Abstract
Rationale
Gender plays a critical role in the effects of drugs and drug abuse liability. Biological factors, including ovarian hormones, may contribute to gender differences in drug abuse. Preclinical and some clinical research suggests that progesterone and its metabolites have activity at the GABAA receptor and may enhance the effect of GABAergic compounds (e.g., benzodiazepines). Because women are exposed to varying levels of progesterone from puberty until menopause, and appear more sensitive to the negative consequences of benzodiazepine use, it is important to understand the impact of progesterone on GABAergic drug effects.
Objectives
The purpose of this experiment was to characterize the behavioral effects of progesterone, alone and in combination with the short-acting benzodiazepine, triazolam, to determine if progesterone potentiates the behavioral effects of triazolam.
Methods
Oral micronized progesterone (0, 100, and 200 mg) and oral triazolam (0.00, 0.12, and 0.25 mg/70 kg) were administered to healthy, premenopausal women (n=11) under conditions of low circulating sex hormones. The subjective, performance and physiological effects of progesterone, alone and in combination with triazolam, were assessed.
Results
Triazolam alone produced prototypical sedative-like effects. Progesterone alone also engendered some sedative effects, although the time course of the effects was more limited than that of triazolam. Progesterone increased and extended the duration of triazolam effects and delayed the onset of triazolam peak effects, most notably at the 0.12 mg/70 kg dose.
Conclusions
Progesterone potentiates the behavioral effects of benzodiazepines and may contribute to benzodiazepine use and abuse among women.
Keywords: Progesterone, Prometrium, Neurosteroid, Benzodiazepine, Triazolam, Subjective effects, Performance effects, Women’s health
Introduction
Gender differences in drug use and abuse are notable, with greater numbers of men reporting non-medical use of drugs than women (Substance Abuse and Mental Health Services Administration 2009). However, the relative risk of developing dependence on sedative-hypnotic drugs (e.g., benzodiazepines) appears to be greater for women. After initiating sedative-hypnotic drug use, women are significantly more likely to develop problematic use patterns relative to their male counterparts, such that women may be nearly twice as likely to develop dependence on these compounds (Anthony et al. 1994; Kandel et al. 1998). In addition, women are prescribed sedative-hypnotic compounds at higher rates than men (Wysowski and Baum 1991; van der Waals et al. 1993; Howes et al. 1996; Nomura et al. 2006), take the medications longer once prescribed (Geiselmann and Linden 1991), and are more likely to use the medications for non-medical purposes (Simoni-Wastila et al. 2004).
Multiple sources of evidence suggest the neurosteroid progesterone and its metabolites might contribute to gender differences in sedative-hypnotic drug abuse. Progesterone is abundantly present in premenopausal women, with plasma concentrations varying as a function of menstrual cycle phase. Progesterone and its primary and derivative metabolites, allopregnanolone and tetrahydrodeoxycorticosterone (TH-DOC), modulate the GABAA receptor complex, which is also the primary mechanism of benzodiazepine effects (Lena et al. 1993; Pluchino et al. 2006). Allopregnanolone and TH-DOC are ligands at extracellular, steroid-specific recognition sites on GABAA receptors, with affinities that are comparable to those of many benzodiazepines (Paul and Purdy 1992). In addition, these neurosteroids dose-dependently increase the effects of benzodiazepines on GABA-induced Cl− currents, indicating that there is a non-genomic, receptor-level interaction between benzodiazepines and neurosteroids (Paul and Purdy 1992; Bertz et al. 1995). The behavioral effects of these neurosteroids also overlap with those engendered by benzodiazepines and include sedation, memory impairment, anxiolysis, depression, stress reduction, and anti-seizure effects (Schumacher et al. 1989; Schumacher and Robert 2002; Rupprecht 2003; Pisu and Serra 2004; Rhodes and Frye 2004). Finally, the behavioral effects of GABAergic drugs are enhanced during menstrual cycle phases in which progesterone levels are elevated. For example, the discriminative stimulus effects of ethanol in nonhuman primates (Grant et al. 1997; Green et al. 1999) and triazolam in humans (Babalonis et al. 2008) are enhanced during the mid-luteal phase of the menstrual cycle when progesterone levels surge. However, menstrual cycle modulation of GABAergic drug effects have not been replicated across all studies (Rukstalis and de Wit 1999; Holdstock and de Wit 2000) and may depend on the experimental conditions (e.g., behavioral measures, drugs, and/or doses that are tested).
Although examining drug effects across the menstrual cycle is a useful method to examine the modulating effects of endogenous ovarian hormones, it does not permit an examination of the direct effects of progesterone, as multiple hormone levels change simultaneously across the cycle. An alternative strategy is to examine the direct effects of exogenous progesterone administration. For example, pre-treatment with exogenous oral micronized progesterone enhanced the sedative, memory, and performance effects of intravenous triazolam in postmenopausal women (McAuley et al. 1995). In addition, combined administration of progesterone and estradiol enhanced sensitivity to the performance impairing effects of alprazolam, lorazepam, and triazolam (Kroboth et al. 1985). There do not appear to be any studies that have examined the independent effects of progesterone on the behavioral effects of sedative-hypnotic drugs in premenopausal women. The purpose of the present study was to determine the subjective and psychomotor effects of progesterone and triazolam, alone and in combination, in healthy adult premenopausal women during the early follicular phase of the menstrual cycle when ovarian hormone levels are at their nadir.
Participants
Healthy, adult premenopausal women were recruited via local newspaper advertisements and with flyer postings on a university campus. All potential participants completed an initial telephone or internet-based questionnaire, and selected respondents were invited for an on-site medical evaluation that included health history and psychological questionnaires, blood chemistry, and urinalysis. Urine samples were also screened for drugs of abuse and pregnancy. Eligibility criteria included age (18 to 35 years), ability to speak and read English, occasional sedative drug use (e.g., alcohol), and use of an oral, hormone-based contraceptive that included a 7 consecutive-day placebo phase. Exclusion criteria included significant medical history (e.g., cardiovascular, neurological, or major psychiatric illnesses, including drug or alcohol dependence), abuse or regular use of drugs or alcohol, pregnant or breastfeeding status, or any other condition that would increase risk for study participation. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document. The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki (2008). All subjects provided sober, written informed consent and the confidentiality of their personal information was maintained throughout. Participants were compensated for their participation.
Design
A double-blind, placebo-controlled, randomized, counterbalanced repeated-measures design was used to assess the subjective, performance and cardiovascular effects of progesterone dose (0, 100, and 200 mg), triazolam dose (0.00, 0.12, and 0.25 mg/70 kg), and time (30, 60, 90, and 120 min after triazolam administration, which correspond to 75, 105, 135, 165, and 195 after progesterone administration) during the early follicular phase of the menstrual cycle, when estrogen and progesterone levels are at their nadir. This study consisted of nine experimental sessions.
Drugs
Doses of progesterone and triazolam were prepared by the University of Kentucky Investigational Pharmacy. Progesterone (0, 100, and 200 mg; Prometrium®) and triazolam (0.00, 0.12, and 0.25 mg/70 kg) were prepared in single size 00 and 0 distinct opaque capsules, respectively, each with cornstarch filler. Placebo capsules contained only cornstarch filler.
The doses of progesterone (100 and 200 mg) were selected to produce systemic progesterone levels that are similar to those occurring during the mid-luteal phase of the menstrual cycle (Simon et al. 1993; Stanczyk 1999). The moderate dose of triazolam (0.12 mg/70 kg) was selected because it is minimally behaviorally active when administered alone, but is sensitive to hormonal variations (Babalonis et al. 2008). The high dose of triazolam (0.25 mg/70 kg) was selected because it is a standard therapeutic dose that has measurable behavioral effects in isolation.
Session schedule
Participants completed one training session to acquaint them with the study procedures and to establish consistent and accurate performance on computerized behavioral tasks. This training session was conducted irrespective of menstrual cycle phase but within 1 week of the initiation of the experimental sessions. The subsequent nine experimental sessions were conducted during the participant’s placebo phases of their oral birth control regimen (e.g., early follicular phase). Each permutation of progesterone and triazolam doses was tested once in random order. One to six experimental sessions occurred during each participant’s placebo phase, with the study spanning across 2–4 months’ time (mean study completion time was 3 months). Each session occurred at approximately the same time of day for each individual participant, although start times varied across participants (e.g., ranged from 7:30 AM to 4:00 PM).
Daily schedule
Participants were instructed to abstain from alcohol and all medications for 24 h and caffeine and food for 4 h prior to their scheduled sessions. At the beginning of each session, participants were asked questions about medication use, birth control regimen and onset of menses, sleep, food consumption, and health status for the preceding 24 h. No sessions were cancelled due to reports of atypical activities. Participants then completed a field sobriety test, provided a breath sample that was tested for alcohol use (Alco-Sensor III; Intoximeters, Inc., St. Louis, MO, USA), and provided a urine sample that was tested for recent use of amphetamine, barbiturates, benzodiazepines, cocaine, marijuana, methadone, methamphetamine, MDMA, and opiates (E–Z Split Key Cup; ACON Laboratories, San Diego, CA, USA), and pregnancy (hCG One Step Pregnancy Test Device; Instant Technologies, Inc., Norfolk, VA, USA). After a baseline assessment, progesterone was administered. Thirty minutes after progesterone administration, participants consumed a moderate-fat snack to enhance progesterone absorption (Simon et al. 1993; Stanczyk 1999). Triazolam was administered 15 min after snack delivery. Assessments were repeated in 30-min intervals for 3 h after triazolam administration. Each assessment consisted of Visual Analog (VAS) and Adjective Rating Scales (ARS), Addiction Research Center Inventory (ARCI), Profile of Mood States (POMS), Digit Symbol Substitution Task (DSST), Balloon Analog Risk Task (BART), and heart rate and blood pressure measurement (Sentry II; NBS Medical, Costa Mesa, CA, USA). After all assessments were complete, participants remained at the laboratory for a minimum of 2 h of rest to allow drug effects to dissipate. Prior to leaving the laboratory, participants completed the field sobriety test in the same manner as their baseline test and reported no remaining drug effects.
Assessment tasks
VAS
Participants rated 32 items presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely”. Items included were: Stimulated, Stressed, Sedated, Anxious, Nervous, Light-headed, Sleepy, Sick to Stomach, Thirsty, Hungry, Shaky, Jittery, Restless, Depressed, Down, High, Euphoric, Active, Alert, Talkative, Friendly, Energetic, Racing/Irregular Heart Rate, Rush, Drug Effect, Good Drug Effect, Bad Drug Effect, Performance Impaired, Performance Improved, Like Drug Effect, Willing to Take Drug Again, and Pay for Drug.
ARCI
The 49-item short form of the true–false inventory (Martin et al. 1971) yielded information on five dimensions: Lysergic Acid Diethylamide (LSD) Scale (a measure of dysphoria and psychomimetic effects), Amphetamine (A) Scale (an amphetamine-sensitive stimulation scale), Benzedrine Group (BG) Scale (a measure of stimulant effects), Morphine-Benzedrine Group (MBG) Scale (a measure of euphoric effects), and the Pentobarbital, Chlorapromazine, Alcohol Group (PCAG) Scale (a measure of sedative effects).
ARS
The Adjective Rating Scale consists of 32 items and contains two subscales: Sedative and Stimulant (Oliveto et al. 1992). In the present study, only the 16 items from the Sedative subscale were presented. Participants rated each item using a numeric keypad to select one of five options: “Not at All”, “A Little Bit”, “Moderately”, “Quite A Bit”, and “Extremely” (scored numerically from 0 to 4, respectively; maximum score=64).
DSST
Participants completed a 2-min computerized version of the DSST adopted from McLeod et al. 1982. Performance on this task is consistently sensitive to both stimulant and sedative effects of drugs (Higgins et al. 1993; Foltin et al. 1993; Greenblatt et al. 2005). Subjects earned $0.02 for each correct trial completed (mean correct trials per assessment=75; mean task earnings per session=$9.00). The dependent measures for this psychomotor task were trial completion rate and accuracy.
BART
During each assessment, participants were presented with 20 individual balloons in succession on a computer screen, as adapted from Lejuez et al. (2002). Participants clicked a mouse to inflate each balloon. Each inflation increased the balloon earnings counter by $0.01 and increased the probability of the next inflation to pop the balloon. Each balloon would pop after a random, unpredictable number of inflations. Participants could collect their balloon earnings prior to a balloon popping by clicking on a “Collect Money” option. However, if a balloon popped, earnings from the balloon were permanently lost. Earnings from each of the six assessments per session were recorded, and participants received earnings from one of the six assessments, based on a random drawing (mean=$5.78). The dependent measures were number of inflation responses per un-popped balloon and number of balloons popped.
Data analysis
Due to the quantity of individual self-report measures, data reduction using a Principal Component Analysis (PCA) was conducted in order to reduce the number of dependent measures. The PCA identified several principal components in the data via extraction with Promax rotation and Kaiser normalization. Each component used in the final analysis was required to have an Eigenvalue greater than 2. Variables that comprised each component, along with their loading scores, are presented in Table 1. Component standard scores were analyzed using repeated measures ANOVA with progesterone, triazolam, and time as factors. Results of this analysis are presented in Table 2. Further analyses were conducted on individual measures that demonstrated the time course of the component’s main effect of progesterone or a progesterone×triazolam interaction. These representative measures, along with cardiovascular and task performance measures, were analyzed using ANCOVA, with progesterone, triazolam, and time as repeated factors, and baseline performance (i.e., performance prior to drug administration) as a covariate. Significant interactions were analyzed as simple effects. Because baseline measures were used as covariates, drug effects were identified either as main effects of drug or drug×time interactions, depending on the time course of the interaction.
Table 1.
Measures and measure loading scores for each principal component
| Positive effects (Eigenvalue 2.20) | |
| VAS Like Drug Effect | 0.85 |
| VAS Good Drug Effect | 0.58 |
| VAS Perform. Improved | 0.65 |
| VAS Pay for Drug | 0.62 |
| Sedative/negative effects (Eigenvalue 2.71) | |
| VAS Sedated | 0.50 |
| VAS Impaired | 0.86 |
| VAS Drug Effects | 0.80 |
| VAS Bad Effects | 0.71 |
| VAS Rush | 0.61 |
| ARCI LSD | 0.58 |
| Sedative/fatigue effects (Eigenvalue 4.48) | |
| ARS Sedation | 0.85 |
| POMS Fatigue | 0.83 |
| VAS Sleepy | 0.78 |
| POMS Arousal | −0.63 |
| Stimulant effects (Eigenvalue 7.82) | |
| POMS Vigor | 0.81 |
| POMS Friendly | 0.90 |
| POMS Elation | 0.70 |
| POMS Total Positive | 0.67 |
| VAS Active | 0.94 |
| VAS Alert | 0.92 |
| VAS Talkative | 0.93 |
| VAS Energetic | 0.86 |
| VAS Friendly | 0.89 |
| Euphoric/dysphoric effects (Eigenvalue 11.86) | |
| VAS Nervous | 0.83 |
| VAS Shaky | 0.85 |
| VAS Jittery | 0.81 |
| VAS High | 0.80 |
| VAS Racing Heart Rate | 0.83 |
| VAS Depression | 0.79 |
| VAS Euphoric | 0.72 |
| VAS Down | 0.64 |
| VAS Stressed | 0.62 |
| VAS Light-headed | 0.71 |
| VAS Sick | 0.63 |
| POMS Confusion | 0.41 |
Table 2.
F values of components and selected measures for which a significant main effect or interaction was obtained (p<0.05)
| Triaz | Time | Tz×Time | Prog | P×Time | Triaz×Prog | Tz×P×Time | |
|---|---|---|---|---|---|---|---|
| Self-report measures | |||||||
| Positive effects | 4.96 | 6.74 | 7.11 | ||||
| Sedative/negative effects | 20.69 | 16.27 | 2.42 | 16.95 | 2.72 | ||
| Sedative/fatigue effects | 5.25 | 4.84 | |||||
| Stimulant effects | 8.25 | 13.24 | 9.94 | ||||
| Euphoric/dysphoric effects | 8.22 | 2.91 | |||||
| Task performance measures | |||||||
| DSST | |||||||
| Total trials | 20.84 | 4.07 | 8.25 | 2.60 | |||
| Prop correct | 4.25 | 3.60 | |||||
| BART | |||||||
| Popped | 3.01 | ||||||
| # Responses | 6.07 | 8.55 | 4.58 | ||||
| Cardiovascular measures | |||||||
| HR | 4.86 | 3.83 | 2.70 | ||||
| Systolic | 10.78 | 3.26 | 1.72 | ||||
| Diastolic | 3.15 | 1.98 | 3.27 | ||||
Results
Participants
Sixteen participants were enrolled in the study. Five participants did not complete the study for reasons unrelated to the protocol, and their data are not included in the analyses. Eleven women (10 Caucasian, one African American) completed the study. The participants ranged in age from 21 to 26 years (median=22 years) and in weight from 51 to 72.4 kg (median=63 kg) (BMI range=19.2 to 26.3, median=22.2). All participants were non-smokers. Alcohol use ranged from less than 1 to 7.5 alcohol drinks per week (median=3 drinks) and caffeine use ranged from 0 to 185 mg of caffeine per day (median=80 mg). One participant reported nonmedical use of Adderall® on two occasions in the month prior to study participation. Participants reported no additional psychoactive drug use in the month prior to study participation, and no drug use was detected during the study with daily urinalysis testing.
Self-report measures
The PCA identified five components (Table 1). Results of the ANOVA with these components are presented in Table 2. One component included measures of positive drug effects (Table 1). Main effects of triazolam and time as well as a progesterone×triazolam interaction were detected on this component. Follow-up testing indicated that the high dose of triazolam increased ratings relative to placebo and the low dose of triazolam (p<0.05). Simple-effects testing of the progesterone×triazolam interaction indicated that 100 mg progesterone increased ratings at placebo and the moderate dose of triazolam, while 200 mg progesterone increased ratings at the high dose of triazolam (p<0.05).
Figure 1 presents VAS Good Drug Effect as a representative measure from this component. A main effect of progesterone was observed on this measure (panel a) with peak effects of progesterone alone occurring at 30 through 90 min (i.e., 75 through 135 min post-progesterone administration). A main effect of triazolam was detected, with the high dose of triazolam increasing subjective ratings 90 through 150 min after dose administration (open squares across panels). A significant interaction between progesterone and triazolam was also detected on this measure, with simple-effects analyses indicating increases when 200 mg was combined with the high dose of triazolam (circles, panel c) (p<0.05). In addition, increases in ratings occurred when 100 mg progesterone was combined with the moderate dose of triazolam (triangles, panel b). Overall, the high dose of progesterone in combination with the high dose of triazolam increased the duration of effect and both delayed and enhanced the peak ratings of VAS Good Drug Effect (circles, panel c) relative to the same dose of progesterone (circles, panel a) or triazolam alone (squares, panel c).
Fig. 1.
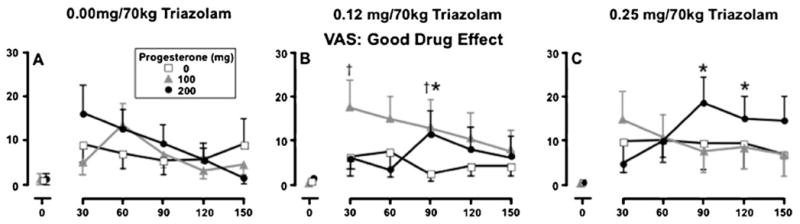
Mean ratings of VAS Good Drug Effect as a function of triazolam dose, progesterone dose and time following triazolam administration. Data points represent means (±SEM denoted by the error bars) of 11 participants. The dagger indicates the mean of the 100 mg progesterone condition is significantly different from the corresponding placebo condition (p<0.05); black asterisks indicate the mean of the 200 mg progesterone condition is significantly different from corresponding placebo condition (p<0.05)
A second PCA component included negative measures of sedative drug effects (Table 1). Main effects of progesterone, triazolam and time, and interaction effects of triazolam by time and progesterone by triazolam were identified (Table 2). Follow-up testing indicated both active doses of triazolam alone and both active doses of progesterone alone increased ratings relative to placebo (p<0.05). Simple-effects analysis of the progesterone× triazolam interaction indicated that both active doses of progesterone increased ratings at the moderate dose of triazolam and the 200 mg dose of progesterone increased ratings at the high dose of triazolam (p<0.05).
Figure 2 presents VAS Drug Effect (top row) and Bad Drug Effect (bottom row) ratings as representative measures of this component. Progesterone increased ratings on both measures (p<0.05), with peak effects occurring at 30 through 90 min (i.e., 75 through 135 min after progesterone administration, panels a and d). Triazo-lam also increased subjective ratings on both measures, as evidenced by main effects of triazolam and triazolam× time interactions (p<0.05). The 0.25 mg/70 kg dose increased ratings 90 through 150 min post-dose (open squares across panels). A significant interaction between progesterone and triazolam was also detected on each measure. Simple-effects analyses indicated that increases occurred when active doses of progesterone were combined with the moderate dose of triazolam (circles and triangles, panel b, panel e) and when 200 mg of progesterone was combined with high dose of triazolam (circles, panel c, panel f) (p<0.05). Compared to progesterone alone (panel a, panel d), the combination of progesterone and triazolam increased the duration of effect and both delayed and enhanced the peak effects relative to either drug alone (panels b, c, e, and f).
Fig. 2.
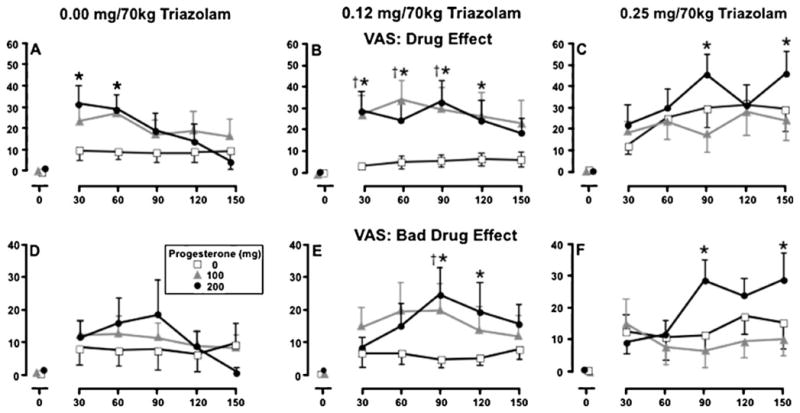
Mean ratings of VAS Drug Effect (top row) and VAS Bad Drug Effect (bottom row) as a function of triazolam dose, progesterone dose, and time following triazolam administration. Data points represent means (±SEM denoted by the error bars) of 11 participants. Different y-axis scales are used across the two measures. The daggers indicate the mean of the 100 mg progesterone condition is significantly different from the corresponding placebo condition (p< 0.05); black asterisks indicate the mean of the 200 mg progesterone condition is significantly different from corresponding placebo condition (p<0.05)
The third PCA component included measures of sedation and fatigue (Table 1). Main effects of progesterone and time were identified (Table 2). Follow-up analyses indicated that both active doses of progesterone increased ratings relative to placebo progesterone (p<0.05).
The fourth PCA component consisted of stimulant-type measures (Table 1). Main effects of progesterone, triazolam, and time were identified (Table 2). Follow-up testing indicated that both active doses of progesterone and both active doses of triazolam decreased ratings (p<0.05). No triazolam×progesterone interactions were observed.
The fifth PCA component included both euphoric and dysphoric effects measures (Table 1). A main effect of triazolam and a progesterone×triazolam interaction were identified (Table 2). Follow-up testing indicated that the high dose of triazolam increased ratings relative to placebo (p<0.05), and the 100 mg progesterone increased ratings at the high dose of triazolam (p<0.05).
Task performance measures
Separate and combined effects of progesterone and triazolam were also observed on measures of psychomotor task performance (Table 2). Figure 3 presents drug effects on DSST trial completions. Triazolam and triazolam×time interaction effects were observed (p<0.05), with decreases in performance occurring at highest dose of triazolam (0.25 mg/70 kg) 90 through 120 min after dose administration (panel c, p<0.05). A main effect of progesterone was also detected (Table 2); however, minimal effects of progesterone were observed in combination with placebo triazolam (panel a). A significant interaction between progesterone and triazolam was also detected, with simple effects analyses indicating that 200 mg progesterone combined with 0.12 mg/70 kg triazolam significantly decreased trial completion (circles, panel b) (p<0.05). Similar to other measures, progesterone increased the duration of effect and both delayed and enhanced triazolam peak effects.
Fig. 3.
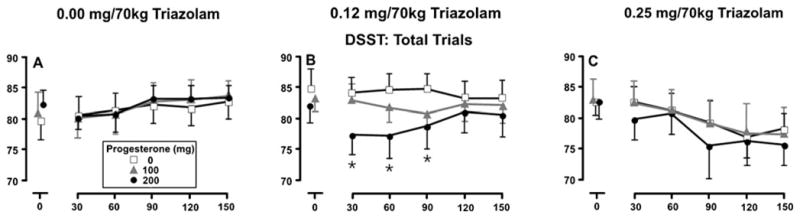
Mean number of trials completed on DSST as a function of triazolam dose, progesterone dose, and time following triazolam administration. Data points represent means (±SEM denoted by the error bars) of 11 participants. Asterisks indicate the mean of the 200 mg progesterone condition is significantly different from the corresponding placebo condition (p<0.05)
Significant main effects of both progesterone and triazolam, as well as a significant interaction between progesterone and triazolam, were observed on number of responses per un-popped balloon on the BART (Table 2). Follow-up testing of the progesterone×triazolam interaction indicated an effect of progesterone at each dose of triazolam and an effect of triazolam only at the 100 mg dose of progesterone (p<0.05). During baseline sessions, variable numbers of responses per balloon were observed, with small magnitude increases in the number of responses per balloon occurring across time following placebo progesterone administration and a small magnitude decrease following either 100 or 200 mg progesterone. This effect was particularly evident at the 0.12 mg/70 kg dose of triazolam. No differences in number of responses per balloon were observed at any assessment, indicating that progesterone and triazolam effects are related to variability in performance as opposed to any reliable pharmacological effects of either compound.
Cardiovascular measures
Separate and combined effects of progesterone and triazolam were also observed on cardiovascular measures (Table 2). Follow-up analyses indicated small magnitude increases in each cardiovascular measure across time, but no systematic changes due to administration of either drug were observed at any assessment. These trends indicate variability at baseline measurements as well as across assessments, as opposed to systematic pharmacological modulation of cardiovascular measures.
Discussion
The present study examined the subjective, performance and cardiovascular effects of exogenous progesterone, alone and in combination with triazolam, in healthy, premenopausal women. The results of this study demonstrated that the effects of both progesterone and triazolam were potentiated when given in combination. In addition, the drug combination extended the duration and delayed the onset of the peak effects of triazolam.
Sedative-like effects of oral progesterone are typically limited to higher oral doses than were used in this study, with peak effects occurring between 2 and 2.5 h after drug administration (Chakmakjian and Zachariah 1987; de Wit et al. 2001; Sofuoglu et al. 2001, 2002). In the present study, 100 and 200 mg oral doses of progesterone engendered significant sedative-like subjective and performance effects, with peak effects occurring between 1.25 and 1.75 h after dose administration (30 and 60 min post-triazolam administration). The most likely factor contributing to the more rapid onset and increased magnitude of sedative-like effects in the present study was the administration of a moderate-fat snack 30 min after progesterone administration, as the administration of a moderate-fat meal has been reported to increase progesterone bioavailability (Simon et al. 1993; Stanczyk 1999). However, other factors, such as participant characteristics (i.e., participants in this study were light drinkers, had limited or no previous benzodiazepine exposure, and were taking oral birth control), could also have contributed to the enhanced pharmacodynamic effects of progesterone observed in the present study.
Triazolam, when administered alone, engendered prototypical sedative-like subjective and performance effects, most consistently at the highest dose (0.25 mg/70 kg), which included increases in sedative, both euphoric and dysphoric, and positive drug effects and decreases in stimulant drugs effects, as well as psychomotor impairment. Peak behavioral effects of 0.25 mg/70 kg triazolam occurred between 1.5 and 2.5 h post-dose, which is consistent with previous reports (e.g., Simpson and Rush 2002; Rush et al. 2003).
The combined effect of progesterone and triazolam enhanced subjective and performance impairment effects, compared to those of triazolam or progesterone in isolation. The combination of progesterone and triazolam also extended the duration of effects and both delayed and enhanced peak effects. In isolation, progesterone effects occurred 75 to 105 min after progesterone administration. However, when progesterone was combined with either active dose of triazolam, the peak effects were enhanced and shifted to time points in the latter portion of the session (135 through 195 min after progesterone administration, or 90 through 150 min after triazolam administration). In addition, peak effects of progesterone in combination with the 0.12 and 0.25 mg/70 kg dose of triazolam occurred at times in which progesterone, alone, had minimal subjective or performance effects.
The enhanced magnitude of triazolam effects occurred at times in which progesterone, alone, had minimal effects. As such, the change in time course of triazolam effects may be associated with circulating levels of the neuroactive progesterone metabolites allopregnanolone and tetrahydro-deoxycorticosterone (TH-DOC), rather than progesterone, per se. These progesterone metabolites are GABAA ligands and can produce behavioral effects similar to alcohol and benzodiazepines (Lambert et al. 1995, 2009; Grant et al. 2008). After oral progesterone administration, peak progesterone and allopregnanolone levels occur at 2 h, with levels remaining elevated above baseline for a total of 5 and 8 h, respectively (Nahoul et al. 1993; Andreen et al. 2006). Although TH-DOC levels rise after progesterone administration, the clinical time course of this hormone derivative has not been established. In the absence of blood levels of progesterone and its metabolites, the relative impact of each neurosteroid in the present study is not clear. However, that the enhanced effect of the combination of progesterone and triazolam occurred during times when progesterone alone engendered minimal subjective or performance effects suggests that progesterone metabolites could be responsible, which merits further investigation.
Although activity at the GABAA receptor complex is the most well-characterized effect of progesterone and its metabolites, other neurotransmitter systems may be involved when progesterone is combined with triazolam. Progesterone and its metabolites may also engender effects via modulation of other neurotransmitter systems, including dopamine, serotonin, opioid, glutamate, and nicotinic acetylcholine (Lena et al. 1993; Pluchino et al. 2006). Further research is necessary to determine the specificity of progesterone modulation of benzodiazepines.
The reinforcing effects of triazolam and other benzodiazepines appear to vary in the general population based on individual difference variables, such as prior sedative use. Progesterone and triazolam yielded similar effects on the positive drug effect component, and ratings were enhanced when the drugs were tested in combination. However, both drugs also increased ratings on the negative measures of sedative drug effect component and the euphoric and dysphoric drug effect component. Many drugs of abuse engender simultaneous positive and negative drug effects, and it has been proposed that the balance between these opposing effects may determine how readily a drug is self-administered (Foltin and Fischman 1991). This profile of results suggests that progesterone may enhance the reinforcing effects of triazolam, but additional research incorporating measures of drug-taking behavior is necessary to more directly determine if the abuse liability of triazolam or other benzodiazepines is modulated by progesterone.
Several limitations to the present study are noted. First, blood levels of triazolam, progesterone, and progesterone metabolites were not obtained during the study. Information on triazolam, progesterone, progesterone metabolites, and other ovarian hormone levels would have allowed further insight into the interactions observed between progesterone and triazolam. Second, both active doses of progesterone engendered some sedative-like effects in isolation and only the highest dose of triazolam produced statistically significant effects compared to placebo; moreover, the interaction between progesterone and triazolam was not always dependent on triazolam dose. Future studies should examine progesterone and benzodiazepine interactions with a wider range of doses that include progesterone doses that engender minimal measureable effects when administered in isolation. A third limitation of this study was the unexpected discordance of the onset of peak effects of progesterone and triazolam, with peak progesterone effects occurring approximately 1 h prior to the peak effects of triazolam. Although the study was designed to examine concomitant peak effects, the separate peak of effects of each compound allowed for the examination of the separate and combined effects of progesterone and triazolam and allowed for interesting interactions to emerge. Fourth, experimental sessions were conducted only during the 7-day placebo phase of participants’ oral contraceptive regimen. While this design allowed for sessions to occur when progesterone and estradiol were at nadir levels, it was not possible to complete the study during one 7-day placebo phase, and sessions were conducted across two to four consecutive months (two to four phases of oral birth control medication). Fifth, although each participant’s sessions were conducted during the same time of day across the study, the starting time of sessions varied across participants, which may have introduced variability in the drug effects. The sample size in the current study was also limited. Although within-subjects design allows for detection of drug effects with relatively few participants and significant effects of both progesterone and triazolam were observed in this study, a larger sample size may have increased the ability to detect subtle drug interactions and/or individual differences in the sensitivity to progesterone and/or triazolam. Another limitation is this study did not include male participants. Although the sedative-like effects of progesterone have been reported in men (Soderpalm et al. 2004; Evans and Foltin 2006), it is unclear if similar interactions between progesterone and benzodiazepines would occur. Lastly, the acute dosing regimen used in this study does not specifically model the hormone exposure that occurs during the mid-luteal phase. During the mid-luteal phase, levels of progesterone and its metabolites are elevated for several days, which causes changes in GABAA receptor sensitivity (Smith et al. 1998; Grobin and Morrow 2000; Rupprecht 2003). Future studies should model this duration of hormone exposure with repeated exogenous hormone administration.
These results, along with a growing body of literature, support the hypothesis that progesterone modulates the behavioral and performance effects of GABAergic drugs (e.g., Kroboth et al. 1985; McAuley et al. 1995; Grant et al. 1997; Green et al. 1999). In addition, these data suggest that progesterone may be one factor among many that contributes to the gender differences observed in benzodiazepine misuse, whereby women are at an elevated risk of developing dependence on benzodiazepine medications compared to men. Future laboratory studies should include examining the role of estrogen, progesterone, and their combination on the effects of benzodiazepines, as well as the role of acute versus chronic progesterone administration. An additional valuable avenue of research would be to more directly assess the influence of neurosteroids on the reinforcing effects of sedative-hypnotic drugs using self-administration procedures. These studies would further inform the role of neurosteroids in gender differences in benzodiazepine use and abuse.
Acknowledgments
The authors wish to thank Stephanie LaBedz, Stephanie Douglas, Dustin Lee, Laura Mudd, Caroline Kimathi, Cleeve Emurian, Glenn Robbins, Phoebe Brown, and Beth Eaves for expert technical assistance. We also thank Steven Sitzlar of the University of Kentucky Investigational Drug Service.
This research and the preparation of this manuscript were supported by grants from the National Institute on Drug Abuse (R36 DA024127, T32 DA007304) and the Center for Biomedical Research Excellence (P20 RR015592). The authors do not have any financial relationship with these funding sources and have no conflict of interest to report.
Contributor Information
Shanna Babalonis, Email: babalonis@uky.edu, Department of Behavioral Science, College of Medicine, University of Kentucky Medical Center, Lexington, KY 40536-0086, USA, Department of Psychology, University of Kentucky College of Arts and Sciences, Lexington, KY, USA.
Joshua A. Lile, Department of Behavioral Science, College of Medicine, University of Kentucky Medical Center, Lexington, KY 40536-0086, USA
Catherine A. Martin, Department of Psychiatry, University of Kentucky Medical Center, Lexington, KY, USA
Thomas H. Kelly, Department of Behavioral Science, College of Medicine, University of Kentucky Medical Center, Lexington, KY 40536-0086, USA, Department of Psychiatry, University of Kentucky Medical Center, Lexington, KY, USA, Department of Psychology, University of Kentucky College of Arts and Sciences, Lexington, KY, USA
References
- Andreen L, Spigset O, Andersson A, Nyberg S, Backstrom T. Pharmacokinetics of progesterone and its metabolites allopreg-nanolone and pregnanolone after oral administration of low-dose progesterone. Maturitas. 2006;54(3):238–244. doi: 10.1016/j.maturitas.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2(3):244–268. [Google Scholar]
- Babalonis S, Emurian CS, Martin CA, Lile JA, Kelly TH. Modulation of the discriminative stimulus effects of triazolam across the menstrual cycle phase in healthy pre-menopausal women. Drug Alcohol Depend. 2008;94(1–3):276–280. doi: 10.1016/j.drugalcdep.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz RJ, Reynolds IJ, Kroboth PD. Effect of neuroactive steroids on [3h]flumazenil binding to the GABAA receptor complex in vitro. Neuropharmacology. 1995;34(9):1169–1175. doi: 10.1016/0028-3908(95)00072-e. [DOI] [PubMed] [Google Scholar]
- Chakmakjian ZH, Zachariah NY. Bioavailability of progester-one with different modes of administration. J Reprod Med. 1987;32 (6):443–448. [PubMed] [Google Scholar]
- de Wit H, Schmitt L, Purdy R, Hauger R. Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women. Psychoneuroendocrinology. 2001;26 (7):697–710. doi: 10.1016/s0306-4530(01)00024-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: a methodological survey. Drug Alcohol Depend. 1991;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- Geiselmann B, Linden M. Prescription and intake patterns in long-term and ultra-long-term benzodiazepine treatment in primary care practice. Pharmacopsychiatry. 1991;24(2):55–61. doi: 10.1055/s-2007-1014439. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3 alpha-hydroxy-5 alpha-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology (Berl) 1997;130 (1):59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Helms CM, Rogers LS, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008;326(1):354–361. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KL, Azarov AV, Szeliga KT, Purdy RH, Grant KA. The influence of menstrual cycle phase on sensitivity to ethanol-like discriminative stimulus effects of GABAA-positive modulators. Pharmacol Biochem Behav. 1999;64(2):379–383. doi: 10.1016/s0091-3057(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Gan L, Harmatz JS, Shader RI. Pharmocokinetics and pharmacodynamics of single-dose triazolam: electroencephalography compared with the digit–symbol substitution test. Br J Clin Pharmacol. 2005;60(3):244–248. doi: 10.1111/j.1365-2125.2005.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Morrow AL. 3alpha-hydroxy-5alpha-pregnan-20-one exposure reduces GABA(a) receptor alpha4 subunit mRNA levels. Eur J Pharmacol. 2000;409(2):R1–R2. doi: 10.1016/s0014-2999(00)00797-4. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Rush CR, Bickel WK, Hughes JR, Lynn M, Capeless MA. Acute behavioral and cardiac effects of cocaine and alcohol combinations in humans. Psychopharmacology (Berl) 1993;111(3):285–294. doi: 10.1007/BF02244943. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology (Berl) 2000;150(4):374–382. doi: 10.1007/s002130000461. [DOI] [PubMed] [Google Scholar]
- Howes JB, Ryan J, Fairbrother G, O’Neill K, Howes LG. Benzodiazepine prescribing in a Sydney teaching hospital. Med J Aust. 1996;165(6):305–308. doi: 10.5694/j.1326-5377.1996.tb124985.x. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Warner LA, Kessler RC. The epidemiology of substance use and dependence among women. In: Wetherington CL, Roman AB, editors. Drug addiction research and the health of women. Diane; Darby: 1998. pp. 105–130. [Google Scholar]
- Kroboth PD, Smith RB, Stoehr GP, Juhl RP. Pharmacodynamic evaluation of the benzodiazepine–oral contraceptive interaction. Clin Pharmacol Ther. 1985;38(5):525–532. doi: 10.1038/clpt.1985.218. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Cooper MA, Simmons RDJ, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34S:48–58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16(9):295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidence for preterminal nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13(6):2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the balloon analogue risk task (BART) J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McAuley JW, Reynolds IJ, Kroboth FJ, Smith RB, Kroboth PD. Orally administered progesterone enhances sensitivity to triazolam in postmenopausal women. J Clin Psychopharmacol. 1995;15(1):3–11. doi: 10.1097/00004714-199502000-00002. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the digit symbol substitution test (DSST) Behav Res Meth Instrum. 1982;14:463–466. [Google Scholar]
- Nahoul K, Dehennin L, Jondet M, Roger M. Profiles of plasma estrogens, progesterone and their metabolites after oral or vaginal administration of estradiol or progesterone. Maturitas. 1993;16(3):185–202. doi: 10.1016/0378-5122(93)90064-o. [DOI] [PubMed] [Google Scholar]
- Nomura K, Nakao M, Sato M, Yano E. Regular prescriptions for benzodiazepines: a cross-sectional study of outpatients at a university hospital. Intern Med. 2006;45(22):1279–1283. doi: 10.2169/internalmedicine.45.1758. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Higgins ST, Fenwick JW. Triazolam as a discriminative stimulus in humans. Drug Alcohol Depend. 1992;30(2):133–142. doi: 10.1016/0376-8716(92)90018-8. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB. 1992;6(6):2311–2322. [PubMed] [Google Scholar]
- Pluchino N, Luisi M, Lenzi E, Centofanti M, Begliuomini S, Freschi L, Ninni F, Genazzani AR. Progesterone and progestins: effects on brain, allopregnanolone and beta-endorphin. J Steroid Biochem Mol Biol. 2006;102(1–5):205–213. doi: 10.1016/j.jsbmb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Pisu MG, Serra M. Neurosteroids and neuroactive drugs in mental disorders. Life Sci. 2004;74(26):3181–3197. doi: 10.1016/j.lfs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Progestins in the hippocampus of female rats have antiseizure effects in a pentylenetetrazole seizure model. Epilepsia. 2004;45(12):1531–1538. doi: 10.1111/j.0013-9580.2004.16504.x. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, de Wit H. Effects of triazolam at three phases of the menstrual cycle. J Clin Psychopharmacol. 1999;19(5):450–458. doi: 10.1097/00004714-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28(2):139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Fillmore MT, Hays LR. Discriminative-stimulus effects of triazolam in light and moderate drinkers. Alcohol Clin Exp Res. 2003;27(4):638–646. doi: 10.1097/01.ALC.0000062742.29158.6E. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, McEwen BS. Regulation of high- affinity GABAA receptors in specific brain regions by ovarian hormones. Neuroendocrinology. 1989;50(3):315–320. doi: 10.1159/000125239. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Robert F. Progesterone: synthesis, metabolism, mechanisms of action, and effects in the nervous system. In: Pfaff DW, editor. Hormones, brain and behavior. Academic; San Diego: 2002. pp. 683–745. [Google Scholar]
- Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertil Steril. 1993;60 (1):26–33. [PubMed] [Google Scholar]
- Simoni-Wastila L, Ross-Degnan D, Mah C, Gao X, Brown J, Cosler LE, Fanning T, Gallagher P, Salzman C, Soumerai SB. A retrospective data analysis of the impact of the New York triplicate prescription program on benzodiazepine use in Medic-aid patients with chronic psychiatric and neurologic disorders. Clin Ther. 2004;26(2):322–336. doi: 10.1016/s0149-2918(04)90030-6. [DOI] [PubMed] [Google Scholar]
- Simpson CA, Rush CR. Acute performance-impairing and subject-rated effects of triazolam and temazepam, alone and in combination with ethanol, in humans. J Psychopharmacol. 2002;16 (1):23–34. doi: 10.1177/026988110201600102. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, French-Mullen JM, Li X. GABAA receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392 (6679):926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, Lindsey S, Purdy RH, Hauger R, de Wit H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29 (3):339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69(1–2):299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72(1–2):431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ. Pharmacokinetics of progesterone administered by the oral and parenteral routes. J Reprod Med. 1999;44(2S):141–147. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-36, HHS Publication No. SMA 09-4434. Office of Applied Studies; Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: national findings. [Google Scholar]
- van der Waals FW, Mohrs J, Foets M. Sex differences among recipients of benzodiazepines in Dutch general practice. Br Med J. 1993;307(6900):363–366. doi: 10.1136/bmj.307.6900.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki Ethical Principle for Medical Research Involving Human Subjects. doi: 10.1310/GTFR-2DRX-M6YE-ELXR. Last revised October 2008. Available at http://www.wma.net/en/30publications/10policies/b3/index.html. [DOI] [PubMed]
- Wysowski DK, Baum C. Outpatient use of prescription sedative-hypnotic drugs in the United States, 1970 through 1989. Arch Intern Med. 1991;151(9):1779–1783. [PubMed] [Google Scholar]


