Abstract
The binding of viridans group streptococci with human platelets was analyzed by two-color flow cytometry. Binding was detected within 15 s of mixing bacteria and platelets. At ratios of bacteria to platelets of 1:1, 10:1, 100:1, and 1,000:1, the percentages of bound streptococci (mean +/- standard deviation) were 93.2% +/- 5.4%, 80.0% +/- 8.6%, 39.8% +/- 11.1%, and 12.5% +/- 2.0%, respectively. Binding of labeled bacteria was reversed by adding a 500-fold excess of unlabeled streptococci. These results demonstrate that streptococcus-platelet binding is rapid, reversible, and saturable, which suggests a specific receptor-ligand interaction.
Full text
PDF
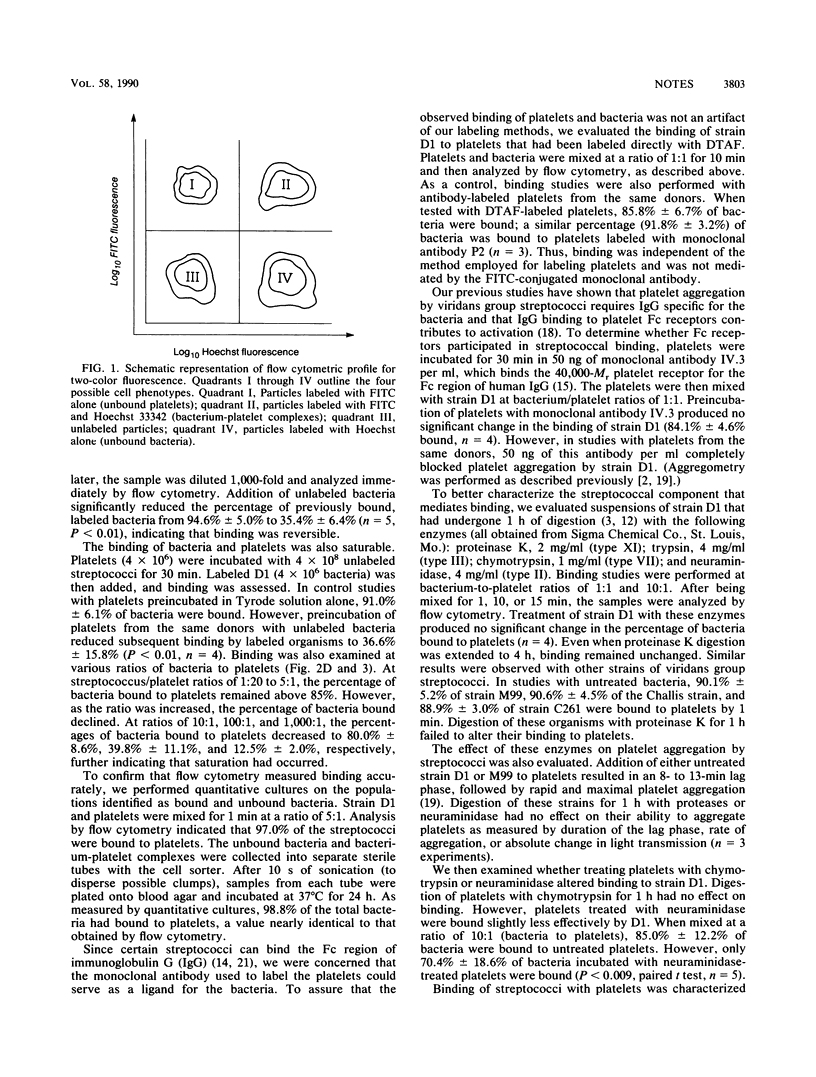
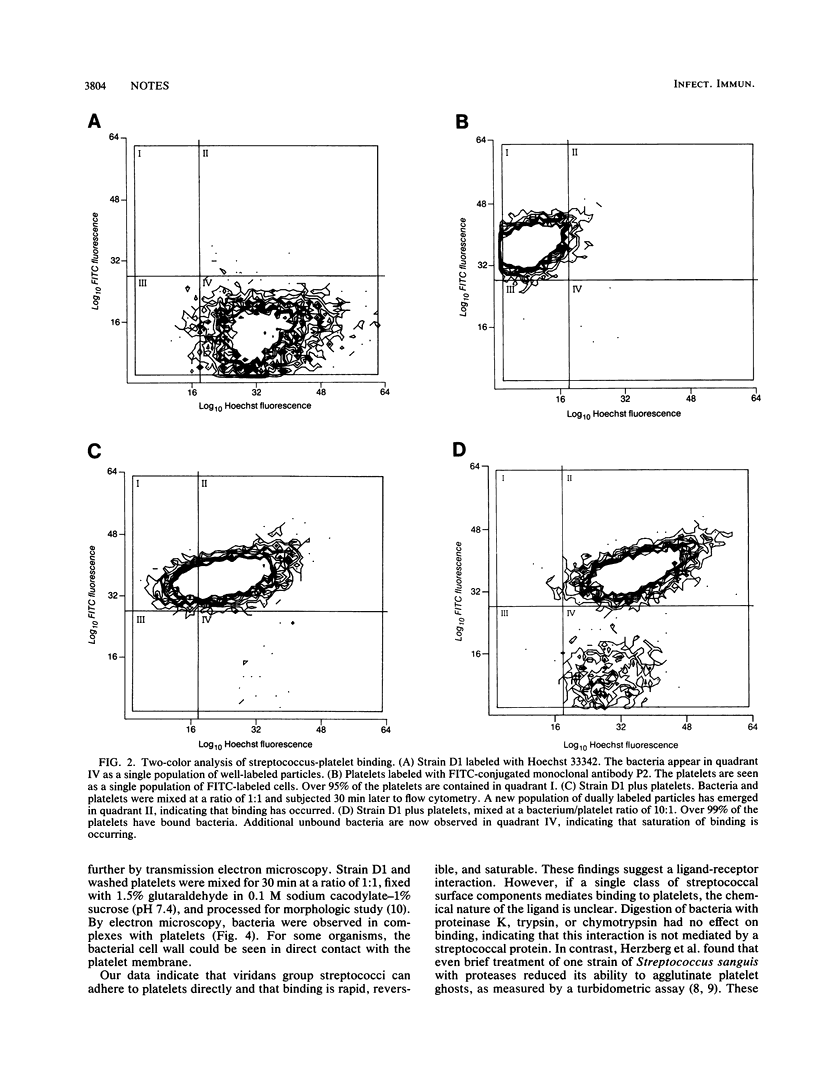
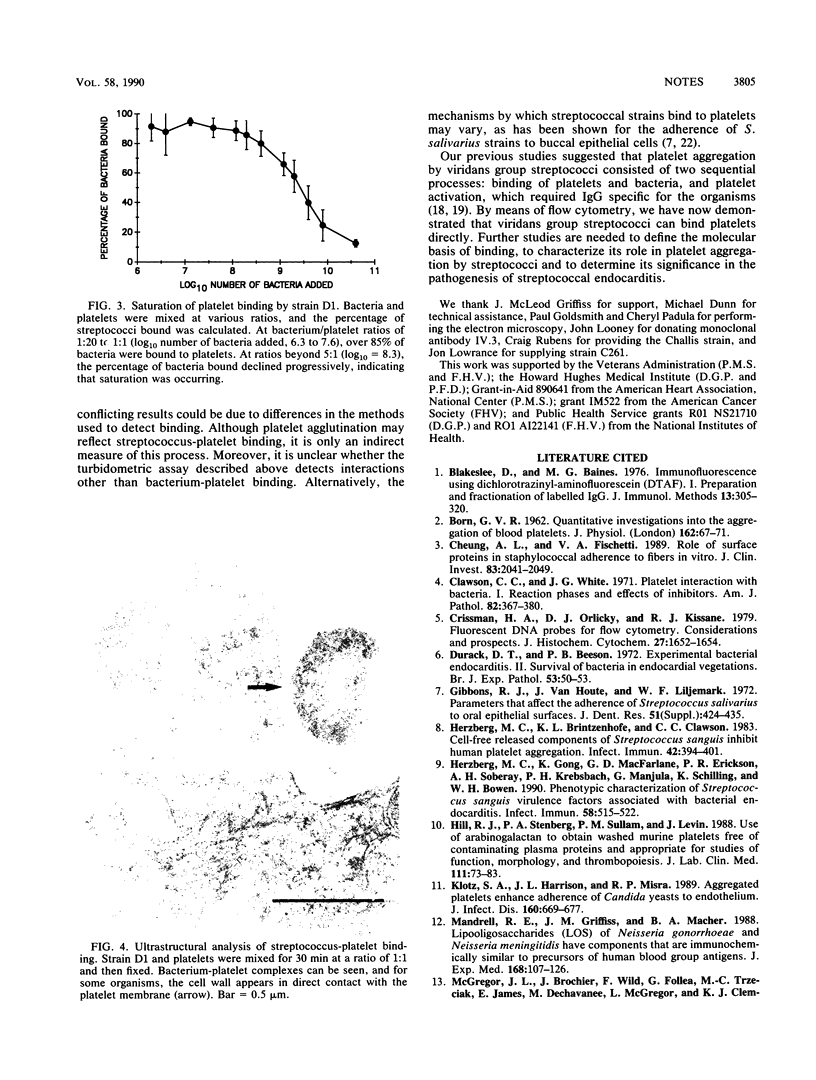

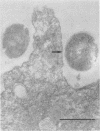
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakeslee D., Baines M. G. Immunofluorescence using dichlorotriazinylaminofluorescein (DTAF). I. Preparation and fractionation of labelled IgG. J Immunol Methods. 1976;13(3-4):305–320. doi: 10.1016/0022-1759(76)90078-8. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Fischetti V. A. Role of surface proteins in staphylococcal adherence to fibers in vitro. J Clin Invest. 1989 Jun;83(6):2041–2049. doi: 10.1172/JCI114115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Orlicky D. J., Kissane R. J. Fluorescent DNA probes for flow cytometry. Considerations and prospects. J Histochem Cytochem. 1979 Dec;27(12):1652–1654. doi: 10.1177/27.12.391999. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. II. Survival of a bacteria in endocardial vegetations. Br J Exp Pathol. 1972 Feb;53(1):50–53. [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Van Houte J., Liljemark W. F. Parameters that effect the adherence of Streptococcus salivarius to oral epithelial surfaces. J Dent Res. 1972 Mar-Apr;51(2):424–435. doi: 10.1177/00220345720510023101. [DOI] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Cell-free released components of Streptococcus sanguis inhibit human platelet aggregation. Infect Immun. 1983 Oct;42(1):394–401. doi: 10.1128/iai.42.1.394-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Gong K., MacFarlane G. D., Erickson P. R., Soberay A. H., Krebsbach P. H., Manjula G., Schilling K., Bowen W. H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990 Feb;58(2):515–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. J., Stenberg P. E., Sullam P. M., Levin J. Use of arabinogalactan to obtain washed murine platelets free of contaminating plasma proteins and appropriate for studies of function, morphology, and thrombopoiesis. J Lab Clin Med. 1988 Jan;111(1):73–83. [PubMed] [Google Scholar]
- Klotz S. A., Harrison J. L., Misra R. P. Aggregated platelets enhance adherence of Candida yeasts to endothelium. J Infect Dis. 1989 Oct;160(4):669–677. doi: 10.1093/infdis/160.4.669. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: description of three major types of receptors for human immunoglobulin G. Infect Immun. 1977 Sep;17(3):475–482. doi: 10.1128/iai.17.3.475-482.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld S. I., Looney R. J., Leddy J. P., Phipps D. C., Abraham G. N., Anderson C. L. Human platelet Fc receptor for immunoglobulin G. Identification as a 40,000-molecular-weight membrane protein shared by monocytes. J Clin Invest. 1985 Dec;76(6):2317–2322. doi: 10.1172/JCI112242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Drake T. A., Sande M. A. Pathogenesis of endocarditis. Am J Med. 1985 Jun 28;78(6B):110–115. doi: 10.1016/0002-9343(85)90373-0. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Jarvis G. A., Valone F. H. Role of immunoglobulin G in platelet aggregation by viridans group streptococci. Infect Immun. 1988 Nov;56(11):2907–2911. doi: 10.1128/iai.56.11.2907-2911.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Valone F. H., Mills J. Mechanisms of platelet aggregation by viridans group streptococci. Infect Immun. 1987 Aug;55(8):1743–1750. doi: 10.1128/iai.55.8.1743-1750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dilla M. A., Langlois R. G., Pinkel D., Yajko D., Hadley W. K. Bacterial characterization by flow cytometry. Science. 1983 May 6;220(4597):620–622. doi: 10.1126/science.6188215. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mering G. O., Boyle M. D. Comparison of type III Fc receptors associated with group C and group G streptococci. Mol Immunol. 1986 Aug;23(8):811–821. doi: 10.1016/0161-5890(86)90066-0. [DOI] [PubMed] [Google Scholar]