0076-6879(08)02004-1. [DOI] [PubMed] [Google Scholar]
- 97.Koh W, Sachidanandam K, Stratman AN, Sacharidou A, Mayo AM, Murphy EA, et al. Formation of endothelial lumens requires a coordinated PKCepsilon-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J Cell Sci. 2009 Jun 1;122(Pt 11):1812–22. doi: 10.1242/jcs.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006 Jul 27;442(7101):453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 99.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, et al. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009 Apr 28;19(8):668–74. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 100.Lampugnani MG, Dejana E. Adherens junctions in endothelial cells regulate vessel maintenance and angiogenesis. Thrombosis research. 2007;120(Suppl 2):S1–6. doi: 10.1016/S0049-3848(07)70124-X. [DOI] [PubMed] [Google Scholar]
- 101.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001 Jun 22;276(25):22614–20. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- 102.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006 Nov;8(11):1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 103.Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004 Nov 5;279(45):46621–30. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- 104.Buchner DA, Su F, Yamaoka JS, Kamei M, Shavit JA, Barthel LK, et al. pak2a mutations cause cerebral hemorrhage in redhead zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2007 Aug 28;104(35):13996–4001. doi: 10.1073/pnas.0700947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hinoki A, Kimura K, Higuchi S, Eguchi K, Takaguri A, Ishimaru K, et al. p21-activated kinase 1 participates in vascular remodeling in vitro and in vivo. Hypertension. 2010 Jan;55(1):161–5. doi: 10.1161/HYPERTENSIONAHA.109.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-Activated Kinases as Targets for Inhibition in Neurofibromatosis Type 2. Cancer Res. 2008 October 1;68(19):7932–7. doi: 10.1158/0008-5472.CAN-08-0866. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siu MK, Wong ES, Chan HY, Kong DS, Woo NW, Tam KF, et al. Differential expression and phosphorylation of Pak1 and Pak2 in ovarian cancer: Effects on prognosis and cell invasion. International journal of cancer. 2009 Oct 28; doi: 10.1002/ijc.25005. [DOI] [PubMed] [Google Scholar]
- 108.Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene. 2006 May 11;25(20):2931–6. doi: 10.1038/sj.onc.1209309. [DOI] [PubMed] [Google Scholar]
- 109.Bostner J, Skoog L, Fornander T, Nordenskjold B, Stal O. Estrogen Receptor-{alpha} Phosphorylation at Serine 305, Nuclear p21-Activated Kinase 1 Expression, and Response to Tamoxifen in Postmenopausal Breast Cancer. Clin Cancer Res. 2010 Mar 1;16(5):1624–33. doi: 10.1158/1078-0432.CCR-09-1733. [DOI] [PubMed] [Google Scholar]
- 110.Bostner J, Waltersson M Ahnstrom, Fornander T, Skoog L, Nordenskjold B, Stal O. Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene. 2007;26(49):6997–7005. doi: 10.1038/sj.onc.1210506. [DOI] [PubMed] [Google Scholar]
- 111.Liu F, Li X, Wang C, Cai X, Du Z, Xu H, et al. Downregulation of p21-activated kinase-1 inhibits the growth of gastric cancer cells involving cyclin B1. International journal of cancer. 2009 Dec 1;125(11):2511–9. doi: 10.1002/ijc.24588. [DOI] [PubMed] [Google Scholar]
- 112.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Experimental cell research. 1999 Nov 25;253(1):210–29. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 113.Weinberg RA. The Biology of Cancer. Garland Science; New York: 2007. [Google Scholar]
- 114.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, et al. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol. 1997 August 1;17(8):4454–64. doi: 10.1128/mcb.17.8.4454. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nheu T, He H, Hirokawa Y, Walker F, Wood J, Maruta H. PAK is essential for RAS-induced upregulation of cyclin D1 during the G1 to S transition. Cell cycle (Georgetown, Tex. 2004 Jan;3(1):71–4. [PubMed] [Google Scholar]
- 116.Hirokawa Y, Tikoo A, Huynh J, Utermark T, Hanemann CO, Giovannini M, et al. A clue to the therapy of neurofibromatosis type 2: NF2/merlin is a PAK1 inhibitor. Cancer J. 2004 Jan-Feb;10(1):20–6. doi: 10.1097/00130404-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 117.Dadke D, Fryer BH, Golemis EA, Field J. Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 2003 Dec 15;63(24):8837–47. [PubMed] [Google Scholar]
- 118.Chen J, Siddiqui A. Hepatitis B Virus X Protein Stimulates the Mitochondrial Translocation of Raf-1 via Oxidative Stress. J Virol. 2007 June 15;81(12):6757–60. doi: 10.1128/JVI.00172-07. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, et al. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000 Nov 17;275(46):36238–44. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 120.Wang R-A, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-[alpha] and promotes hyperplasia in mammary epithelium. EMBO J. 2002;21(20):5437–47. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deacon K, Mistry P, Chernoff J, Blank JL, Patel R. p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell. 2003 May;14(5):2071–87. doi: 10.1091/mbc.E02-10-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jakobi R, Moertl E, Koeppel MA. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J Biol Chem. 2001 May 18;276(20):16624–34. doi: 10.1074/jbc.M007753200. [DOI] [PubMed] [Google Scholar]
- 123.Menard RE, Jovanovski AP, Mattingly RR. Active p21-activated kinase 1 rescues MCF10A breast epithelial cells from undergoing anoikis. Neoplasia. 2005 Jul;7(7):638–45. doi: 10.1593/neo.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, et al. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000 Jan;20(2):453–61. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin S, Zhuo Y, Guo W, Field J. p21-activated Kinase 1 (Pak1)-dependent phosphorylation of Raf-1 regulates its mitochondrial localization, phosphorylation of BAD, and Bcl-2 association. J Biol Chem. 2005 Jul 1;280(26):24698–705. doi: 10.1074/jbc.M413374200. [DOI] [PubMed] [Google Scholar]
- 126.Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000 Mar 31;275(13):9106–9. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- 127.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007 Oct 15;120(Pt 20):3700–12. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 128.Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, et al. Autocrine laminin-5 ligates {alpha}6{beta}4 integrin and activates RAC and NF{kappa}B to mediate anchorage-independent survival of mammary tumors. J Cell Biol. 2003 December 22;163(6):1397–407. doi: 10.1083/jcb.200302023. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bachelder RE, Ribick MJ, Marchetti A, Falcioni R, Soddu S, Davis KR, et al. P53 Inhibits {alpha}6{beta}4 Integrin Survival Signaling by Promoting the Caspase 3-Dependent Cleavage of Akt/PKB. J Cell Biol. 1999 November 29;147(5):1063–72. doi: 10.1083/jcb.147.5.1063. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bradley EW, Ruan MM, Oursler MJ. PAK1 is a novel MEK-independent raf target controlling expression of the IAP survivin in M-CSF-mediated osteoclast survival. J Cell Physiol. 2008 Dec;217(3):752–8. doi: 10.1002/jcp.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008 Apr;12(4):463–76. doi: 10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- 132.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008 Nov;18(11):560–74. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 133.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Molecular Aspects of Medicine. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia. 2008 Apr;10(4):314–29. doi: 10.1593/neo.07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou L, Yan C, Gieling RG, Kida Y, Garner W, Li W, et al. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated kinase-1. BMC Immunol. 2009;10:15. doi: 10.1186/1471-2172-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Seminars in Cell & Developmental Biology. 2008;19(1):52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 137.Kandel ES, Lu T, Wan Y, Agarwal MK, Jackson MW, Stark GR. Mutagenesis by reversible promoter insertion to study the activation of NF-kappaB. Proceedings of the National Academy of Sciences of the United States of America. 2005 May 3;102(18):6425–30. doi: 10.1073/pnas.0502463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McPhie DL, Coopersmith R, Hines-Peralta A, Chen Y, Ivins KJ, Manly SP, et al. DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. J Neurosci. 2003 Jul 30;23(17):6914–27. doi: 10.1523/JNEUROSCI.23-17-06914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998 Sep;20(1):25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- 140.Zhao L, Ma Q-L, Calon F, Harris-White ME, Yang F, Lim GP, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9(2):234–42. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 141.Kreis P, Barnier J-V. PAK signalling in neuronal physiology. Cellular signalling. 2009;21(3):384–93. doi: 10.1016/j.cellsig.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 142.Boda B, Alberi S, Nikonenko I, Node-Langlois R, Jourdain P, Moosmayer M, et al. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J Neurosci. 2004 Dec 1;24(48):10816–25. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma Q-L, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, et al. p21-activated Kinase-aberrant Activation and Translocation in Alzheimer Disease Pathogenesis. Journal of Biological Chemistry. 2008 May 16;283(20):14132–43. doi: 10.1074/jbc.M708034200. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, et al. Altered cortical synaptic morphology and impaired memory consolidation in forebrain- specific dominant-negative PAK transgenic mice. Neuron. 2004 Jun 10;42(5):773–87. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 145.Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, et al. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul 3;104(27):11489–94. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luo S, Mizuta H, Rubinsztein DC. p21-activated kinase 1 promotes soluble mutant huntingtin self-interaction and enhances toxicity. Hum Mol Genet. 2008 Mar 15;17(6):895–905. doi: 10.1093/hmg/ddm362. [DOI] [PubMed] [Google Scholar]
- 147.Eriguchi M, Mizuta H, Luo S, Kuroda Y, Hara H, Rubinsztein DC. alpha Pix enhances mutant huntingtin aggregation. J Neurol Sci. 2010 Mar 15;290(1-2):80–5. doi: 10.1016/j.jns.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 148.Van den Broeke C, Radu M, Chernoff J, Favoreel HW. An emerging role for p21-activated kinases (Paks) in viral infections. Trends Cell Biol. 2010 Mar;20(3):160–9. doi: 10.1016/j.tcb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leis J, Johnson S, Collins LS, Traugh JA. Effects of phosphorylation of avian retrovirus nucleocapsid protein pp12 on binding of viral RNA. J Biol Chem. 1984 Jun 25;259(12):7726–32. [PubMed] [Google Scholar]
- 150.Fu X, Phillips N, Jentoft J, Tuazon PT, Traugh JA, Leis J. Site-specific phosphorylation of avian retrovirus nucleocapsid protein pp12 regulates binding to viral RNA. Evidence for different protein conformations. J Biol Chem. 1985 Aug 15;260(17):9941–7. [PubMed] [Google Scholar]
- 151.Sawai ET, Baur A, Struble H, Peterlin BM, Levy JA, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1994 February 15;91(4):1539–43. doi: 10.1073/pnas.91.4.1539. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nunn MF, Marsh JW. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J Virol. 1996 Sep;70(9):6157–61. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fackler OT, Lu X, Frost JA, Geyer M, Jiang B, Luo W, et al. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol Cell Biol. 2000 Apr;20(7):2619–27. doi: 10.1128/mcb.20.7.2619-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arora VK, Molina RP, Foster JL, Blakemore JL, Chernoff J, Fredericksen BL, et al. Lentivirus Nef specifically activates Pak2. J Virol. 2000 Dec;74(23):11081–7. doi: 10.1128/jvi.74.23.11081-11087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Renkema GH, Manninen A, Mann DA, Harris M, Saksela K. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr Biol. 1999 Dec 2;9(23):1407–10. doi: 10.1016/s0960-9822(00)80086-x. [DOI] [PubMed] [Google Scholar]
- 156.Foster J, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5(1):84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schindler M, Rajan D, Specht A, Ritter C, Pulkkinen K, Saksela K, et al. Association of Nef with p21-activated kinase 2 is dispensable for efficient human immunodeficiency virus type 1 replication and cytopathicity in ex vivo-infected human lymphoid tissue. J Virol. 2007 Dec;81(23):13005–14. doi: 10.1128/JVI.01436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lang SM, Iafrate AJ, Stahl-Hennig C, Kuhn EM, Nisslein T, Kaup FJ, et al. Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat Med. 1997 Aug;3(8):860–5. doi: 10.1038/nm0897-860. [DOI] [PubMed] [Google Scholar]
- 159.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000 Oct;11(10):3341–52. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Amstutz B, Gastaldelli M, Kalin S, Imelli N, Boucke K, Wandeler E, et al. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. Embo J. 2008 Apr 9;27(7):956–69. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mercer J, Helenius A. Vaccinia Virus Uses Macropinocytosis and Apoptotic Mimicry to Enter Host Cells. Science. 2008 April 25;320(5875):531–5. doi: 10.1126/science.1155164. 2008. [DOI] [PubMed] [Google Scholar]
- 162.Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, et al. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proceedings of the National Academy of Sciences of the United States of America. 2009 May 26;106(21):8707–12. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smith SD, Jaffer ZM, Chernoff J, Ridley AJ. PAK1-mediated activation of ERK1/2 regulates lamellipodial dynamics. J Cell Sci. 2008 Nov 15;121(Pt 22):3729–36. doi: 10.1242/jcs.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, et al. Directional Sensing Requires G[beta][gamma]-Mediated PAK1 and PIX[alpha]-Dependent Activation of Cdc42. Cell. 2003;114(2):215–27. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 165.Rudrabhatla RS, Sukumaran SK, Bokoch GM, Prasadarao NV. Modulation of myosin light-chain phosphorylation by p21-activated kinase 1 in Escherichia coli invasion of human brain microvascular endothelial cells. Infect Immun. 2003 May;71(5):2787–97. doi: 10.1128/IAI.71.5.2787-2797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Allen JD, Jaffer ZM, Park S-J, Burgin S, Hofmann C, Sells MA, et al. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009 March 19;113(12):2695–705. doi: 10.1182/blood-2008-06-160861. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Frost JA, Khokhlatchev A, Stippec S, White MA, Cobb MH. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J Biol Chem. 1998 Oct 23;273(43):28191–8. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- 168.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008 Nov;10(11):1356–64. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 169.He H, Hirokawa Y, Manser E, Lim L, Levitzki A, Maruta H. Signal therapy for ras-induced cancers in combination of ag 879 and pp1, specific inhibitors for erbb2 and src family kinases, that block pak activation. Cancer Journal. 2001;7(3):191. 05. [PubMed] [Google Scholar]
- 170.Maruta H, He H, Tikoo A, Nur-e-Kamal M. Cytoskeletal tumor suppressors that block oncogenic RAS signaling. Ann N Y Acad Sci. 1999;886:48–57. doi: 10.1111/j.1749-6632.1999.tb09399.x. [DOI] [PubMed] [Google Scholar]
- 171.Thullberg M, Gad A, Beeser A, Chernoff J, Stromblad S. The kinase-inhibitory domain of p21-activated kinase 1 (PAK1) inhibits cell cycle progression independent of PAK1 kinase activity. Oncogene. 2007 Mar 15;26(12):1820–8. doi: 10.1038/sj.onc.1209983. [DOI] [PubMed] [Google Scholar]
- 172.Gartel AL, Kandel ES. RNA interference in cancer. Biomolecular engineering. 2006 Mar;23(1):17–34. doi: 10.1016/j.bioeng.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 173.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008 Jan;26(1):127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 174.Porchia LM, Guerra M, Wang YC, Zhang Y, Espinosa AV, Shinohara M, et al. 2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl} acetamide (OSU-03012), a celecoxib derivative, directly targets p21-activated kinase. Mol Pharmacol. 2007 Nov;72(5):1124–31. doi: 10.1124/mol.107.037556. [DOI] [PubMed] [Google Scholar]
- 175.Nheu TV, He H, Hirokawa Y, Tamaki K, Florin L, Schmitz ML, et al. The k252a derivatives, inhibitors for the pak/mlk kinase family, selectively block the growth of ras transformants. Cancer Journal. 2002;8(4):328. doi: 10.1097/00130404-200207000-00009. 07. [DOI] [PubMed] [Google Scholar]
- 176.Maksimoska J, Feng L, Harms K, Yi C, Kissil J, Marmorstein R, et al. Targeting large kinase active site with rigid, bulky octahedral ruthenium complexes. J Am Chem Soc. 2008 Nov 26;130(47):15764–5. doi: 10.1021/ja805555a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008 Apr;15(4):322–31. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther. 2009 Sep 1; doi: 10.1158/1535-7163.MCT-09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–33. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Birukova AA, Alekseeva E, Cokic I, Turner CE, Birukov KG. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. American journal of physiology. 2008 Oct;295(4):L593–602. doi: 10.1152/ajplung.90257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Banerjee M, Worth D, Prowse DM, Nikolic M. Pak1 phosphorylation on t212 affects microtubules in cells undergoing mitosis. Curr Biol. 2002 Jul 23;12(14):1233–9. doi: 10.1016/s0960-9822(02)00956-9. [DOI] [PubMed] [Google Scholar]
- 182.Thiel DA, Reeder MK, Pfaff A, Coleman TR, Sells MA, Chernoff J. Cell cycle-regulated phosphorylation of p21-activated kinase 1. Curr Biol. 2002 Jul 23;12(14):1227–32. doi: 10.1016/s0960-9822(02)00931-4. [DOI] [PubMed] [Google Scholar]
- 183.Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998 Oct 23;273(43):28238–46. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 184.Papakonstanti EA, Stournaras C. Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. Mol Biol Cell. 2002 Aug;13(8):2946–62. doi: 10.1091/mbc.02-01-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hubsman M Weisz, Volinsky N, Manser E, Yablonski D, Aronheim A. Autophosphorylation-dependent degradation of Pak1, triggered by the Rho-family GTPase, Chp. The Biochemical journal. 2007 Jun 15;404(3):487–97. doi: 10.1042/BJ20061696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proceedings of the National Academy of Sciences of the United States of America. 2000 Jan 4;97(1):185–9. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leisner TM, Liu M, Jaffer ZM, Chernoff J, Parise LV. Essential role of CIB1 in regulating PAK1 activation and cell migration. The Journal of cell biology. 2005 Aug 1;170(3):465–76. doi: 10.1083/jcb.200502090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leeuw T, Wu C, Schrag JD, Whiteway M, Thomas DY, Leberer E. Interaction of a G-protein beta-subunit with a conserved sequence in Ste20/PAK family protein kinases. Nature. 1998 Jan 8;391(6663):191–5. doi: 10.1038/34448. [DOI] [PubMed] [Google Scholar]
- 189.Lise MF, Srivastava DP, Arstikaitis P, Lett RL, Sheta R, Viswanathan V, et al. Myosin-Va-interacting protein, RILPL2, controls cell shape and neuronal morphogenesis via Rac signaling. J Cell Sci. 2009 Oct 15;122(Pt 20):3810–21. doi: 10.1242/jcs.050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Talukder AH, Meng Q, Kumar R. CRIPak, a novel endogenous Pak1 inhibitor. Oncogene. 2006 Mar 2;25(9):1311–9. doi: 10.1038/sj.onc.1209172. [DOI] [PubMed] [Google Scholar]
- 191.Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proceedings of the National Academy of Sciences of the United States of America. 2001 May 22;98(11):6174–9. doi: 10.1073/pnas.101137298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lightcap CM, Kari G, Arias-Romero LE, Chernoff J, Rodeck U, Williams JC. Interaction with LC8 is required for Pak1 nuclear import and is indispensable for zebrafish development. PloS one. 2009;4(6):e6025. doi: 10.1371/journal.pone.0006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ku GM, Yablonski D, Manser E, Lim L, Weiss A. A PAK1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. Embo J. 2001 Feb 1;20(3):457–65. doi: 10.1093/emboj/20.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, et al. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. The Journal of cell biology. 1999 May 17;145(4):851–63. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS letters. 2003 Jan 30;535(1-3):6–10. doi: 10.1016/s0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- 196.Frost JA, Xu S, Hutchison MR, Marcus S, Cobb MH. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol Cell Biol. 1996 Jul;16(7):3707–13. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. Embo J. 2002 Oct 15;21(20):5437–47. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, et al. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO reports. 2002 Aug;3(8):767–73. doi: 10.1093/embo-reports/kvf157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002 Mar 22;277(12):10394–9. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 200.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Molecular cell. 2005 Oct 28;20(2):237–49. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 201.Tran NH, Wu X, Frost JA. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem. 2005 Apr 22;280(16):16244–53. doi: 10.1074/jbc.M501185200. [DOI] [PubMed] [Google Scholar]
- 202.Gallagher ED, Xu S, Moomaw C, Slaughter CA, Cobb MH. Binding of JNK/SAPK to MEKK1 is regulated by phosphorylation. J Biol Chem. 2002 Nov 29;277(48):45785–92. doi: 10.1074/jbc.M207702200. [DOI] [PubMed] [Google Scholar]
- 203.Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem. 2004 Apr 30;279(18):18392–400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- 204.Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK) Genes Cells. 2002 Feb;7(2):91–7. doi: 10.1046/j.1356-9597.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- 205.Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001 Jan 19;276(3):1677–80. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- 206.Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005 Apr 1;280(13):12152–61. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- 207.Foster DB, Shen LH, Kelly J, Thibault P, Van Eyk JE, Mak AS. Phosphorylation of caldesmon by p21-activated kinase. Implications for the Ca(2+) sensitivity of smooth muscle contraction. J Biol Chem. 2000 Jan 21;275(3):1959–65. doi: 10.1074/jbc.275.3.1959. [DOI] [PubMed] [Google Scholar]
- 208.Takizawa N, Koga Y, Ikebe M. Phosphorylation of CPI17 and myosin binding subunit of type 1 protein phosphatase by p21-activated kinase. Biochemical and biophysical research communications. 2002 Oct 4;297(4):773–8. doi: 10.1016/s0006-291x(02)02302-1. [DOI] [PubMed] [Google Scholar]
- 209.Ohtakara K, Inada H, Goto H, Taki W, Manser E, Lim L, et al. p21-activated kinase PAK phosphorylates desmin at sites different from those for Rho-associated kinase. Biochemical and biophysical research communications. 2000 Jun 16;272(3):712–6. doi: 10.1006/bbrc.2000.2854. [DOI] [PubMed] [Google Scholar]
- 210.Buscemi N, Foster DB, Neverova I, Van Eyk JE. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circ Res. 2002 Sep 20;91(6):509–16. doi: 10.1161/01.res.0000035246.27856.53. [DOI] [PubMed] [Google Scholar]
- 211.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999 Sep;1(5):253–9. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 212.Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, et al. Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J Biol Chem. 2002 Nov 15;277(46):44417–30. doi: 10.1074/jbc.M203754200. [DOI] [PubMed] [Google Scholar]
- 213.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Molecular cell. 2004 Jul 2;15(1):117–27. doi: 10.1016/j.molcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 214.Vadlamudi RK, Manavathi B, Singh RR, Nguyen D, Li F, Kumar R. An essential role of Pak1 phosphorylation of SHARP in Notch signaling. Oncogene. 2005 Jun 30;24(28):4591–6. doi: 10.1038/sj.onc.1208672. [DOI] [PubMed] [Google Scholar]
- 215.Liberali P, Kakkonen E, Turacchio G, Valente C, Spaar A, Perinetti G, et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. Embo J. 2008 Apr 9;27(7):970–81. doi: 10.1038/emboj.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005 Apr 15;65(8):3179–84. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 217.Gururaj A, Barnes CJ, Vadlamudi RK, Kumar R. Regulation of phosphoglucomutase 1 phosphorylation and activity by a signaling kinase. Oncogene. 2004 Oct 21;23(49):8118–27. doi: 10.1038/sj.onc.1207969. [DOI] [PubMed] [Google Scholar]
- 218.Manavathi B, Rayala SK, Kumar R. Phosphorylation-dependent regulation of stability and transforming potential of ETS transcriptional factor ESE-1 by p21-activated kinase 1. J Biol Chem. 2007 Jul 6;282(27):19820–30. doi: 10.1074/jbc.M702309200. [DOI] [PubMed] [Google Scholar]
- 219.Wang RA, Vadlamudi RK, Bagheri-Yarmand R, Beuvink I, Hynes NE, Kumar R. Essential functions of p21-activated kinase 1 in morphogenesis and differentiation of mammary glands. The Journal of cell biology. 2003 May 12;161(3):583–92. doi: 10.1083/jcb.200212066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sakurada K, Kato H, Nagumo H, Hiraoka H, Furuya K, Ikuhara T, et al. Synapsin I is phosphorylated at Ser603 by p21-activated kinases (PAKs) in vitro and in PC12 cells stimulated with bradykinin. J Biol Chem. 2002 Nov 22;277(47):45473–9. doi: 10.1074/jbc.M206673200. [DOI] [PubMed] [Google Scholar]
- 221.Ahmed S, Prigmore E, Govind S, Veryard C, Kozma R, Wientjes FB, et al. Cryptic Rac-binding and p21(Cdc42Hs/Rac)-activated kinase phosphorylation sites of NADPH oxidase component p67(phox) J Biol Chem. 1998 Jun 19;273(25):15693–701. doi: 10.1074/jbc.273.25.15693. [DOI] [PubMed] [Google Scholar]
- 222.Shalom-Barak T, Knaus UG. A p21-activated kinase-controlled metabolic switch up-regulates phagocyte NADPH oxidase. J Biol Chem. 2002 Oct 25;277(43):40659–65. doi: 10.1074/jbc.M206650200. [DOI] [PubMed] [Google Scholar]
- 223.Ito M, Nishiyama H, Kawanishi H, Matsui S, Guilford P, Reeve A, et al. P21-activated kinase 1: a new molecular marker for intravesical recurrence after transurethral resection of bladder cancer. J Urol. 2007 Sep;178(3 Pt 1):1073–9. doi: 10.1016/j.juro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 224.Balasenthil S, Sahin AA, Barnes CJ, Wang R-A, Pestell RG, Vadlamudi RK, et al. p21-activated Kinase-1 Signaling Mediates Cyclin D1 Expression in Mammary Epithelial and Cancer Cells. Journal of Biological Chemistry. 2004 January 9;279(2):1422–8. doi: 10.1074/jbc.M309937200. 2004. [DOI] [PubMed] [Google Scholar]
- 225.Bekri S, Adelaide J, Merscher S, Grosgeorge J, Caroli-Bosc F, Perucca-Lostanlen D, et al. Detailed map of a region commonly amplified at 11q13-->q14 in human breast carcinoma. Cytogenet Cell Genet. 1997;79(1-2):125–31. doi: 10.1159/000134699. [DOI] [PubMed] [Google Scholar]
- 226.Holm C, Rayala S, Jirstrom K, Stal O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006 May 17;98(10):671–80. doi: 10.1093/jnci/djj185. [DOI] [PubMed] [Google Scholar]
- 227.Lundgren K, Holm K, Nordenskjold B, Borg A, Landberg G. Gene products of chromosome 11q and their association with CCND1 gene amplification and tamoxifen resistance in premenopausal breast cancer. Breast Cancer Res. 2008;10(5):R81. doi: 10.1186/bcr2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Salh B, Marotta A, Wagey R, Sayed M, Pelech S. Dysregulation of phosphatidylinositol 3-kinase and downstream effectors in human breast cancer. International journal of cancer. 2002 Mar 1;98(1):148–54. doi: 10.1002/ijc.10147. [DOI] [PubMed] [Google Scholar]
- 229.Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, et al. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003 Sep;163(3):985–92. doi: 10.1016/S0002-9440(10)63458-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Carter JH, Douglass LE, Deddens JA, Colligan BM, Bhatt TR, Pemberton JO, et al. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin Cancer Res. 2004 May 15;10(10):3448–56. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- 231.Aoki H, Yokoyama T, Fujiwara K, Tari AM, Sawaya R, Suki D, et al. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin Cancer Res. 2007 Nov 15;13(22 Pt 1):6603–9. doi: 10.1158/1078-0432.CCR-07-0145. [DOI] [PubMed] [Google Scholar]
- 232.Ching Y-P, Leong VYL, Lee M-F, Xu H-T, Jin D-Y, Ng IO-L. P21-Activated Protein Kinase Is Overexpressed in Hepatocellular Carcinoma and Enhances Cancer Metastasis Involving c-Jun NH2-Terminal Kinase Activation and Paxillin Phosphorylation. Cancer Res. 2007 April 15;67(8):3601–8. doi: 10.1158/0008-5472.CAN-06-3994. 2007. [DOI] [PubMed] [Google Scholar]
- 233.Mao X, Onadim Z, Price EA, Child F, Lillington DM, Russell-Jones R, et al. Genomic alterations in blastic natural killer/extranodal natural killer-like T cell lymphoma with cutaneous involvement. J Invest Dermatol. 2003 Sep;121(3):618–27. doi: 10.1046/j.1523-1747.2003.12406.x. [DOI] [PubMed] [Google Scholar]
- 234.Brown LA, Kalloger SE, Miller MA, Shih Ie M, McKinney SE, Santos JL, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer. 2008 Jun;47(6):481–9. doi: 10.1002/gcc.20549. [DOI] [PubMed] [Google Scholar]
- 235.O’Sullivan GC, Tangney M, Casey G, Ambrose M, Houston A, Barry OP. Modulation of p21-activated kinase 1 alters the behavior of renal cell carcinoma. International journal of cancer. 2007;121(9):1930–40. doi: 10.1002/ijc.22893. [DOI] [PubMed] [Google Scholar]