Abstract
A flow type quartz crystal microbalance (QCM) chemical sensor was developed for monitoring of heavy metal ions in aqueous solutions (that is suitable for environmental monitoring). The sensor is based upon surface chelation of the metal ions at multifunctional polymer modified gold electrodes on 9 MHz AT-cut quartz resonators, functioning as a QCM. New processes have been developed which enable to obtain surface-modified gold electrodes with high heavy metal ions complexing ability. These polymer grafted QCM sensors can selectively adsorb heavy metal ions, such as copper lead chrome and cadmium, from solution over a wide range from 0.01 to 1000 ppm concentration by complexation with functional groups in the polymers. Cations typically present in natural water did not interfere with the detection of heavy metals. X-Ray Reflectivity (XRR) and Total Reflection X-ray Fluorescence (TXRF) were carried out to characterise the unmodified and modified gold surfaces as well as to verify the possibility to selectively bond and remove metal ions.
Keywords: polymer grafted quartz crystal microbalance, multifunctional polymers, heavy metal ions detection
1. INTRODUCTION
Total concentrations of the heavy metals in the natural water can be measured using a number of analytical techniques such as e.g. atomic absorption spectroscopy [1], high performance liquid chromatography [2], anodic stripping voltammetry [3], which are quite expensive and cannot be used for continuous measurements in situ. In general the advantages of chemical sensors include the possibility to perform real-time continuous measurements and relatively inexpensive apparatus. On the other hand, there are a great number of urgent practical problems which demand the continuous application of sensors directly in the analysing media, for example on site measurements on both natural and waste water. However existing sensors can fail in such a complex media analysis due to a series of difficulties. First of all, selectivity of the majority of sensors for liquid media composition analysis is often poor when the determining species are present in solution simultaneously and/or in comparable concentrations. Also, the composition of natural and waste waters can be very complex and it is just impossible to predict or to consider all interactions between analytes. Besides, a very high chemical stability of sensor materials is required considering the commonly low levels of concentration of components and pollutants, such as heavy metals, in the measured media.
The QCM is an unsophisticated, economical, real time responding, high resolution and durable method in gravimetric sensing. The changes of the resonant frequency are directly proportional to the surface mass changes [4] resulting from affinity complex formation in the sensing area. The QCM principle was developed and utilised in various fields such as environmental protection [5,6], medicine [7] and biotechnology [8] in both gas and liquid phases analyses [9]. In fact, the possibility to utilise ad hoc designed receptor films to modify quartz crystal electrode permits to construct functional and efficient chemical sensors and greatly expands the range of their properties and applications. It is crucial to obtain an appropriate immobilisation approach to ensure the high sensitivity and stability responses for practical QCM applications.
In this study new process has been developed which enables to obtain surface-modified electrodes with high heavy metal ions complexing ability. The synthetic approach is based on grafting of multifunctional polymers of polyamidoamine (PAA) type on preaminated gold via covalent bonding. PAAs are synthetic water-soluble polymers, characterized by the presence of amido and tertiary amino groups regularly arranged along their macromolecular chain. These polymers have been extensively studied because of their abilities to form complexes with DNA [10], moreover the versatility of their chemical structures has allowed a variety of applications ranging from drug delivery [11], to metal ion complexation [12] and gas sensing [13].
The aim of this paper is to report the realisation of a modified gold electrode by covalent anchoring of multifunctional polymer chains and its preliminary employment in the fabrication of a QCM sensor for the determination of heavy metal ions in the aqueous solutions. In order to identify the best sensing performances of the modified gold electrodes and to investigate film quality, a comparative study on different grafting procedures has also been performed.
2. MATERIAL AND METHODS
2.1 Chemicals
All starting reagents were purchased from Fluka and used without further purification with the exception of 2,2-bis(acrylamido) acetic acid which was synthesised as previously described [12].
2.2 Synthesis of vinyl-terminated macromonomers
2,2-Bis(acrylamido) acetic acid (BAC) (26.5 mmole) was dissolved in NaOH 3 M (8.83 ml) and cooled with an ice bath. The solution was subjected to several vacuum-nitrogen-cycles and, under stirring, added of triethylamine (TEA) (9.42 ml) and ethylenediamine-N,N’-diacetic acid (EDDA) (22.5 mmole). The reaction mixture was thoroughly mixed in water (2 ml of water each gram of monomers). The reaction was run under nitrogen atmosphere to avoid discolorations and it was kept 24 hrs at 25°C. The product was isolated and fractionated by ultrafiltration in an Amicon system with YM 3 membrane and finally lyophilized with a yield of 70%.
2.3 QCM crystal surface modification
As first step the activation of the sensing area of the QCM crystal requires the surface modification. This was realised by soaking the front side Au-electrode of a clean QCM crystal in a 0.222 M cystamine aqueous solution for 72 hrs at room temperature (22°C); after that the crystal was careful washed with bi-distilled water to remove the soluble unreacted reagent. The frequency values of the crystal were taken before and after the cystamine treatment observing a decrease ranging from 90 to 120 Hz.
2.4 In situ polymerization on modified QCM - Sensor S
2,2-Bis(acrylamido) acetic acid (BAC) (26.5 mmole) was dissolved in NaOH 3 M (8.83 ml) and cooled in ice bath. After several vacuum-nitrogen-cycles, triethylamine (TEA) (9.42 ml) and ethylenediamine-N,N’-diacetic acid (EDDA) (22.5 mmole) were added. The reaction mixture was thoroughly mixed in water (2 ml/g of the sum of the monomers) and added in the modified QCM (prepared as described in 2.3). The mixture, degassed and purged in nitrogen atmosphere, was kept (in the dark) at 25 °C for three days. Finally, the QCM was careful washed. The frequency values of the crystal were taken before and after the polymer grafting observing a decrease ranging from 650 to 750 Hz.
2.5 Grafting of Vinyl-terminated macromonomers on modified QCM - Sensor P
A solution of vinyl-terminated PAA (100 mg) in water (1 ml), was added to a modified QCM (prepared as reported in 2.3). The reaction mixture, degassed and purged in nitrogen atmosphere, was allowed to react in the dark at room temperature for three days. The polymer grafted QCM was careful washed. The frequency values of the crystal were taken before and after the polymer grafting observing a decrease ranging from 60 to 80 Hz.
2.6 Apparatus and measuring procedures
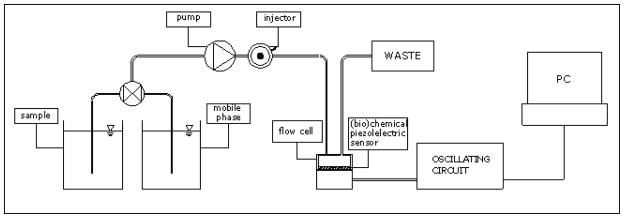
A schematic illustration of the QCM setup is shown in Scheme 1. The QCM measurement system (RQCM/CTH-100, Maxtek, INC., Santa Fe Springs, CA) is equipped with a flow cell (FC-550, Maxtek, INC., Santa Fe Springs, CA) to create a flow chamber of 0.1 ml, in which the temperature is controlled by the mobile phase. The Maxtek 1 inch quartz crystal oscillator was 9 MHz AT-cut quartz with gold electrodes and a configuration allowing both electrical contacts on the backside of the crystal, thus allowing measurements in conducting liquids.
Scheme 1.
Experimental set up
The commercial RQCM instrument has been modified to realise a flow-injection QCM (FI-QCM) system. It consists of a fluid circuit with a volumetric pump (Knauer, Berlin, Germany), to maintain a flow rate of 0.4 ml/min and an injection valve with a 100 γl sample loop for injection of the solutions; before the pump there is a three way control device which enables to use as mobile phase bi-distilled or contaminated water. Teflon tubes (Tygon Norton Co, Akron OH, USA), internal diameter 1 mm, were used for connections. The output frequency was registered by using a RS-232 standard serial port.
With this set-up (Scheme 1) two types of experiments have been carried out:
The flow injection experiments consist of 5–10 min flow natural water as the mobile phase to stabilise the baseline signal, followed by consecutive injections of the analyte solutions into the flowing water. The concentrations of the heavy metal ions (copper (Cu++) lead (Pb++), chrome (Cr+++) and cadmium (Cd++)) ranged between 102 and 103 mg/L.
Real-time continuous measurements consist of flowing the mobile phase (natural water at 24°C) to stabilise the baseline signal, followed by flowing measured media containing heavy metals. All experiments were carried out at 24°C with a flow rate of 0.4 ml/min; the concentrations of heavy metal ions ranged between 1 10−2 and 1 mg/L; injections of HCl 0.1 M were used to regenerate the sensing surface.
2.7 Surface characterisation
X-Ray Reflectivity (XRR) measurements were collected with a Bruker D8 Advance reflectometer equipped with a Göbel mirror. The X-ray beam was a Cu Kα radiation, λ = 0.154 nm. XRR profiles were analysed with the IMD [14] software in order to evaluate layer density, thickness and roughness.
Total X-Ray Fluorescence (TXRF) measurements were performed with the Bruker TXRF system S2 Picofox, air cooled, Mo tube, Silicon-Drift Detector, operating values 50kV and 1000μA, using an acquisition time of 600 seconds.
3. RESULTS AND DISCUSSION
3.1 Preparation of the sensitive units
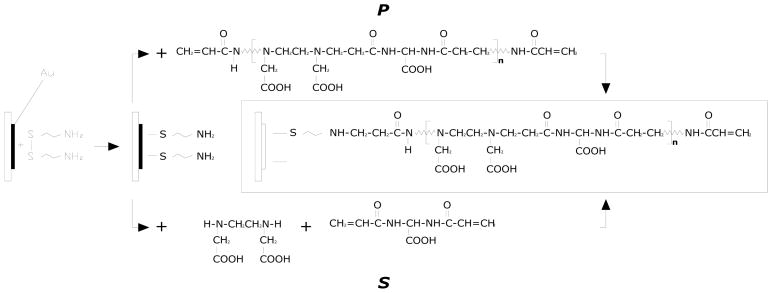
The surface modification of gold electrode constituting the active layer (Scheme 2) has been accomplished in the light of two requirements: the presence at the same time of a termination with an end group able to generate a stable bond with the gold electrode (i.e. Au-S interaction, thiolate bond) and, on the other side, of great number of functional groups prone to interact reversibly with heavy metal ions in aqueous solutions.
Scheme 2.
Reaction scheme
The adopted chemical approach involves, as a first step, the functionalization of the front side Au-electrode with amino groups (−NH2) via cystamine method [15]; the next activation step involves the anchoring of a selected sensing layer onto preaminated gold surface. A structurally related PAA multifunctional polymer which, in linear form, has been shown to possess excellent complexing ability and selectivity for toxic heavy metal ions [12], was selected to obtain new modified electrode having improved specific properties, in particular, heavy metal ions detection ability.
Two different synthetic approaches were employed to perform PAA grafting: the first one (namely S in Scheme 2) by in situ polymerization, that is simultaneous grafting and growing polymer chains onto the aminated gold surface, and a second one (P in Scheme 2) which enables the grafting of selected presynthesized macromonomers. The electrode modification process concerns the introduction of the active layer by grafting the vinyl-terminated macromonomers onto the amino groups. The grafting reaction was carried out in water without catalist at 25 °C where the polymerization of linear PAAs is known to yield higher molecular weight products [12].
The resonant frequency was evaluated after every step of synthetic procedure. As expected, both gold functionalization and polymer grafting induce a decrease of electrode resonance frequency. For instance the resonance decreases from 9,011.545 kHz for unmodified QCM to 9,011.427± 0.025 kHz for aminated QCM, and to 9,011.357 ±0.018 or 9,010.691± 0.166 kHz after polymer grafting via P or S method respectively. As far as the amount of grafted polymer is concerned, considering that the principle of the QCM detection lies in recording the frequency decrease corresponding to weight increase on the electrode surface, the PAA content of sensor P was always considerably smaller than that obtained with procedure S. This may occur for the reason that grafting hardly proceeds because of the heterogeneous reaction system and of the steric hindrance exerted by grafted chains. Accordingly, grafting by in situ polymerization (sensor S, Scheme 2), where low molecular weight monomers are involved, leads to modified QCM with at least a frequency decrease about ten time higher than that recorded following procedure P.
The starting gold electrode as well as the polymer grafted product have been fully characterised by XRR and TXRF measurements as reported below. It has been found that the corresponding polymer is effectively grafted onto the surface through the formation of real chemical bonds [16]. Moreover the immobilisation of the active layer by covalent anchoring the chain ends onto the gold electrode, overcome the inconvenience of loss of functional material over time, a typical drawback recurrent when the electrode modification is realised through physisorbed films.
3.2 Detection of heavy metal ions
Linear PAAs as well as polymer-grafted silica are extensively studied for their heavy metal ions chelating properties. In particular, PAA chains retain their complexing ability even in the grafted form and the complexing capacity is of the same order (one metal ion per repeating unit) as in their free counterparts [12].
In order to identify the best sensing performances of the modified gold electrodes and to investigate film quality, a comparative study on different grafting procedures has been performed. The polymer grafted gold electrode was placed in a microflow cell assembled in a one-side configuration and the commercial QCM instrument was modified to obtain an apparatus operating in flow injection analysis mode; the setup is shown in Scheme 1. The flow-injection modification is an improvement of the original system in comparison with the batch operating mode, because it allows to carry out continuous analyses as well as injections of small samples and to obtain responses in real time. These features together with the simplicity, the rapidity and the cheapness of the QCM, make this system a valid alternative to the usual methods of investigation, such as atomic adsorption spectroscopy, high performance liquid chromatography or anodic stripping voltammetry.
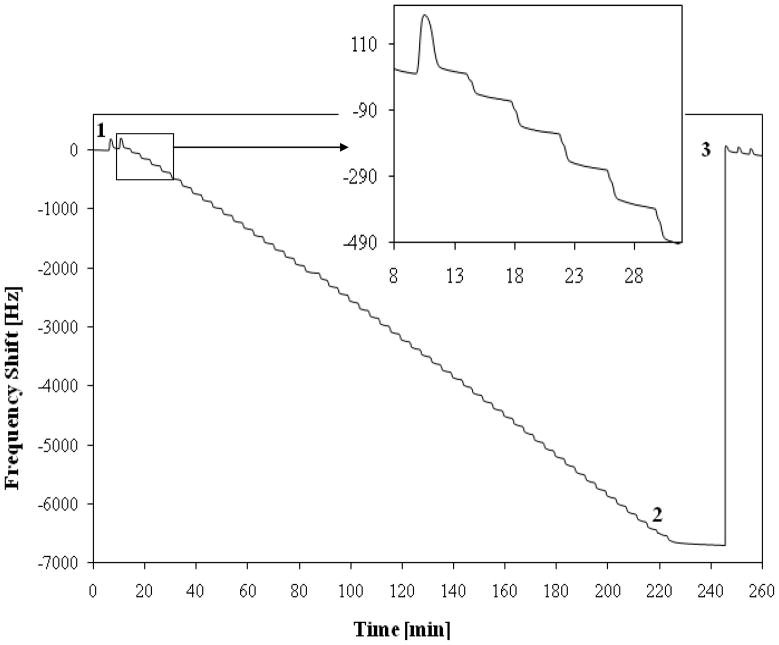
The sensing performances of the modified gold electrodes were investigated by monitoring the frequency variation induced by the presence of heavy metal ions, such as copper (Cu++), lead (Pb++), chrome (Cr+++) and cadmium (Cd++), in aqueous media. Figure 1 shows a typical measured signal obtained by Sensor P for more than fifty subsequent injection of 100 γl Cr+++ solution (249 mg/L) using natural water as mobile phase at 0.4 ml/min. Similar experiment were previously executed by using bi-distilled water as mobile phase and it was verified that cations present in natural water don’t interfere with heavy cations chelation. After the injections it was detected very close values of frequency decreases, indicating the accuracy and reproducibility of the detection method; in Table 1 the frequency decreases after a single injection (100 µl) of above mentioned ions are also reported. Furthermore the amount of metal ion injected didn’t exceed the linear detection range of the sensor also after a frequency decrease more than 6000 Hz. Nevertheless the resonant frequency of the crystal returns closely to the base line frequency after HCl regeneration (standard deviation = ± 34 Hz), indicating the complete removal of chelated metal ions from the surface of the active layer as well as the impossibility to washing off or hydrolyse the grafted multifunctional polymer. This behaviour was confirmed for more than 50 cycles of adsorption and desorption (standard deviation = ± 86 Hz). In addition sensor P detects metal ions in flow injection mode with analogous results even if a double flow rate is used. This behaviour means that the total amount of chelated metal ions do not change indicating that the sensing layer possesses a very high complexing capacity and the complexes thus formed are stable: as a consequence in all cases the responses are fast, reversible and reproducible.
Figure 1.
Measurement curve of the QCM modified sensor: (1) coated surface in equilibrium with natural water flow (0.4 ml/min); (2) frequency decrease due to 54 subsequent injection of 100 µl Cr+++ solution 249 mg/L; (3) successful regeneration by 100 µl HCl solution 0.1 M.
Table 1.
Frequency decreases after a single ion injection (100 µl)
| Ion | Concentration (mg/L) | ΔF (Hz) |
|---|---|---|
| Cu2+ | 150 | 19 ± 2 |
| Cr3+ | 249 | 119 ± 7 |
| Pb2+ | 338 | 161 ± 5 |
| Cd2+ | 376 | 207 ± 18 |
Similar results, in terms of frequency decrease, were obtained with sensor S by flow-injection experiments of heavy cations. Instead, some difficulties were encountered during heavy cations removal and regeneration of polymeric layer by HCl injection because the resonant frequency unlikely returns to the base line frequency after a number of HCl injection and also after few hours soaking the modified QCM in 0.1M HCl solution. This may be because grafting by in situ polymerization, where low molecular weight monomers are involved, leads to a considerably higher polymer content than that shown by the same modified detector prepared by polymer grafting (sensor P) were controlled molecular weight chains are involved but in this case probably more complex interactions not completely reversible, over ions chelation by polymeric layer, occur.
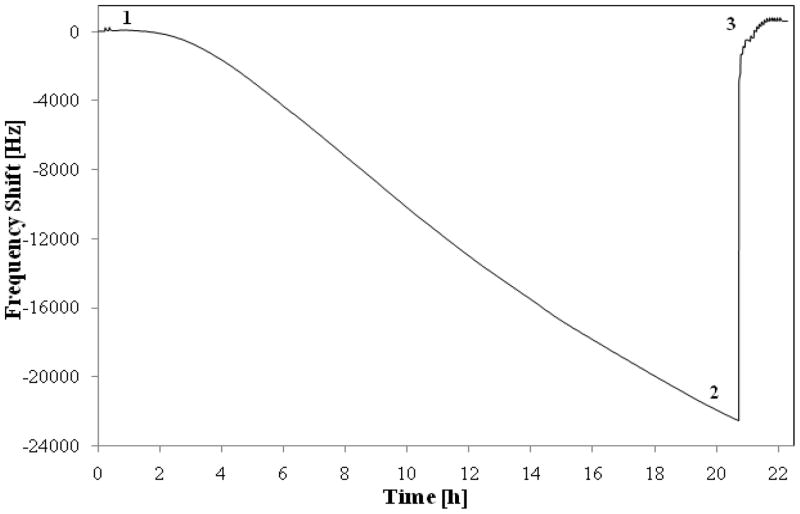
When solutions with low metal ions concentration have been used (i.e. less than 1 mg/L) in the flow injection experiments, no frequency variation was observed. In this case two possible operation mode can be considered: the increase of the sample loop volume, which is an obvious resolution, or, more interesting, the use of the sample solution as mobile phase. In the second case, the QCM modified sensor is used as an accumulator, in other words the sample solution is eluted through the flow cell for settled elution time and then the sensing layer is regenerated by HCl injections. The results obtained are very interesting: as example, Figure 2 shows the binding and the regeneration curves obtained by sensor P when a Pb++ solution 0.363 mg/L was eluted for more than 20 hrs, after flowing natural water to stabilise the base line. First of all, it is possible to note the continuous frequency decrease all over the time of the sample elution, secondly a complete regeneration process is obtained by HCl injections. Also in this case the responses are fast reversible and reproducible. In such a way, the modified chemical sensor can become an efficient, easy and inexpensive apparatus for real time liquid monitoring (i.e. heavy metal ions detection).
Figure 2.
Typical time course adsorption and regeneration curve of QCM modified sensor: (1) coated surface in equilibrium with natural water flow (0.4 ml/min); (2) frequency decrease due to flowing Pb++ solution 0.363 mg/L for 20 hrs; (3) regeneration by 100 µl HCl solution 0.1 M.
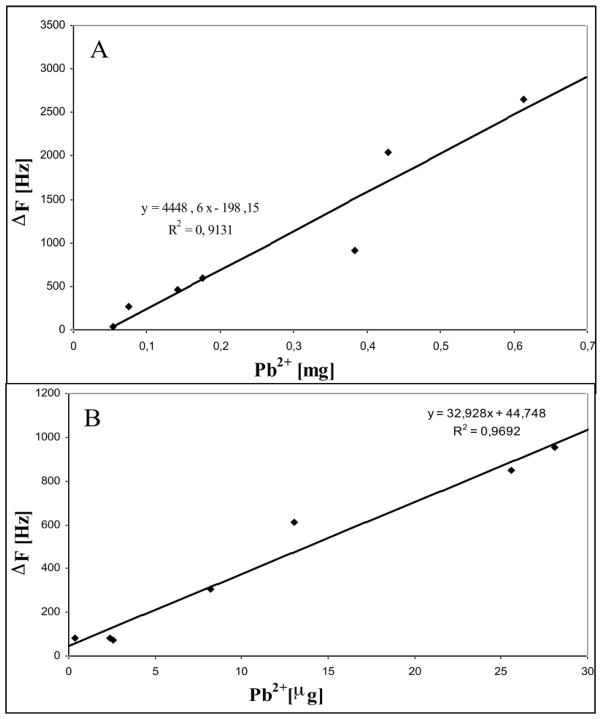
Finally, by means of a set of experiments, using Pb++ as model ion, it was possible to construct the calibration curves as reported in Figure 3 both for flow injection experiments (fig. 3A) and using sample solution as mobile phase (Fig. 3B). A very good correlation was observed between the data of each experimental mode, so PAA modified QCM can consider a promising solution for environmental monitoring if used in a sensor arrays or a multisensor systems.
Figure 3.
Calibration curve constructed with frequency shifts measured after Pb++ adsorption from different solutions: flow injection mode experiments (A); sample solutions were used as mobile phase (B).
Similar results were obtained considering the corresponding frequency shifts after ions desorption and regeneration steps instead of absorption: in this way, it should be possible to check and eliminate some potential noises due to experimental conditions.
3.3 Surface Characterization
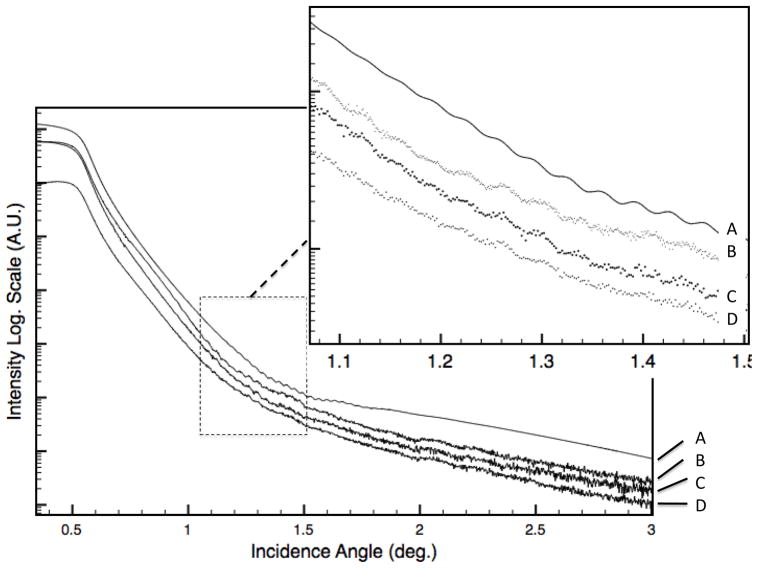
X-Ray Reflectivity (XRR) is a very powerful technique for thin films characterisation, because it can provide thickness, density and roughness of investigated thin films samples [17, 18]. It was extensively applied to characterise polymers layers, even of few nanometers [19]. Figure 4 reports experimental and simulated XRR patterns collected on P sensor, at different functionalisation and operation stages. At low incidence angles all incident radiation is totally reflected. At 0.55° the intensity of XRR patterns starts to decrease, due to the critical angle of total reflection, and X-Rays start to penetrate in the sample. Considering patterns A and B (respectively simulated and experimental XRR spectra collected on P sample) we can see Kiessing fringes, that appear at about 1° of incidence; they have a periodic modulation that corresponds to a layer thickness of about 120 nm. In the XRR simulation (pattern A) we considered a SiO2 substrate with a layer of Cr (20 nm) and Au (about 100 nm, in accord to the Maxtek, INC., Santa Fe Springs, CA indications). Simulation of experimental spectrum B, allowed to verify that the critical angle corresponds to the more dense layer (i.e. gold density of 19.3 g/ cm3), and that Kiessig fringes are due to the gold layers. Gold layer resulted 120 nm thick. Over this substrate (SiO2, Cr/Au), the polymer layer has been considered and its structural properties were extracted from simulation. From the fitting procedure, the polymer layer density (about 1 g/cm3), thickness (3 nm) and roughness (0.9 nm) were estimated. These data allow to calculate a grafted polymer mass per unit area of 300 ng/cm2 . This value is close to the mass change obtained by using the well known Sauerbrey equation [4]:
were Δf is the frequency change (Hz) corresponding to a mass loading Δm and Cf represent the collection of constants that define the crystal sensitivity (0.1834 Hz/ng/cm 2).
Figure 4.
Experimental (B) and simulated (A) XRR patterns collected on sample P. XRR patterns after Pb injections (D) and after ions desorption and regeneration (C) on the same sample are also reported. To well enhance the effect of the regeneration process XRR spectra B and C are close.
In fact, assuming that the polymer layer has the same acousto-elastic properties as quartz, we calculate a mass increase on the crystal surface of 327ng/cm2.
An higher thickness (5 nm) was observed on XRR spectra on S sample, but in this case there is not correspondence with the mass change obtained using Sauerbrey equation probably because grafting reaction doesn’t produce an uniform, rigid, thin film and actually the system exhibit more complicated frequency-mass correlations.
In Figure 4 XRR of sensor P after subsequent injections of 100 µl Pb++ solution 338 mg/L is also reported (pattern D). It can be clearly shown that interference fringes (related to the gold thickness) completely disappear. The disappearance of Kiessig fringes may be related to two main factors: increase in the surface layer roughness and/or decrease of the XRR sensitivity, due, for example to a change in the penetration depth of X-Rays. XRR simulation showed that an increase in the roughness of sensing polymer layer is not sufficient to justify the disappearing of Kissig fringes. This may be more probably related to the presence of lead in surface layer: because of the high atomic weight of the element, a thin Pb layer decreases the X-Ray penetration depth, preventing to detect the contribution of the gold layer. After regeneration procedure (see Figure 4, pattern C), that involve the elimination of Pb from the sensing layer, the XRR pattern resulted identical to that collected on the P sensor, before the lead anchoring. This strongly suggests that the process is completely reversible. Total Reflection X-ray Fluorescence (TXRF) is a well established technique for chemical analysis, and it is extremely sensitive to metals [20]. The reflection of X-Rays close to the critical angle makes this technique surface sensitive and with a higher detection sensitivity to contaminants if compared to standard XRF [21].
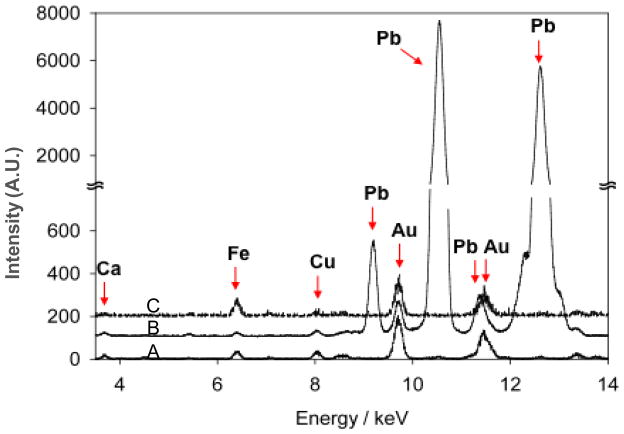
Figure 5 shows TXRF spectra of (A) sensor P, (B) sensor P after subsequent injections of 100 µl Pb++ solution 338 mg/L, and (C) sensor P after Pb++ injections and regeneration procedure (polymer film do not show contribution in the TXRF patterns). There are several peaks in the TXRF patterns: Au (and Cr) peaks can be attributed to the sample substrate and Fe, Cu, and Ca are commonly present in the natural water (Figure 5, pattern A). All patterns were normalised in respect to the gold main peak, that is considered an internal standard, because the gold layer remains unaltered after all sample treatments. After the injection of Pb analyte solution, TXRF clearly shows the appearance of Pb main peaks (pattern B), indicating an high surface concentration of this metal. It is very important to notice that the peaks intensities of all other elements remain almost unchanged, indicating that their concentration remains constant. This highlights the high sensor sensitivity for Pb. TXRF pattern of sample P collected after regeneration procedure (pattern C) corresponds to the pattern collected on the same sample, before analyte injection. This supports the complete reversibility of the process.
Figure 5.
TXRF spectra of (A) polymer grafted QCM - Sensor P, (B) the same sensor after the injection of Pb++ solution, and (C) the same sensor after regeneration procedure.
4. CONCLUSIONS
New processes have been developed which enable to obtain polymer grafted gold electrodes with high heavy metal ions complexing ability. These surface-modified QCM sensors can selectively adsorb heavy metal ions; investigations in the presence of copper lead chrome and cadmium, have evidenced fast reversible and reproducible responses to analytes in aqueous media. Cations typically present in natural water did not interfere with the detection of heavy metals. XRR and TXRF were carried out to characterise the unmodified and modified gold surfaces, besides the experiments are consistent with the possibility to selectively bond and removed metal ions.
Acknowledgments
The authors acknowledge Dr. Paolo Colombi for the useful discussions about the paper.
This work is partially supported by Award Number R01ES019222 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hwang TJ, Jiang SJ. Determination of copper, cadmium and lead in biological samples by isotope dilution inductively coupled plasma mass spectrometry after online pre-treatment by anodic stripping voltammetry. J Anal At Spectrosc. 1996;11:353–357. [Google Scholar]
- 2.Malik AK, Kaur V, Verma N. A Review on Solid Phase Microextraction–High Performance Liquid Chromatography as a Novel Tool for the Analysis of Toxic Metal Ions. Talanta. 2006;68(3):842–849. doi: 10.1016/j.talanta.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Mcgaw EA, Swain GM. A Comparison of Boron-Doped diamond Thin Film and Hg-Coated Glassy Carbon Electrodes for Anodic Stripping Voltammetric Determination of Heavy Metal Ions in Aqueous Media. Anal Chim Acta. 2006;575(2):180–189. doi: 10.1016/j.aca.2006.05.094. [DOI] [PubMed] [Google Scholar]
- 4.Sauerbrey G. Verwendung von Schwingsquarzen zur Wägung dunner Schichten und zur Mikrowägung. Z Phys. 1959;155:206–212. [Google Scholar]
- 5.Karousos NG, Aouabdi S, Way AS, Reddy SM. Quartz crystal microbalance determination of organophosphorus and carbamate pesticides. Anal Chim Acta. 2002;469:189–196. [Google Scholar]
- 6.Casilli S, Malitesta C, Conoci S, Petralia S, Sortino S, Valli L. Piezoelectric sensor functionalised by a self-assembled bipyridinium derivative: characterisation and preliminary applications in the detection of heavy metal ions. Biosens Bioelectron. 2004;20:1190–1195. doi: 10.1016/j.bios.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Tsai WC, Lin IC. Development of a piezoelectric immunosensor for the detection of alpha-fetoprotein. Sens Actuators, B. 2005;106:455–460. [Google Scholar]
- 8.Liu Yung-Chuan, Hsiung Chih-Ming Wang Kuang-Pin. Comparison of different protein immobilization methods on Quartz Crystal Microbalance surface in flow injection immunoassay. Anal Biochem. 2001;299:130–135. doi: 10.1006/abio.2001.5409. [DOI] [PubMed] [Google Scholar]
- 9.Janshoff A, Steinem C. Quartz Crystal Microbalance for Bioanalytical Application. Sensor Update. 2001;9:313–354. [Google Scholar]
- 10.Jones NA, Hill IRC, Stolnik S, Davis SS, Garnett MC. Polymer Chemical Structure is a Key Determinant of Physicochemical and Colloidal Properties of Polymer-DNA Complexes for Gene Delivery. Biochim Biophys Acta-Gene Struct Expr. 2000;1517(1):1–18. doi: 10.1016/s0167-4781(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 11.Ferruti P, Ranucci E, Sartore L, Bignotti F, Marchisio MA, Bianciardi P, Veronese FM. Recent Results on Functional Polymers and Macromonomers of Interest as Biomaterials or For Biomaterial Modification. Biomaterials. 1994;15(15):1235–1241. doi: 10.1016/0142-9612(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 12.Sartore L, Penco M, Bignotti F, Peroni I, Gil MH, Ramos A, D'Amore A. Grafting of selected presynthesized macromonomers onto various dispersions of silica particles. J Appl Polym Sci. 2002;85(6):1287–1296. [Google Scholar]
- 13.Sartore L, Penco M, Della Sciucca S, Borsarini G, Ferrari V. New Carbon Black Composite Vapor Detectors Based on Multifunctional Polymers. Sens Actuators B. 2005;111–112:160–165. [Google Scholar]
- 14.Windt DL. IMD-software for modelling the optical properties of multilayer films. Comput Phys. 1998;12:360–370. [Google Scholar]
- 15.Cohen Y, Levi S, Rubin S, Willner I. Modified monolayer electrodes for electrochemical and piezoelectric analysis of substrate-receptor interactions: novel immunosensor electrodes. J Electroanal Chem. 1996;417:65–75. [Google Scholar]
- 16.Sartore L, Barbaglio M, Penco M, Bergese P, Bontempi E, Colombi P, Depero LE. Polymer-coated quartz crystal microbalance chemical sensor for heavy cations in water. J Nanosci Nanotechnol. 2009;9:1164–1168. doi: 10.1166/jnn.2009.c111. [DOI] [PubMed] [Google Scholar]
- 17.Bontempi E. X-Ray Reflectivity for the characterization of thin films. Recent Res Devel Chem Physics. 2004;5:461–488. [Google Scholar]
- 18.Colombi P, Gibaud A, Jergel M, Krumrey M, Lafford TA, Lamperti A, Ma T, Fujimoto T. Reproducibility in X-ray Reflectometry: results from the first world-wide round robin experiment. J Appl Crystallogr. 2008;41(1):143–152. [Google Scholar]
- 19.Bergese P, Bontempi E, Chiari M, Colombi P, Damin F, Depero LE, Oliviero G, Pirri G, Zucca M. Investigation of a biofunctional polymeric coating deposited onto silicon microcantilevers. Appl Surf Sci. 2007;253(9):4226–4231. [Google Scholar]
- 20.Borgese L, Zacco A, Bontempi E, Pellegatta M, Vigna L, Patrini L, Riboldi L, Rubino FM, Depero LE. Use of total reflection X-ray fluorescence (TXRF) for the evaluation of heavy metal poisoning due to the improper use of a traditional ayurvedic drug. J Pharm Biomed Anal. 2010;52(5):787–790. doi: 10.1016/j.jpba.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Borgese L, Zacco A, Bontempi E, Colombi P, Bertuzzi R, Ferretti E, Tenini S, Depero LE. Total reflection of x-ray fluorescence (TXRF): a mature technique for environmental chemical nanoscale metrology. Meas Sci Technol. 2009;20(8) [Google Scholar]