Summary
Genetic lesions in chromosomal region 3p21.3 marks one of the earliest events in human lung cancer development. It is hypothesized that one or more tumor suppressor genes reside in this region. Identification and characterization of these genes are important for the understanding of lung cancer initiation. UBE1L (UBA7) is a long-suspected 3p21.3 residing tumor suppressor gene. It encodes the key enzyme that activates ISGylation, a novel, ubiquitination-like, post-translational protein modification system that is inducible by interferon. It has been implicated that ISGylation plays a variety of biological roles ranging from viral defense to tumor surveillance. Here we tested the possible function of ISGylation during lung cancer development by using the Ube1l-deficient mice and the K-rasLA2 lung cancer mice. Protein ISGylation levels were largely unchanged during lung cancer progression. Ube1l deficiency neither altered the lung cancer progression nor affected the overall survival of K-rasLA2 lung cancer mice. Our study suggests that Ube1l is not a tumor suppressor gene in K-rasLA2 lung cancer mouse model. However, as described in the discussion, additional studies with other lung cancer mouse models will be necessary to elucidate the potential tumor suppressor function of UBE1L in K-RAS mutation independent human lung cancers.
Keywords: Tumor suppressor gene, UBE1L, ISG15, ISGylation, K-ras, mouse cancer model
1. Introduction
Lung cancer, a virulent type of cancer with a high metastasis rate and a poor prognosis, is the most common cancer form worldwide and the leading cause of cancer death [1]. Numerous genomic lesions have been detected in lung cancer, the most prevalent among which is the loss of allelic heterozygosity (LOH) in chromosome 3p21.3 [2]. LOH in 3p21.3 is observed in 70–80% Non-small-cell lung cancers (NSCLC) and 90–100% small cell lung cancers (SCLC). Transfer of a normal 3p DNA into human cancer cells lead to cancer suppression [3,4], an observation initiating the speculation of tumor suppressor genes (TSGs) in this region [5]. As a matter of fact, several 3p21.3 TSGs, such as LUCA2, RASSF1 and NPRL2, have been cloned and confirmed [6]. Inactivation of these genes is achieved via gene deletion and/or epigenetic modifications.
UBEL is one of the TSG candidates in 3p21.3. Similar to other known TSGs in this region, the expression of UBE1L was largely reduced in many lung cancer cell lines [7,8,9]. In addition, UBE1L was identified as a target gene important for lung cancer chemoprevention. Overexpression of UBE1L correlated with cyclin D1 repression [10,11], further supporting the hypothesis that UBE1L is a TSG. The only known biological function for UBE1L is that it catalyzes ISG15 conjugation. ISG15 is a ubiquitin-like small molecule encoded by an interferon (IFN)-stimulated gene. Similar to ubiquitin, it modifies substrate proteins post-translationally by forming convalent conjugations (ISGylation) [12]. The three sequential reaction steps - activation, conjugation and ligation - are catalyzed by enzyme E1, E2 and E3, respectively. UBE1L is the only E1 enzyme for ISGylation. Consistently, Ube1l knockout (KO) mice are deficient in ISGylation [13]. An array of essential proteins with diverse biological functions are targeted by ISGylation, underlining the biological importance of ISGylation [14,15,16,17]. Intriguingly, Ube1l KO mice appear to be fertile with no obvious phenotypes. The discrepancy may be attributed to the indigenous inducible nature of ISGylation system itself. Certain biological stresses, such as viral infection or cancer development, may be required to reveal the biological functions of ISGylation.
K-RAS was firstly discovered as a proto-oncogene with homology to the transforming gene from Kirsten Rat Sarcoma virus [18]. Functionally, K-RAS is a critical molecule that transduces signals from activated cell surface receptors and regulates a range of cellular functions, including transcription and apoptosis [19]. It is a typical small GTPase. The intrinsic hydrolysis activity keeps the active state transient through self-inactivation. Oncogenic K-RAS isoforms are constitutively active mutants with reduced GTPase activity. K-RAS is the one of the most frequently activated oncogene: about 20% of human tumors contain activation mutations in this gene. For example, 70–90% pancreas and 25–50% lung carcinomas were positive with K-RAS mutation [20].
Interferons have been used for almost half a century as a treatment for a variety of cancers [21]. As one of the most prominently induced pathways of IFNs, ISGylation may be important for cancer development. In the present study, we tested whether the ISGylation activation enzyme, Ube1l, is truly a TSG using K-rasLA2 mice cancer model. K-rasLA2 mice carry a latent, oncogenic allele of K-ras, K-rasG12D, which only activates upon the occurrence of spontaneous somatic recombination events [22]. These mice developed lung tumors and were also prone to thymic lymphoma. Here we generated the Ube1l-deficient K-rasLA2 mice and compared cancer progression between Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice. Loss of ISGylation did not affect tumor spectrum, tumor pathology or survival of K-rasLA2 mice, suggesting that Ube1l is not a TSG, at least in this particular mice cancer model.
2. Materials and methods
2.1. Antibodies and immunofluorescence staining
OCT-frozen sections were blocked at room temperature for 30 minutes in PBS containing 3% BSA. They were then incubated with rabbit anti-mouse ISG15 antibody diluted (1:100) in PBS containing 3%BSA or in a RPMI complete medium with monoclonal antibody G8.8 for 1 hour at room temperature. Following incubation, sections were washed twice for a total 10 minutes with PBS. Sections were then incubated with Alexa fluor 488 goat anti-rabbit IgG(H+L) antibodies (1:400 dilution, Molecular Probes, catalog # A-11008) with or without Alexa fluor 594 goat anti-rat IgG antibodies (1:400 dilution, Molecular Probes, catalog # A-11007) for 1 hour at room temperature. Sections were then counter stained for 5 minutes with DAPI. Staining with second antibodies alone was used as a negative control. Rabbit anti-mouse ISG15 polyclonal antibody has been described previously [15]. G8.8 is a monoclonal antibody that stains murine medullary thymic epithelial cells (a gift from Dr. Andrew Farr) [23]. Adjacent frozen sections were stained with haematoxylin and eosin (H&E) to visualize the cell morphology.
2.2. Protein expression analysis
About 0.1 g thymus tissues were harvested from mice and minced by scissors. These samples were then treated with 0.5 ml ACK buffer (NH4Cl 8 mg/ml, KHCO3 0.1 mg/ml and disodium EDTA 2H2O 0.0372 mg/ml) for 5 mins on the ice to remove red blood cells. After a PBS wash, tissue pellets were stored at −80°C before use. To prepare the whole cell lysates, tissues were homogenized by sonication in 1× sample buffer containing 62.5 mM Tris-HCl (pH 6.8), 10% glycerol, 2% SDS and 2 mM phenylmethylsulfonyl fluoride. Protein concentrations were measured using Micro BCA™ protein assay kit from PIERCE (catalog # 23235). Samples were diluted further with 1× sample buffer to generate equal protein concentrations. 30–40 μg proteins from each sample were resolved on 8–18% gradient gels and transferred to nitrocellulose membranes (Amersham Biosciences Inc.) for western blotting. Where indicated, membranes were stripped after the first blot in 50 mM Tris-HCl, pH 7.0, 2% SDS, and 50 mM dithiothreitol at 55°C for 30 min. Antibody for tubulin-α was from Sigma (catalog # T9026).
2.3. Generation of mice
Ube1L and K-ras double-heterozygous (Ube1L+/−/K-rasLA2) mice were generated by crossing K-rasLA2 (heterozygous) mice [22] and Ube1L−/− mice [13]. Ube1l+/−/K-rasLA2 mice were intercrossed to generate Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice. All mice used for this study were in a mixed background of C57BL/6 and 129Sv. Mice were housed in a pathogen-free facility and procedures were approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute.
2.4. Genotype analysis
Mouse genomic DNA was isolated from tail biopsies and subjected to PCR for genotyping. PCR reactions were performed in a 15 μl solution containing 10 mM Tris-Cl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton, 0.2 mM dNTP, 1.3 μM total primers and 1uL Taq enzyme. PCR products were analyzed on 2% agarose gel and visualized by ethidium bromide staining. The forward primer used in Ube1L genotyping was ACCTCTTCTCTGCTGAGCATGG. The reverse primers were CTACTGAGGGACTAGAGGATGG (wild-type) and CTTCCTCGTGCTTACGGTATCG (knockout). Two bands were generated from the PCR: one is about 400 bp, indicating the wild-type allele; the other is about 700 bp, showing the KO allele. The forward primers used for K-ras genotyping was TGCACAGCTTAGTGAGACCC. The reverse primers were GACTGCTCTCTT TCACCTCC (Wild-type) and GGAGCAAAGCTGCTATTGGC (LA2). Two bands were generated from the PCR: one is about 220 bp, indicating the wild-type allele; the other is about 390 bp, showing the LA2 allele of K-ras.
2.5. Tumorigenesis analysis
Littermate controlled Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice were monitored weekly. Animals were sacrificed when they show signs of illness or moribund, such as tumors on the skin, rough fur, hunched, or difficulty in breathing. The averaged age for sacrificed mice was 30 weeks. Thymuses and lungs were dissected out, fixed with PBS containing 4% Formaldehyde and subjected to paraffin sectioning. The sections were stained with H&E and examined microscopically. Slides were visualized using a microscope (LEICA DMLB) at 100×, 200× and 400× magnifications. Images were captured with a SPOT digital camera (Diagnostic Instrument).
2.6. Statistical analysis
Statistical significance of tumor incident rate was calculated using Fisher’s Exact Test (http://www.matforsk.no/ola/fisher.htm). Statistical significance of the survival curves was calculated by chi squared test using the program Prism 4 (Graphpad Software Inc.).
3. Results
3.1. The level of ISG15 expression and ISGylation in lung tissues was largely unchanged during tumor progression
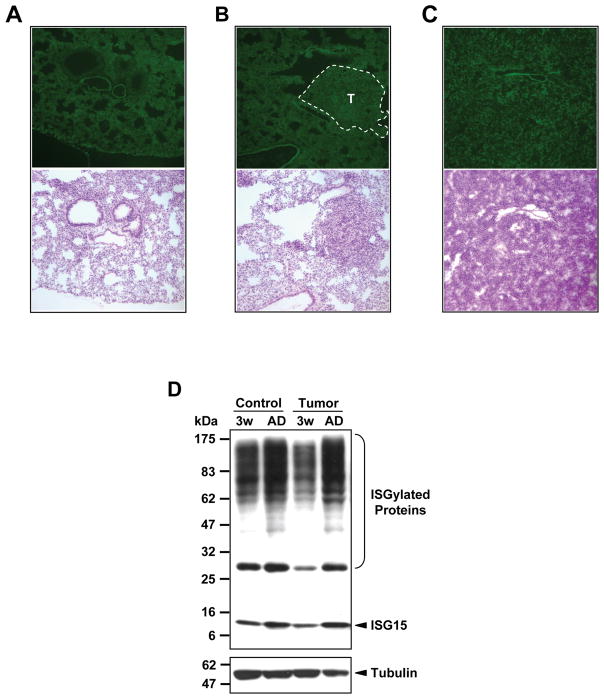
To check whether lung cancer development modulates protein ISGylation levels, we performed immunofluorescence staining on frozen sections of harvested lung tissues. ISG15 expressions were detected at similar levels in lungs from healthy wild type mice, lungs from 2–3 week old K-rasLA2 mice consisting of both normal tissues and newly developed tumor nodules, and lungs from moribund K-rasLA2 mice consisting of mostly cancer cells (Fig. 1A-C). Due to the unique morphology of tumor cells, the spatial distributions of ISG15 staining in cancerous tissues were different from those in normal tissues. However, the overall staining intensities were similar, as was also illustrated by western-blotting analysis (Fig. 1D). The levels of free ISG15 and ISGylation in lung tissues were not different between wild type mice and K-rasLA2 mice at comparable ages. Taken together, these results suggest that protein ISGylation is not modulated by lung tumor progression.
Figure 1. Expression of ISG15/ISGylation in K-rasLA2 lungs.
A-C, Representative immunofluoresence images (100×) showing ISG15 expression in normal and cancerous lungs. Images on the top, mISG15 staining; images at the bottom, H&E staining of adjacent frozen sections. A. a normal lung from a wild-type mouse, showing ectopic ISG15 expression in lung. B. ISG15 expression in tumor nodules (T) and adjacent healthy lung tissues in a lung isolated from a 3-week old K-rasLA2 mouse. C. ISG15 expression in a lung isolated from a moribund K-rasLA2 mouse. D. Western blotting analysis comparing the level of ISG15 and ISG15 conjugates in the lungs of wild type and KrasLA2 mice at 3-week old (lane 1 and lane 3) and adult age (lane 2 and lane 4).
3.2. The level of ISG15 expression and ISGylation was dramatically reduced in thymus with lymphoma
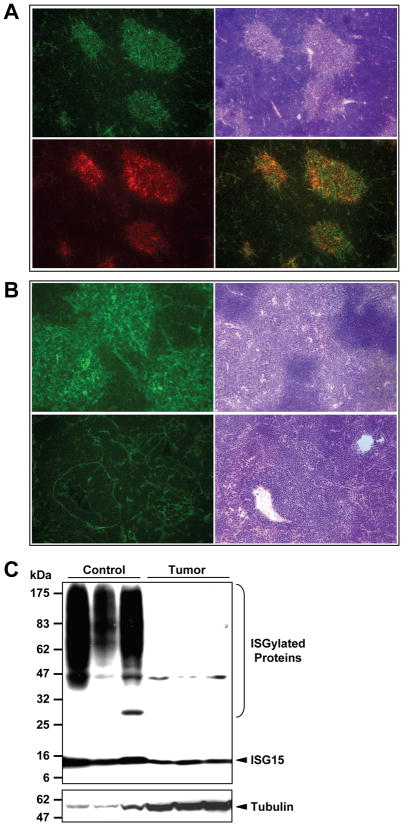
K-rasLA2 mice are also prone to thymic lymphoma development. In normal thymuses, ISG15 positive cells clustered in areas that appeared to be the medulla, as illustrated by the eosinophilic color in H&E staining (Fig. 2A. top two images). Indeed, these areas were stained positively with a medullary marker (Fig. 2A bottom two images). In contrast, there was no definable medullary structure in thymuses with lymphoma. Instead, ISG15 positive cells were less in number and scattered throughout the thymuses (Fig. 2B bottom two images). Giant in size and irregular in shape, these ISG15 positive cells differ significantly from those in healthy thymuses (Fig. 2B top two images). Western blotting analysis revealed a significant reduction of both free ISG15 and ISGylated protein conjugates in thymic lymphoma (Fig. 2C).
Figure 2. Expression of ISG15/ISGylation in K-rasLA2 thymic tumors.
A&B, Representative immunofluorescence images showing ISG15 expression in normal and cancerous mouse thymuses. A. 100×, ISG15 expression is predominantly localized to the medulla of a normal thymus. Top left, ISG15 staining; top right, H&E staining of the adjacent frozen section; bottom left, staining of a medullary marker (G8.8); bottom right, merged image of ISG15 and G8.8 staining. B. 200×, ISG15 expression in a normal (top) and cancerous thymus (bottom). Images on the right are H&E staining of adjacent tissue sections. C. Western blotting analysis of ISG15 and protein ISGylation levels in normal (lanes 1–3) and cancerous (lanes 4–6) thymic tissues. The membrane was blotted with anti-tubulin α antibody first before it was striped and reprobed with anti-ISG15 antibody.
3.3. Ube1l deficiency did not alter tumor progression in K-rasLA2 mice
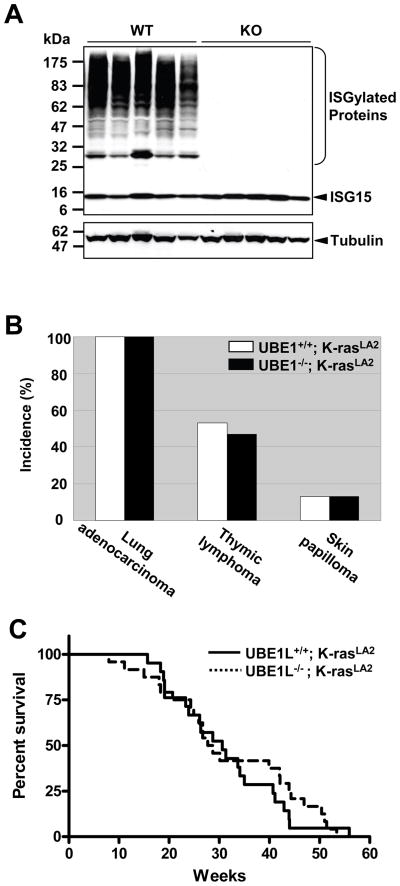
To test whether Ube1l is a tumor suppressor gene in K-rasLA2 mice tumor model, we generated Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice. Moribund Ube1l+/+/K-rasLA2 mice (n = 15) and Ube1l−/−/K-rasLA2 mice (n = 15) were analyzed pathologically. As expected, ISGylation was absent in lung tissues harvested from Ube1l−/−/K-rasLA2 mice (Fig. 3A). No differences in the tumor spectrums were detected (Fig. 3B). All 30 mice had lung cancer. Eight (53%) Ube1l+/+/K-rasLA2 mice and seven (47%) Ube1l−/−/K-rasLA2 mice had thymic lymphoma. In addition, two mice (13%) from each group had skin papilloma. The initiation of lung tumor was observed as early as 2 week after birth in both groups (n > 3). Survival curves were not different either (logrank test, P > 0.6, figure 3C). The mean life span of Ube1l+/+/K-rasLA2 mice was 30.6 weeks (n = 21), and the mean life span of Ube1l−/−/K-rasLA2 mice was 28.2 weeks (n = 24). The tumor spectrums and life spans of these mice were close to those previously reported [22].
Figure 3. Absence of ISGylation did not alter the tumor spectrum or survival in K-rasLA2 mice.
A. Western blotting analysis showing the absence of ISGylation in lung tissues from Ube1l−/−/K-rasLA2 mice (lanes 6–10) compared to Ube1l+/+/K-rasLA2 mice (lanes 1–5). The membranes were blotted with anti-tubulin α antibody first before it was striped and reprobed with anti-ISG15 antibody. B. The incident rate of different tumor types in K-rasLA2 moribund mice (n = 15 for both groups). C. The survival curves of Ube1l+/+/K-rasLA2 mice (solid line, n = 21) and Ube1l−/−/K-rasLA2 mice (dashed line, n = 24). Mice were observed weekly for death events and tumor development. There are no differences in survival between the two genotypes (P = 0.21, Logrank test)
3.4. Absence of ISGylation did not change tumor pathology
The tumor pathology was further analyzed in detail. Tumors from Ube1l+/+/K-rasLA2 mice and Ube1l−/−/K-rasLA2 mice were fixed in formaldehyde, sectioned and stained with H&E. Representative images were shown in Figure 4. Figure 4A shows the morphology of normal and cancerous lungs. A-(a) shows the morphology of the normal lung from a wild type mouse. A-(b) and A-(c) are representative images of lung sections from 12-week old K-rasLA2 mice showing the morphology of lung alveolar adenomas. A-(d) and A-(e) are representative images of lung sections from K-rasLA2 moribund mice. Compared to lung tumors at early stage, those at late stage lack clear tumor boundary and were irregular in cell shape. Overall, lung tumors in Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice had similar morphologies. Figure 4B shows the morphology of thymuses. B-(a) shows the medullary structure of a normal thymus. Thymuses with lymphoma lack distinctive medullary structure. Instead, they contain a sheet of lymphocytes with compacted nucleus and some star-shaped cells scattered in between. The thymic lymphomas in Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice had similar morphology [Fig. 4B-(b) and B-(c)]. In some moribund K-rasLA2 mice, the lymphoma cells also infiltrated into adjacent lungs, a phenotype observed in both Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice [Fig. 4B-(d) and B-(e)].
Figure 4. Ube1l+/+/K-rasLA2 and Ube1l−/−/K-rasLA2 mice exhibited similar tumor pathology.
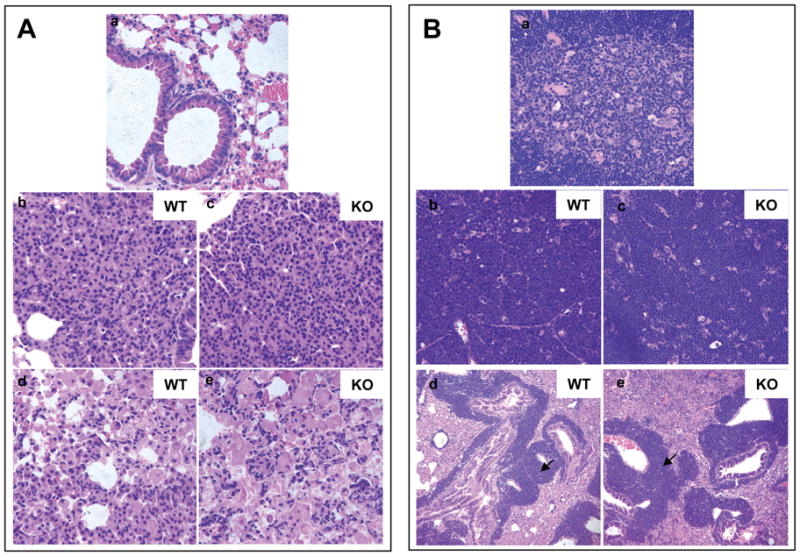
A. H&E staining images of lung tissues. a. A normal lung from a wild type mouse. b&c. Lung alveolar adenomas from 12-week old mice. d&e. Lung lesions in moribund mice showing irregular cell shapes. B. H&E staining images of normal thymuses and thymic lymphomas. a. A normal thymus showing the medulla structure. b&c. A thymus with lymphoma showing a sheet of lymphocytes with scattered star-like cells. d&e. Infiltration of lymphoma cells (indicated by arrows) in the lung. b&d are representative images for Ube1l+/+/K-rasLA2 mice. c&e are representative images for Ube1l−/−/K-rasLA2 mice. All images are at 400× magnification except figure B-(d) and figure B-(e), which are at 100× magnification.
4. Discussion
Ubiquitin and ubiquitin-like molecules (Ubls), such as SUMO-1 and NEDD-8, are signaling messengers that control almost all aspects of cellular functions including proliferation and apoptosis. Deregulation of ubiquitin and Ubl pathways leads to the development of diseases including cancers [24]. For example, covalent modification of tumor suppressor p53 by ubiquitin, SUMO-1, or NEDD8 regulates its degradation, nuclear export or transcriptional activity [25,24]. An ample amount of genetic evidence suggests that deregulation of ubiquitin/Ubl enzymes are involved in cancer development. MDM2, ubiquitylation E3 enzyme, is associated with risks of NSCLCs and colorectal cancers [26,27]. The E2 enzyme for Sumoylation, Ubc9, is associated with ovarian cancer [28]. The expression of E1 enzyme for ISGylation, UBE1L, is associated with lung cancer [8,29,30,10]. In addition to UBE1L, it was reported that both ISG15 and its conjugates were highly elevated in certain oncogene transformed cells, tumor biopsies and many tumor cell lines, including breast cancer and ovarian cancer cell line [31]. The expression of ISG15 was also found to be stage-associated in bladder cancer [32].
Compared to the research in ubiquitylation and Sumoylation field, the work in ISGylation field is far behind. Interferon-inducible ISG15 has been discovered almost three decades ago [33]. ISG15 is reported important in viral defense and immune response [34,35,36,37,38]. Still, little is known about the molecular mechanisms underlying these functions. Similarly, UBE1L has been a lung cancer tumor suppressor candidate since 1993 [8], but this hypothesis has not been tested directly. Our present study aimed to fill in this knowledge gap and test this hypothesis in vivo using K-rasLA2 mouse lung cancer model.
Ube1l KO mice are normal and fertile [13]. No abnormal cancerous growth was observed in these mice. All mice harboring a K-rasLA2 allele developed lung cancer as early as 2 weeks after birth. Lung cancer progression did not alter the overall ISGylation level in lungs. Deficiency in Ube1l did not affect lung cancer initiation time, cancer pathology or survival in K-rasLA2 mice. Our data do not support a function of Ube1l as a lung cancer tumor suppressor in K-rasLA2 mice lung cancer model. However, it is important to note that K-RAS mutation is reported in 30–50% of human lung adenocarcinoma (NSCLC), never in SCLC, and rarely in other lung cancer subtypes [39,40]. LOH of 3p21 (containing UBE1L gene) was reported in 70–80% of human NSCLC and almost 100% of human SCLC [41]. Therefore, no K-RAS mutation is identified in human SCLC that lacks normal UBE1L expression. It is possible that loss of UBE1L and K-RAS mutation are two separate pathways in lung cancer development. Additional work is necessary to characterize the role of UBE1L in lung cancer with other available mouse lung cancer models [42].
Fifty percent of K-rasLA2 mice developed thymic lymphoma. Most ISG15 positive cells clustered in the medulla of normal thymuses, suggesting ISG15/ISGlyation signaling might play a role in regulating the proliferation and/or maturation of T-cells. In contrary to medullary cells, surrounding T-lymphocytes show much less ISG15 expression. This is consistent with the low ISG15 expression levels in thymuses with lymphoma, where most of thymic structures were deformed and replaced by cancerous T-lymphocytes. Despite the high ISG15/ISGylation expression level detected in thymus medulla, ISGylation deficiency introduced by Ube1l KO alleles did not change the incident rate or pathology of thymic lymphomas in KrasLA2 mice. This data further denies the tumor suppressor function of UBE1l in K-rasLA2-derived cancers.
Although UBE1L has been a strong 3p21.3 TSG candidate of human lung cancer for more than a decade, it has never been verified by any genetic methods. Our study is the first report that tested this hypothesis genetically at the animal model level. Our data clearly demonstrated that Ube1L does not suppress the development of lung adenoma or thymic lymphoma in K-rasLA2 cancer model. However, animal tumor models may not fully recapitulate the lung cancer development in humans. Additional genetic studies using human lung cancer cell lines can be performed in the future in order to fully understand the role of UBE1L in lung cancer. Furthermore, UBE1L is an interferon-inducible gene. It may not have an obvious impact on the tumor development under physiological conditions. Rather, it may present an interesting therapeutic target in interferon or chemo-reagent-mediated cancer therapy or prevention. Future studies focusing on uncovering the molecular mechanisms of UBE1L-mediated cancer prevention may provide insight into the interplay between UBE1L and cancer therapy.
Acknowledgments
We wish to thank members of Zhang lab for valuable discussions and Dr. Joseph Biggs for critical editing. This work was supported by research funding from National Institutes of Health (GM066955 & CA102625). The Stein Endowment Fund has partially supported the departmental molecular biology service laboratory for DNA sequencing and oligonucleotide synthesis. This is manuscript #19110 from The Scripps Research Institute.
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93(4):277–283. doi: 10.1093/jnci/93.4.277. [DOI] [PubMed] [Google Scholar]
- 2.Massior PP. Genomic alterations in lung cancer. In: Pass HI, Carbone DP, Johnson DH, Minna JD, Turrisi AT III, editors. Lung cancer: principles and practice. Philadelphia: Lippincott William & Wilkins; 2005. pp. 77–96. [Google Scholar]
- 3.Satoh H, Lamb PW, Dong JT, Everitt J, Boreiko C, Oshimura M, et al. Suppression of tumorigenicity of A549 lung adenocarcinoma cells by human chromosomes 3 and 11 introduced via microcell-mediated chromosome transfer. Mol Carcinog. 1993;7(3):157–164. doi: 10.1002/mc.2940070306. [DOI] [PubMed] [Google Scholar]
- 4.Todd MC, Xiang RH, Garcia DK, Kerbacher KE, Moore SL, Hensel CH, et al. An 80 Kb P1 clone from chromosome 3p21.3 suppresses tumor growth in vivo. Oncogene. 1996;13(11):2387–2396. [PubMed] [Google Scholar]
- 5.Zabarovsky ER, Lerman MI, Minna JD. Chromosome 3 Abnormalities in lung cancer. In: Pass HI, Carbone DP, Johnson DH, Minna JD, Turrisi AT III, editors. Lung cancer: principles and practice. Philadelphia: Lippincott William & Wilkins; 2005. pp. 118–134. [Google Scholar]
- 6.Hesson LB, Cooper WN, Latif F. Evaluation of the 3p21.3 tumour-suppressor gene cluster. Oncogene. 2007;26(52):7283–7301. doi: 10.1038/sj.onc.1210547. [DOI] [PubMed] [Google Scholar]
- 7.Kok K, van den BA, Buchhagen DL, Carritt B, Buys CH. A PCR-aided transcript titration assay revealing very low expression of a gene at band 3p21 in 33 cells lines derived from all types of lung cancer. Eur J Hum Genet. 1993;1(2):156–163. doi: 10.1159/000472402. [DOI] [PubMed] [Google Scholar]
- 8.Kok K, Hofstra R, Pilz A, van den BA, Terpstra P, Buys CH, et al. A gene in the chromosomal region 3p21 with greatly reduced expression in lung cancer is similar to the gene for ubiquitin-activating enzyme. Proc Natl Acad Sci U S A. 1993;90(13):6071–6075. doi: 10.1073/pnas.90.13.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carritt B, Kok K, van den BA, Osinga J, Pilz A, Hofstra RM, et al. A gene from human chromosome region 3p21 with reduced expression in small cell lung cancer. Cancer Res. 1992;52(6):1536–1541. [PubMed] [Google Scholar]
- 10.Pitha-Rowe I, Petty WJ, Feng Q, Koza-Taylor PH, Dimattia DA, Pinder L, et al. Microarray analyses uncover UBE1L as a candidate target gene for lung cancer chemoprevention. Cancer Res. 2004;64(21):8109–8115. doi: 10.1158/0008-5472.CAN-03-3938. [DOI] [PubMed] [Google Scholar]
- 11.Dragnev KH, Feng Q, Ma Y, Shah SJ, Black C, Memoli V, et al. Uncovering novel targets for cancer chemoprevention. Recent Results Cancer Res. 2007;174:235–243. doi: 10.1007/978-3-540-37696-5_21. [DOI] [PubMed] [Google Scholar]
- 12.Dao CT, Zhang DE. ISG15: a ubiquitin-like enigma. Front Biosci. 2005;10:2701–2722. doi: 10.2741/1730. [DOI] [PubMed] [Google Scholar]
- 13.Kim KI, Yan M, Malakhova O, Luo JK, Shen MF, Zou W, et al. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol Cell Biol. 2006;26(2):472–479. doi: 10.1128/MCB.26.2.472-479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannakopoulos NV, Luo JK, Papov V, Zou W, Lenschow DJ, Jacobs BS, et al. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem Biophys Res Commun. 2005;336(2):496–506. doi: 10.1016/j.bbrc.2005.08.132. [DOI] [PubMed] [Google Scholar]
- 15.Malakhov MP, Kim KI, Malakhova OA, Jacobs BS, Borden EC, Zhang DE. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J Biol Chem. 2003;278(19):16608–16613. doi: 10.1074/jbc.M208435200. [DOI] [PubMed] [Google Scholar]
- 16.Wong JJ, Pung YF, Sze NS, Chin KC. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc Natl Acad Sci U S A. 2006;103(28):10735–10740. doi: 10.1073/pnas.0600397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci U S A. 2005;102(29):10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Der CJ, Krontiris TG, Cooper GM. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A. 1982;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 20.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 21.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 22.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410(6832):1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 23.Farr A, Nelson A, Truex J, Hosier S. Epithelial heterogeneity in the murine thymus: a cell surface glycoprotein expressed by subcapsular and medullary epithelium. J Histochem Cytochem. 1991;39(5):645–653. doi: 10.1177/39.5.2016514. [DOI] [PubMed] [Google Scholar]
- 24.Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer. 2006;6(10):776–788. doi: 10.1038/nrc1994. [DOI] [PubMed] [Google Scholar]
- 25.Watson IR, Irwin MS. Ubiquitin and ubiquitin-like modifications of the p53 family. Neoplasia. 2006;8(8):655–666. doi: 10.1593/neo.06439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menin C, Scaini MC, De Salvo GL, Biscuola M, Quaggio M, Esposito G, et al. Association between MDM2-SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J Natl Cancer Inst. 2006;98(4):285–288. doi: 10.1093/jnci/djj054. [DOI] [PubMed] [Google Scholar]
- 27.Lind H, Zienolddiny S, Ekstrom PO, Skaug V, Haugen A. Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int J Cancer. 2006;119(3):718–721. doi: 10.1002/ijc.21872. [DOI] [PubMed] [Google Scholar]
- 28.Mo YY, Yu Y, Theodosiou E, Rachel Ee PL, Beck WT. A role for Ubc9 in tumorigenesis. Oncogene. 2005;24(16):2677–2683. doi: 10.1038/sj.onc.1208210. [DOI] [PubMed] [Google Scholar]
- 29.Kitareewan S, Pitha-Rowe I, Sekula D, Lowrey CH, Nemeth MJ, Golub TR, et al. UBE1L is a retinoid target that triggers PML/RARalpha degradation and apoptosis in acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(6):3806–3811. doi: 10.1073/pnas.052011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitha-Rowe I, Hassel BA, Dmitrovsky E. Involvement of UBE1L in ISG15 conjugation during retinoid-induced differentiation of acute promyelocytic leukemia. J Biol Chem. 2004;279(18):18178–18187. doi: 10.1074/jbc.M309259200. [DOI] [PubMed] [Google Scholar]
- 31.Desai SD, Haas AL, Wood LM, Tsai YC, Pestka S, Rubin EH, et al. Elevated expression of ISG15 in tumor cells interferes with the ubiquitin/26S proteasome pathway. Cancer Res. 2006;66(2):921–928. doi: 10.1158/0008-5472.CAN-05-1123. [DOI] [PubMed] [Google Scholar]
- 32.Andersen JB, Aaboe M, Borden EC, Goloubeva OG, Hassel BA, Orntoft TF. Stage-associated overexpression of the ubiquitin-like protein, ISG15, in bladder cancer. Br J Cancer. 2006;94(10):1465–1471. doi: 10.1038/sj.bjc.6603099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrell PJ, Broeze RJ, Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979;279(5713):523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- 34.D’Cunha J, Knight E, Jr, Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci U S A. 1996;93(1):211–215. doi: 10.1073/pnas.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Cunha J, Ramanujam S, Wagner RJ, Witt PL, Knight E, Jr, Borden EC. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J Immunol. 1996;157(9):4100–4108. [PubMed] [Google Scholar]
- 36.Owhashi M, Taoka Y, Ishii K, Nakazawa S, Uemura H, Kambara H. Identification of a ubiquitin family protein as a novel neutrophil chemotactic factor. Biochem Biophys Res Commun. 2003;309(3):533–539. doi: 10.1016/j.bbrc.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Lenschow DJ, Giannakopoulos NV, Gunn LJ, Johnston C, O’Guin AK, Schmidt RE, et al. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol. 2005;79(22):13974–13983. doi: 10.1128/JVI.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci U S A. 2007;104(4):1371–1376. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28(2 Suppl 4):3–13. [PubMed] [Google Scholar]
- 40.Mills NE, Fishman CL, Rom WN, Dubin N, Jacobson DR. Increased prevalence of K-ras oncogene mutations in lung adenocarcinoma. Cancer Res. 1995;55(7):1444–1447. [PubMed] [Google Scholar]
- 41.Wakamatsu N, Devereux TR, Hong HH, Sills RC. Overview of the molecular carcinogenesis of mouse lung tumor models of human lung cancer. Toxicol Pathol. 2007;35(1):75–80. doi: 10.1080/01926230601059993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64(7):2307–2316. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]