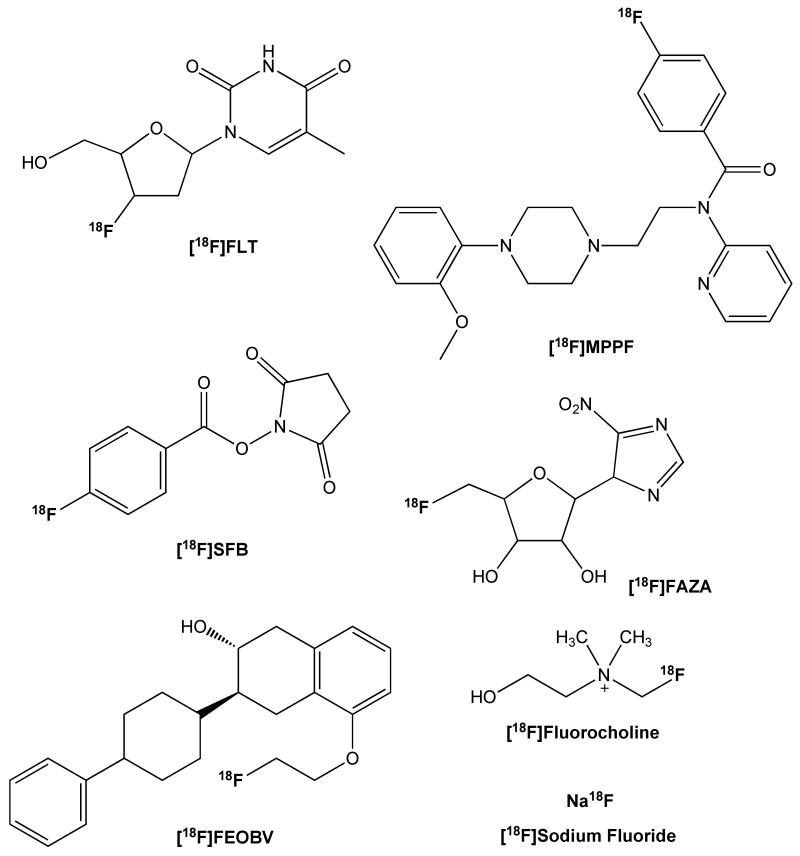
Abstract
The field of radiochemistry is moving towards exclusive use of automated synthesis modules for production of clinical radiopharmaceutical doses. Such a move comes with many advantages, but also presents radiochemists with the challenge of re-configuring synthesis modules for production of radiopharmaceuticals that require non-conventional radiochemistry whilst maintaining full automation. This review showcases the versatility of the Tracerlab FXFN synthesis module by presenting simple, fully automated methods for producing [18F]FLT, [18F]FAZA, [18F]MPPF, [18F]FEOBV, [18F]sodium fluoride, [18F]fluorocholine and [18F]SFB.
Keywords: positron emission tomography, automated radiopharmaceutical synthesis, fluorine-18, radiochemistry
1. Introduction
The field of radiochemistry is increasingly migrating towards the use of commercially available automated synthesis modules for production of clinical radiopharmaceutical doses. The advantages of such a strategy over more traditional ‘manual’ synthetic approaches are clear and include: (i) automation of radiochemical syntheses providing robust, repeatable processes; (ii) the ability to handle multiple Curies of radioactivity safely enabling PET Centers to produce and distribute hundreds of doses daily; (iii) facilitated regulatory compliance through manufacturer IQ/OQ/PQ and scheduled maintenance protocols performed on synthesis modules by authorized personnel; and (iv) improved radiation safety through elimination of manual operations. Despite these numerous significant advantages, the move towards exclusive use of automated synthesis modules also has some associated challenges. Chief amongst these is the issue of re-configuring synthesis modules for production of radiopharmaceuticals that require non-conventional radiochemistry (such radiochemical strategies have been the subject of several recent major review articles[1-10]) whilst maintaining full automation. Furthermore, what is the best method to simplify making such frequently non-trivial changes in module configuration so that multiple operators can make them with a minimal chance for error?
At the University of Michigan PET Center, fluorine-18 labeled radiopharmaceuticals (with the exception of [18F]FDG) are prepared using General Electric Medical Systems (GEMS) TracerLab FXFN automated synthesis modules. As the demand for clinical doses of different fluorine-18 labeled radiopharmaceuticals, many of which are prepared using complex radiochemical techniques, continues to increase, we[11-13] and others[14-22] have had to address the issue of frequent synthesis module reconfiguration. For example, recently we reported reconfiguration of a TracerLab FXC-Pro to allow preparation of multiple carbon-11 labeled radiopharmaceuticals without the need to open the hot-cell door between syntheses,[12] and modifications to a TracerLab FXFN enabling production of [18F]sodium fluoride to address the ongoing technetium-99m isotope shortage.[13]
In this overview we report configurational changes to a TracerLab FXFN that allow for straightforward switching between production of [18F]-labeled radiopharmaceuticals using routine methods and those prepared using less common radiochemical techniques (e.g. gas phase reactions). Proof-of-concept is demonstrated through the simple, fully automated, production of multiple different [18F]labeled radiopharmaceuticals using a single TracerLab FXFN (Figure 1).
Figure 1.
Fluorine-18 Labeled Radiopharmaceuticals
2. Automated Radiopharmaceutical Production using a Tracerlab FXFN
2.1 Introduction
2.1.1 General Modifications to the Tracerlab FXFN
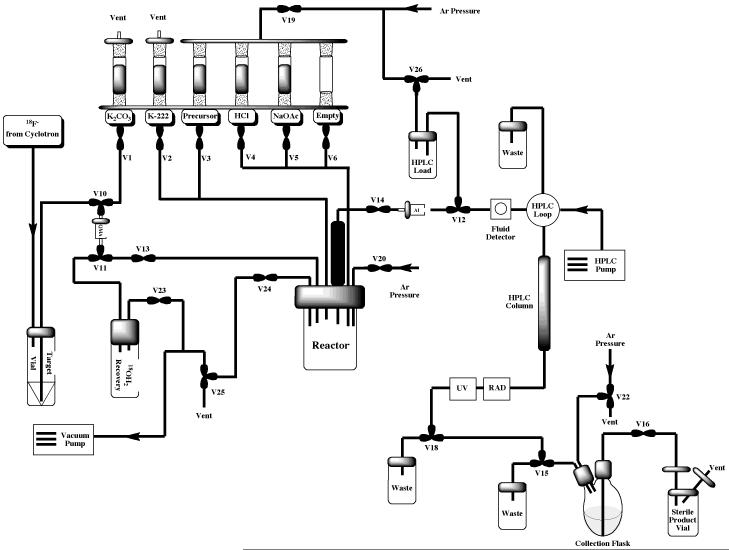
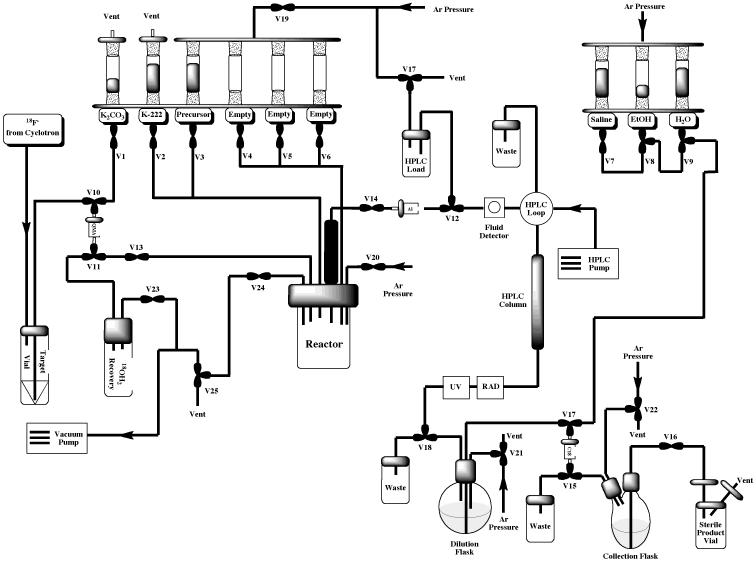
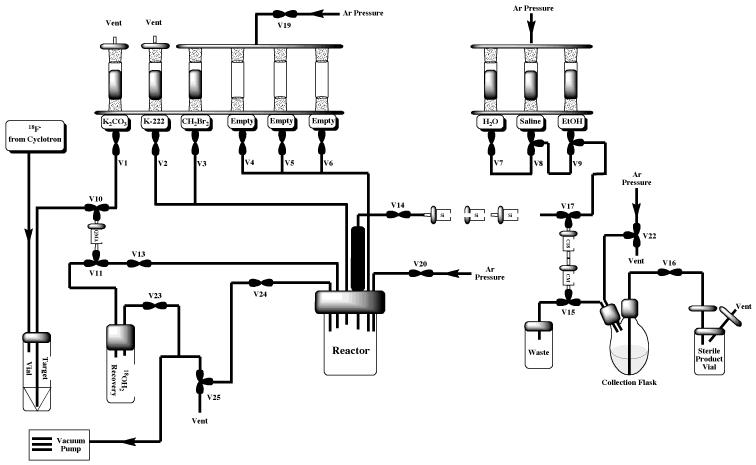
The Tracerlab FXFN synthesis module is installed “out-of-the-box” in its most basic configuration illustrated in Figure 3. This configuration is suitable for straightforward production of radiopharmaceuticals requiring HPLC purification (e.g. FLT or FAZA, discussed in Section 2.2) and can easily be reconfigured to incorporate a reformulation (e.g. for production of MPPF or FEOBV, discussed in Section 2.3 and illustrated in Figure 7). However, with some simple modifications the TracerLab FXFN can also be used to produce radiopharmaceuticals using other strategies such as the gas phase reactions outlined elsewhere in this article.
Figure 3.
Standard HPLC Tracerlab FXFN Configuration for Production of [18F]FLT or [18F]FAZA
Figure 7.
Standard HPLC / Reformulation Tracerlab FXFN Configuration for Production of [18F]MPPF or [18F]FEOBV
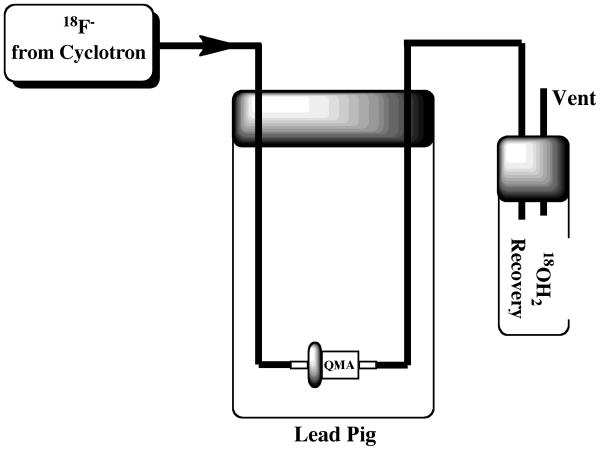
The first modification involves the delivery line from the cyclotron target. This line was originally just pushed through a hole in the septum of the target vial. However, if this line is clipped in half and the two parts re-connected using luer lock fittings, then target delivery can easily be switched between the target vial and, for example, the round-bottom dilution flask (necessary for production of [18F]sodium fluoride as discussed in Section 2.4) or directly on to a QMA Sep-Pak, with minimal risk of damaging the delivery line. The latter approach of trapping a target dump directly onto a QMA Sep-Pak has been adapted by our group for straightforward and safe transportation of fluoride to satellite PET centers. The QMA Sep-Pak is housed in a lead pig prior to fluoride-18 delivery (Figure 2), and the luer lock fitting on the cyclotron delivery line is connected to the lines of the QMA delivery system. After fluoride delivery, the delivery line can easily be disconnected and the pig safely transferred to a DOT approved Type A container for transport.
Figure 2.
Set-up for Fluoride-18 Distribution
The second modification to the Tracerlab FXFN is similar, and involves incorporating luer lock fittings into both the line connecting V-18 to the round-bottom dilution flask and also the line connecting V-17 to the round-bottom dilution flask. These fittings are essential for production of [18F]sodium fluoride, [18F]fluorocholine and [18F]SFB, as discussed in Section 2.4. Reflecting the fact that these modifications are only to tubing and fittings, defined as consumables and customer responsible maintenance items by GEMS, they do not invalidate the manufacturer IQ/OQ. In order to verify that the alternative synthesis module configurations are suitable, three process verification (qualification) runs are completed for each radiopharmaceutical prior to preparation of clinical doses.
2.1.2 General Synthesis Considerations
Unless otherwise stated, reagents and solvents were commercially available and used without further purification: sodium chloride, 0.9% USP and Sterile Water for Injection, USP were purchased from Hospira; ethanol was purchased from American Regent; anhydrous acetonitrile, potassium carbonate, sodium acetate, hydrochloric acid, sodium dihydrogenphosphate, dribromomethane, acetic acid, DMSO, DIPEA, tetrapropylammonium hydroxide and TSTU were purchased from Sigma Aldrich; kryptofix-2.2.2 was purchased from Acros Organics and HPLC grade acetonitrile was purchased from Fisher Scientific.
Precursors and standards were commercially available unless otherwise indicated. FLT (standard and DMT-Boc-Nosyl / cyclic precursors), MPPF (standard and precursor), fluorocholine (standard) and SFB (standard and precursor) were purchased from ABX Advanced Biochemicals. Sodium fluoride and dimethylamino ethanol (fluorocholine precursor) were purchased from Sigma Aldrich. Boc-Boc-Nosyl FLT precursor is the intellectual property of Siemens Molecular Imaging and Biomarker Research. [18F]FAZA precursor and unlabeled reference standard were purchased from Prof. Friedrich Hammerschmidt (Universität Wien, Austria) and Prof. Hans-Jürgen Machulla (Steinbeis Transfer Center Radiopharmacy, Germany), and used as received. [18F]FEOBV standard and precursor were synthesized in house.
Other synthesis components were obtained as follows: sterile filters were obtained from Millipore; sterile product vials were purchased from Hollister-Stier; [18O]H2O was purchased from ABX Advanced Biochemical Compounds; silica, plus-CM light, alumina-light, C18-light and QMA-light Sep-Paks were purchased from Waters Corporation. Plus-CM light, alumina-light, C18-light and QMA-light Sep-Pak’s were flushed with 10 mL of ethanol followed by 10 mL of sterile water prior to use. Silica Sep-Paks were used for preparation of [18F]fluorocholine without pre-conditioning.
2.1.3 General procedure for Azeotropic Drying of [18F]Fluoride
Fluoride-18 at the University of Michigan was produced via the 18O(p,n)18F nuclear reaction using a GEMS PETTrace cyclotron equipped with a high yield fluorine-18 target. Fluoride-18 was delivered from the cyclotron (in a 1.5 mL bolus of [18O]H2O) and trapped on a QMA-light Sep-Pak to remove [18O]H2O. Fluoride-18 was then typically eluted into the reaction vessel using aqueous potassium carbonate (3.0 – 3.5 mg in 0.4 – 0.5 mL water). A solution of kryptofix-2.2.2 (15 – 20 mg in 1 mL of acetonitrile) was then added to the reaction vessel and the fluoride-18 was dried by evaporating the water - acetonitrile azeotrope. Evaporation of the azeotrope was achieved by heating the reaction vessel to 80 °C and drawing full vacuum for 4 min. After this time, the reaction vessel was cooled to 60 °C and subjected to both an argon stream and the vacuum simultaneously for an additional 4 min.
2.2 Tracerlab Production with HPLC Purification
2.2.1 Production of [18F]FLT
Introduction
3′-Deoxy-3′-[18F]-fluorothymidine ([18F]FLT) is a PET tracer based upon the DNA base thymidine. FLT does not get incorporated into DNA directly, but is similar enough to thymidine that it gets phosphorylated by the same enzyme (thymidine kinase-1) and trapped in the cell. Therefore, it can be used to image tumor cell proliferation. FLT was originally reported by Shields et al. in 1998[23] and, after extensive development over the last decade, is now in global clinical trials. For example, FLT is in Phase 2/3 clinical trials in the United States and the Society of Nuclear Medicine (SNM) has a multi-center Investigational New Drug (IND) application approved by the U. S. Food and Drug Administration (FDA). These factors all indicate that FLT is en route to becoming a standard of care PET biomarker for oncologists and to date it has been used to effectively diagnose and monitor many different cancers including brain, lung, breast and esophageal tumors. A Scifinder search for [18F]FLT returns over 200 articles and referencing all of them is beyond the scope of this paper. However, the extensive use of [18F]FLT in oncological PET imaging has been recently reviewed by Been and colleagues.[24]
A number of synthetic routes and precursors have been reported for the production of [18F]FLT with varying degrees of success.[17, 25-29] The [18F]FLT production method disclosed herein has been tested using the Boc-Boc-Nosyl, DMT-Boc-Nosyl and 2,3′-anhydro-cyclic precursors and all can be used to prepare [18F]FLT. We have found the Boc-Boc-Nosyl precursor, reported by Walsh and Padgett,[25] very effective because it provides [18F]FLT in high yields (23% non-decay corrected) and eliminates purification and QC issues associated with DMT contaminants, although to the best of our knowledge this precursor is not yet commercially available. Therefore, in addition to the Boc-Boc-Nosyl method, a synthetic strategy using the standard commercially available 2,3′-anhydro-cyclic precursor is also reported herein.
Synthesis Procedures
Method A: Boc-Boc-Nosyl Precursor
To prepare FLT using Method A, the Tracerlab synthesis module was used in the original “out-of-the-box” configuration illustrated in Figure 3 and reagent vials were loaded as follows: Vial 1: potassium carbonate (3.0 mg in 0.4 mL water); Vial 2: kryptofix-2.2.2 (20 mg in 1 mL MeCN); Vial 3: Boc-Boc-Nosyl FLT precursor (17.5 mg in 1.0 mL MeCN); Vial 4: HCl (1M, 1.0 mL); Vial 5: Aq. NaOAc (2M, 0.5 mL).
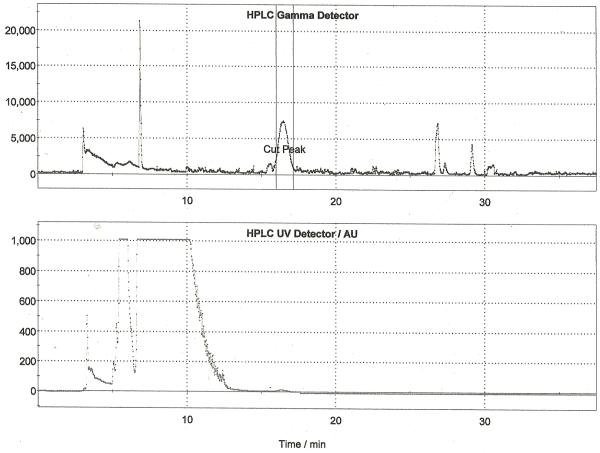
Following drying of the fluoride using the general procedure, Boc-Boc-Nosyl FLT precursor (17.5 mg in 1 mL MeCN) was added and the reaction vessel was heated at 135 °C for 3 min. The acetonitrile reaction solvent was then evaporated and 1M HCl (1.0 mL) was added to remove the Boc groups. The deprotection was heated at 110°C for 5 min after which time the reaction vessel was cooled to room temperature and neutralized with 2M NaOAc (0.5 mL). The crude reaction mixture was diluted, passed through an alumina Sep-Pak to remove unreacted fluoride-18 and purified by semi-preparative HPLC (column: Phenomonex Synergi Hydro-RP, 250 × 10 mm; mobile phase: 8% EtOH: 92% 21mM sodium phosphate (v/v); flow rate: 5.5 mL/min). A typical trace is shown in Figure 4. The peak corresponding to [18F]FLT was collected into the collection vial of the TracerLab, from which it was then passed through an alumina Sep-Pak and sterile filter into a sterile dose vial. Doses were released for quality control testing as described in Section 3, and the yield of [18F]FLT using this method is 23% (non-decay corrected, n = 5).
Figure 4.
Semi-preparative HPLC Trace for [18F]FLT (Method A)
Method B: 2,3′-Anhydro-cyclic Precursor
To prepare FLT using Method B, the Tracerlab synthesis module was used in the original “out-of-the-box” configuration illustrated in Figure 3 and reagent vials were loaded as follows: Vial 1: potassium carbonate (3.5 mg in 0.5 mL water); Vial 2: kryptofix-2.2.2 (15 mg in 1 mL MeCN); Vial 3: 2,3′-anhydro-cyclic precursor (10 mg in 1.0 mL DMSO); Vial 4: NaOH (0.25M, 0.35 mL); Vial 5: aq. sodium dihydrogen phosphate (0.25M, 0.75 mL); Vial 6: semi-preparative HPLC mobile phase (5% EtOH: 95% 10mM sodium dihydrogenphosphate, 1.75 mL).
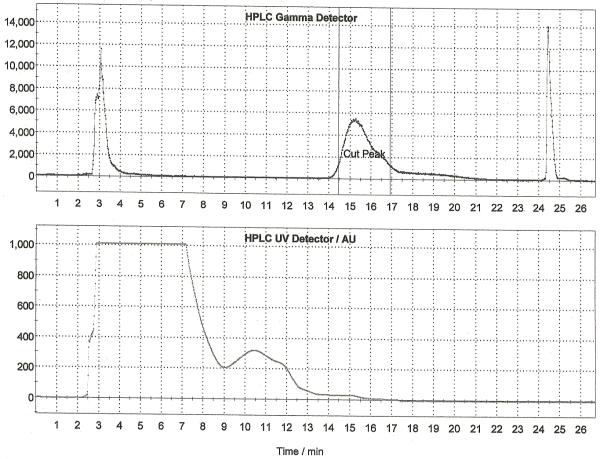
Following drying of the fluoride using the general procedure, 2,3′-anhydro-cyclic FLT precursor (10 mg in 1 mL DMSO) was added and the reaction vessel was heated at 160 °C for 10 min. After this time the reaction was cooled to 50 °C and 0.25M HCl (0.35 mL) was added for deprotection. The deprotection was heated at 50 °C for 4 min after which time the reaction was neutralized with 0.25 M aq. sodium dihydrogen phosphate (0.75 mL). The crude reaction mixture was diluted with 1.75 mL of HPLC mobile phase, passed through an alumina Sep-Pak to remove unreacted fluoride-18 and purified by semi-preparative HPLC (column: Alltech Econosphere C18 10μ, 250 × 10 mm; mobile phase: 5% EtOH : 95% 10mM sodium dihydrogenphosphate (v/v); flow rate: 5.0 mL/min). A representative HPLC trace is illustrated in Figure 5. The peak corresponding to [18F]FLT was collected into the collection vial of the TracerLab, from which it was then passed through a sterile Millipore Millex-GS filter into a sterile dose vial. Doses were released for quality control testing as described in Section 3, and the yield of [18F]FLT using this method is 4.2% (non-decay corrected, n = 25).
Figure 5.
Semi-preparative HPLC Trace for [18F]FLT (Method B)
2.2.2 Production of [18F]FAZA
Introduction
Tumor hypoxia results when tumor cells have been deprived of oxygen, frequently occuring when a tumor has outgrown its blood supply. It is important to determine if a tumor is hypoxic as soon as possible, because such tumors are typically resistant to radiotherapy and chemotherapy and require alternative treatment strategies. 2-Nitroimidazoles are an important class of compound known to be retained in higher levels in hypoxic tumor cells than the corresponding normoxic cells. The reason for the increased uptake is that in a hypoxic environment 2-nitroimidazoles undergo intracellular reduction which leads to fragmentation into species which are unable to pass back out of the cell. An analogous process is also known to occur in myochardial ischemia. 2-Nitroimidazoles have therefore been adapted for use in molecular imaging of hypoxia. For example, radioactive 2-nitroimidazole derivatives have been prepared, such as [18F]-fluoroazomycin arabinoside ([18F]FAZA),[30-35] that are finding widespread use in diagnosis of tumor hypoxia and monitroring subsequent response to therapy.
Synthesis Procedures
To prepare FAZA, the Tracerlab synthesis module was used in the original “out-of-the-box” configuration illustrated in Figure 3 and reagent vials were loaded as follows: Vial 1: potassium carbonate (3.5 mg in 0.5 mL water); Vial 2: kryptofix-2.2.2 (15 mg in 1 mL MeCN); Vial 3: FAZA precursor (5 mg in 0.7 mL DMSO); Vial 4: NaOH (0.1M, 1.0 mL); Vial 5: aq. sodium dihydrogen phosphate (0.25M, 0.5 mL); Vial 6: semi-preparative HPLC mobile phase (3% EtOH: 97% 10mM sodium dihydrogenphosphate, 1.0 mL).
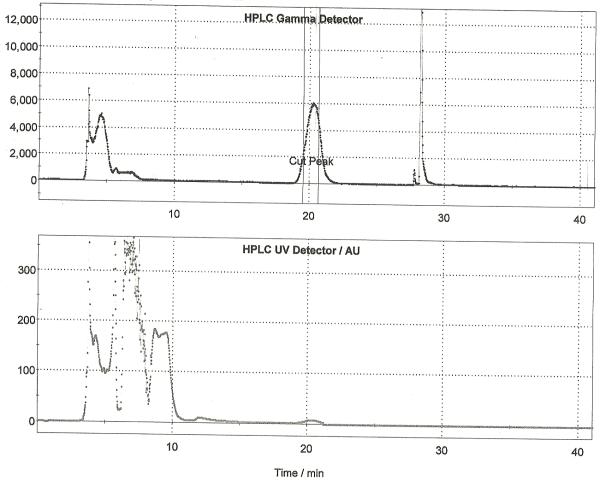
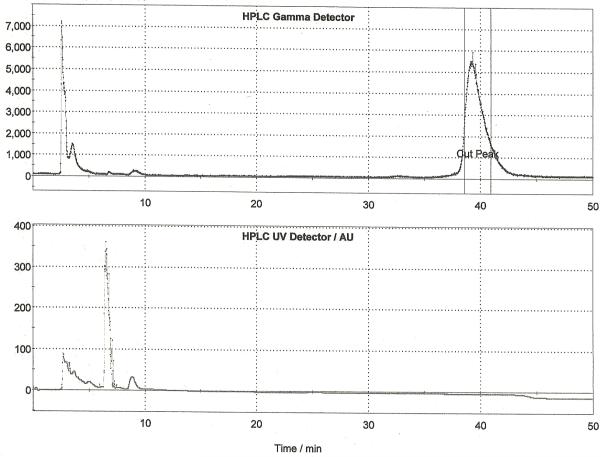
Following drying of the fluoride using the general procedure, a solution of [18F]FAZA precursor (5 mg) in anhydrous DMSO (0.7 mL) was added to the dried [18F]fluoride and the reaction was heated to 100 °C with stirring for 10 min. After this time, the reaction was cooled to 40 °C and 0.1 M aqueous sodium hydroxide (1 mL) was added. The reaction was stirred for 5 min at 40 °C to hydrolyze the acetate protecting groups. After hydrolysis, the crude reaction mixture was diluted with 0.25 M aqueous sodium dihydrogen phosphate solution (0.5 mL) and HPLC mobile phase (1 mL). The diluted reaction mixture was passed through an alumina Sep-P ak cartridge and then purified by semi-preparative HPLC (column: Alltech Econsosphere C18 10μ, 250 × 10 mm; mobile phase: 3% ethanol : 97% 10 mM sodium dihydrogenphosphate (v/v); flow rate: 4 mL/min). The fraction corresponding to [18F]FAZA (typically eluting between 20 and 23 minutes as shown in Figure 6) was collected for 1 minute into a vial pre-charged with 0.9% sodium chloride, USP (3.8 mL) and sodium phosphates, USP (0.2 mL). The final formulation (8 mL) was then passed through a sterile filter into a sterile vial to provide [18F]FAZA in an isotonic solution released for quality control testing as outlined in Section 3. Typical yield of [18F]FAZA prepared using this method is 4.8% (non-decay corrected, n = 25).
Figure 6.
Semi-preparative HPLC Trace for [18F]FAZA
2.3 Tracerlab Production with HPLC Purification and SPE Reformulation
2.3.1 Production of [18F]MPPF
Introduction
The serotonin (5-hydroxytryptamine, 5-HT) receptors are involved in a range of CNS functions but conversely have also been implicated in a range of psychiatric disorders such as depression, epilepsy, anxiety, Alzheimer’s disease and schizophrenia. Therefore, PET radiopharmacueticals capable of monitoring 5-HT receptor activity in the brain have potential for diagnosing and monitoring response to treatment, a concept recently reviewed by Pike.[36] Indeed, [carbonyl-11C]WAY-100635 has been used routinely to detect 5-HT1A receptors for many years now. However, the synthesis of [carbonyl-11C]WAY-100635 involves a Grignard reagent and, despite the efforts of many groups[37-40] and recent improvements,[41] in our hands production has always proven somewhat unreliable. Therefore, we wished to make an alternative radiopharmaceutical available to those physicians wishing to quantify 5-HT1A receptor activity, with the aim ultimately of phasing out production of [carbonyl-11C]WAY-100635. With this goal in mind, we have just completed site qualification for clinical production of [18F]MPPF, a fluorinated analog of WAY, at the University of Michigan.
[18F]MPPF has been shown to have very high selectivity for 5-HT1A receptors (Ki = 3.3 ± 0.8 nM) and its use in both clinical and pre-clinical PET imaging of 5-HT1A receptors has been recently reviewed.[42] Originally, the iodinated derivative ([125I]MPPI) was reported in 1994 by Kung and co-workers[43] and subsequently, in 1997, they also prepared the fluorinated analog ([18F]MPPF).[44] In contrast to WAY, [18F]MPPF can simply be prepared by nucleophilic fluorination, HPLC purification and subsequent reformulation – such conditions have been optimized by Le Bars et al.[45]
Synthesis Procedures
To prepare [18F]MPPF, the Tracerlab FXFN was configured as shown in Figure 7, incorporating the dilution flask for the reformulation process, and reagent vials were loaded as follows: Vial 1: potassium carbonate (3.5 mg in 0.5 mL water); Vial 2: kryptofix-2.2.2 (15 mg in 1 mL MeCN); Vial 3: MPPF precursor (10 mg in 0.5 mL DMSO); Vial 6: HPLC mobile phase (3.0 mL), Vial 7: 0.9% sodium chloride for injection, USP (9.5 mL); Vial 8: ethanol (0.5 mL); Vial 9: sterile water for injection, USP (12 mL); round-bottomed flask: water (50 mL).
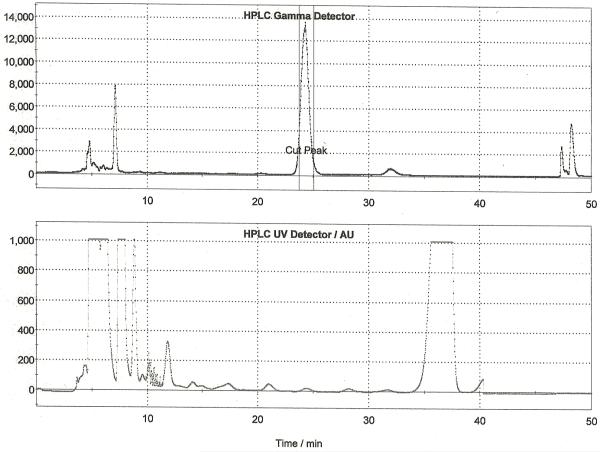
Following drying of the fluoride using the general procedure, MPPF precursor (10 mg in 0.5 mL DMSO) was then added and the reaction vessel was heated at 140 °C for 20 min. After this time, the reaction vessel was cooled to room temperature and the crude reaction mixture was diluted, passed through an alumina Sep-Pak and purified by semi-preparative HPLC (column: Phenomonex Luna C18, 250 × 10 mm; mobile phase: 55% 50 mM NaOAc (pH = 5) : 18% THF : 27% MeOH (v/v); flow rate: 5 mL/min). A representative HPLC trace is shown in Figure 8. The fraction corresponding to [18F]MPPF was collected into the round-bottom dilution flask and simultaneously diluted into sterile water. This solution was then passed through a Waters SDB-XC Sep-Pak to trap the [18F]MPPF whilst washing away acetonitrile left over from the HPLC buffer. Following trapping, the cartridge was rinsed with sterile water (12 mL). [18F]MPPF was then eluted into the collection vial with ethanol (0.5 mL) and diluted with sterile saline (9.5 mL). The dose was then passed through a sterile filter into a sterile dose vial and released for QC testing as per Section 3. Typical yields of [18F]MPPF using this method were 8.8% (non-decay corrected, n = 28).
Figure 8.
Semi-preparative HPLC Trace for [18F]MPPF
2.3.2 Production of [18F]FEOBV
Introduction
Loss of cholinergic neurons has been documented in a number of prevalent neurodegenerative diseases such as Alzheimer’s Disease and the parkinsonian dementias.[46-49] Furthermore, cholinergic function has been shown to correlate with cognitive function parameters.[50, 51] There has therefore been great interest in developing imaging agents to reliably quantify cholinergic function in vivo. One such approach targets the vesicular ACh transporter (VAChT) in cholinergic terminals with radiolabeled forms of the drug vesamicol.[52] A mounting body of evidence points to VAChT as being a very specific marker of cholinergic terminals, spurring a search for suitable radioligands for measuring VAChT in vivo. With this goal in mind, we have studied a class of analogs called benzovesamicols, discovered by Rogers et al.,[53] and identified a number of novel radiolabeled benzovesamicol analogs which distribute in vivo in patterns that correlate closely with the densities of marker proteins of the cholinergic nerve terminal.[54] Analogs with radioiodine, carbon-11, or fluorine-18 labels at the 5 position of the 2-hydroxy-3-N-(4-phenyl)-piperidino-tetralin frame-work, which we term 5-substituted-benzovesamicols, have shown particularly favorable imaging properties. Human SPECT imaging studies with the [123I]labeled benzovesamicol analogue [123I]IBVM show decreases in tracer binding in several brain regions of AD patients.[55, 56] These studies offer encouragement that appropriate benzovesamicol tracers can map cholinergic neurons and gauge the functional integrity of cholinergic synapses in human subjects.
With the aim of extending human benzovesamicol imaging studies to a higher level of precision with PET, we have developed the novel fluorine-18 labeled agent, 5-fluoroethoxy-benzovesamicol ([18F]-(−)FEOBV). We have conducted extensive pre-clinical evaluation of [18F]FEOBV,[57, 58] and the results have been extremely promising. Therefore we have recently submitted an exploratory investigational new drug (IND) application to the U. S. FDA to permit introduction of [18F]FEOBV as an investigational cholinergic synaptic tracer for human PET imaging studies.
Synthesis Procedures
To prepare [18F]FEOBV, the Tracerlab FXFN was configured as shown in Figure 7, incorporating the dilution flask for the reformulation process, and reagent vials were loaded as follows: Vial 1: potassium carbonate (3.5 mg in 0.5 mL water); Vial 2: kryptofix-2.2.2 (15 mg in 1 mL MeCN); Vial 3: FEOBV precursor (0.2 – 0.5 mg in 0.5 mL DMSO); Vial 6: sterile water (2.0 mL) / acetonitrile (1.5 mL); Vial 7: 0.9% sodium chloride for injection, USP (9.5 mL); Vial 8: ethanol (0.5 mL); Vial 9: sterile water for injection, USP (10 mL); round-bottomed dilution flask: sterile water (40 mL).
Following drying of the fluoride using the general procedure FEOBV precursor (0.2 – 0.5 mg) dissolved in anhydrous DMSO (0.5 mL) was then added to the reactor vessel. The reaction mixture was then heated at 120 °C for 10 min and then cooled to 50 °C. The resulting mixture was diluted with 3.5 mL of an aqueous acetonitrile solution. The diluted reaction mixture was passed through a Sep-Pak Light Alumina-N, and loaded onto a semi-preparative HPLC (column: Phenomenex Synergi Polar-RP, 10 × 250mm; mobile phase: 45% MeCN : 55% aqueous 50mM ammonium acetate; flow rate = 4 mL/min). The single peak corresponding to [18F]FEOBV eluted between 30 and 40 min (Figure 9). That product fraction was collected, diluted into 40 mL of sterile water, and passed through a C-18 Sep-Pak which was then washed with 10mL of sterile water. [18F]FEOBV was then eluted with 0.5mL of EtOH (USP for injection) and collected in the Tracerlab FX-FN product vial. The Sep-Pak was washed with 9.5mL of saline to bring the final formulation volume to 10 mL. The final drug product was dispensed into a septum sealed, sterile, pyrogen-free glass vial through a 0.22 μm sterile filter and submitted for QC testing as outlined in Section 3. Typical yields of [18F]FEOBV using this method were 9.4% (non-decay corrected, n = 10).
Figure 9.
Semi-preparative HPLC Trace for [18F]FEOBV
2.4 Tracerlab Production with SPE Purification
2.4.1 Production of [18F]Sodium Fluoride
Introduction
Globally, about 70,000 nuclear medicine diagnostic imaging procedures, including bone imaging, are performed using technetium-99m every day.[59] Technetium-99m is obtained as the decay product from molybdenum-99, but despite this demand, only six nuclear reactors worldwide are capable of producing molybdenum-99. During 2009, the Chalk River nuclear reactor in Canada has been shutdown for unscheduled repairs leaving thousands of hospitals in North America without a supply of technetium-99m and a crisis in the molecular imaging community.[59, 60]
Consequently, alternatives to technetium-99m scans are in high demand and [18F]sodium fluoride represents an easily accessible positron emission tomography (PET) imaging alternative to technetium-99m bone imaging agents.[61, 62] In an effort to address the worldwide shortage of technetium-99m, the U.S. Centers for Medicare and Medicaid Services (CMS) are investigating the effectiveness of [18F]sodium fluoride as an imaging agent for bone metastasis (to monitor the spread of, for example, breast cancer to the bone) and is re-evaluating their non-coverage policy. In 2010 they agreed limited reimbursement for those patients on a clinical trial and therefore, PET centers worldwide have an immediate and urgent need for easy access to [18F]sodium fluoride suitable for clinical use.
Synthesis Procedures
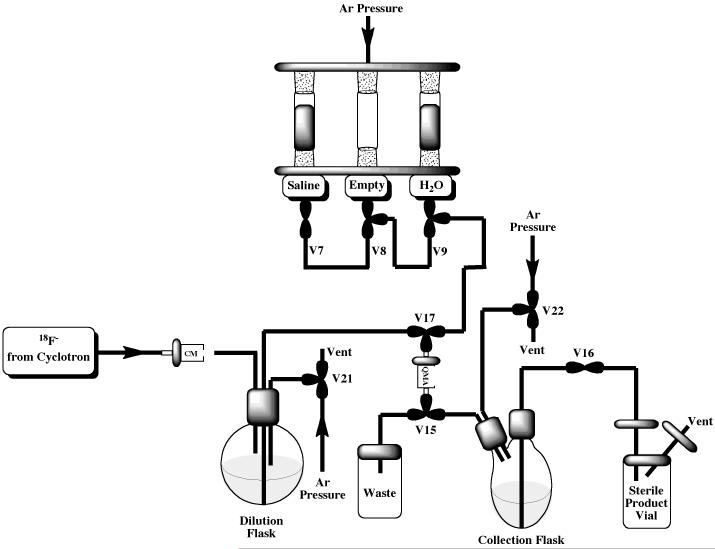
We recently reported a preparation of [18F]sodium fluoride,[13] in which a Tracerlab FXFN was reconfigured to allow full automation of the method for producing sodium fluoride reported by Nandy and co-workers.[63] The Tracerlab was configured as shown in Figure 10 and vials were loaded as follows: vial 7: 0.9% sodium chloride, USP for injection (10 mL); vial 9: sterile water for injection, USP (10 mL); dilution flask sterile water for injection, USP (10 mL). The delivery line from the cyclotron was connected to the dilution flask via plastic luer lock fittings which enable easy switching between delivery of fluoride to the target vial for a routine synthesis, and delivery to the dilution flask for preparation of [18F]sodium fluoride. Furthermore, these fittings allow easy incorporation of Sep-Paks into the target delivery line.
Figure 10.
Modified Tracerlab FXFN Configuration for [18F]Sodium Fluoride Production
A QMA-light Sep-Pak, pre-conditioned by flushing with ethanol (10 mL) followed by Sterile Water for Injection, USP (10 mL), was placed in the C18 cradle (between V15 and V17), a Plus-CM cation exchange cartridge was incorporated into the target delivery line and a sterile product vial with appropriate sterile filters was attached to the product delivery line. Note that since [18F]sodium fluoride is prepared using the reformulation portion of the Tracerlab FXFN synthesis module, a feature that is common to other Tracerlab modules including the Tracerlab FXc-pro, this method can easily be transferred to other synthesis modules.
Fluoride-18 was delivered to the dilution flask through an in-line Plus-CM cation exchange cartridge (to remove positively charged recoil nuclei generated concomitantly with fluoride-18 in the cyclotron target). Following completion of the cyclotron delivery, the solution of fluoride-18 was then transferred (Ar pressure) through the QMA-light Sep-Pak, trapping the fluoride-18 and passing the water to waste. Following trapping, the QMA-light Sep-Pak was then washed with an additional 10 mL of Sterile Water for Injection, USP to remove residual [18O]H2O. The fluoride-18 was then eluted into the Tracerlab collection vial using 0.9% Sodium Chloride (10 mL) with concomitant generation of [18F]sodium fluoride. The 10 mL dose was passed through a sterile Millipore Millex-GS filter into a sterile dose vial and submitted for quality control testing (see experimental section). Typical yields of [18F]sodium fluoride were >95% (non-decay corrected, n = 10).
2.4.2 Production of [18F]Fluorocholine
Introduction
Choline is a salt that is found in all cells where it is essential for the biosynthesis of phospholipids.[64] Such phospholipids are then incorporated into cell membranes and therefore radiolabeled analogs of choline can be used to monitor the rate of production of cell membranes in vivo, and thus rate of cell proliferation. Like [18F]FLT, radiolableled choline derivatives find widespread use in clinical PET imaging of tumors and [11C]choline has been used as a biomarker to image human brain, lung and prostrate tumors.[65, 66]
However, as the field of PET imaging expands and demand for PET tracers at hospitals not possessing a cyclotron continues to grow, there is a concerted effort towards developing the corresponding fluorine-18 labeled analogs of high demand carbon-11 labeled radiopharmaceuticals. The favorable half-life of fluorine-18 (110 min), when compared to that of carbon-11 (20 min), facilitates distribution of radiopharmaceuticals from radiochemistry production facilities to satellite PET centers that do not possess cyclotrons. With this in mind, in 2001 DeGrado and co-workers reported a synthesis of [18F]fluorocholine from the reactive intermediate [18F]fluorobromomethane.[67, 68] Subsequently, others have also prepared [18F]fluorocholine from [18F]fluoromethyl triflate.[69] DeGrado’s method has also been adapted for SPE purification, allowing automated production of [18F]fluorocholine on a TracerLab MXFDG, by Kryza et al.[15]
Since the demand for [18F]fluorocholine from our PET center has increased heavily of late, we decided to adapt Kryza’s method for use on a TracerLab FXFN, and herein report the first results from these research efforts.
Synthesis Procedures
The TracerLab FXFN was configured as shown in Figure 11 and the reagent vials were loaded as follows: Vial 1: potassium carbonate (3.5 mg in 0.5 mL water); Vial 2: kryptofix-2.2.2 (15 mg in 1 mL MeCN); Vial 3: dibromomethane (300 μL in 0.7 mL MeCN); Vial 7: sterile water for injection, USP (15 mL); Vial 8: 0.9% sodium chloride for injection, USP (3.0 mL); Vial 9: ethanol (10.0 mL); C-18 Sep-Pak light: pre-charged with N,N-dimethylamino ethanol (1 mL).
Figure 11.
Modified Tracerlab FXFN Configuration for [18F]Fluorocholine Production
The front end of the synthesis module was configured as for a typical radiofluorination reaction. The line from the reactor needle out through V14 (normally connected to an alumina cartridge as shown in Figures 3 and 4) was connected to 3 x silica Sep-Pak cartridges connected in series. The third silica cartridge was then connected (by luer fitting) to V17. N,N-Dimethylamino ethanol (400 μL) was loaded onto a C18 Sep-Pak and then this was stacked on top of a CM-plus Sep-Pak in the Tracerlab FXFN C18 cradle.
Following drying of the fluoride using the general procedure, dibromomethane precursor was added to the reactor and the reaction was heated to 95 °C for 5 min. After this time, the reactor was cooled (40 °C), and the [18F]fluorobromomethane was distilled through 3 x silica Sep-Paks, C18 Sep-Pak and, finally, the CM Sep-Pak to waste. The argon supply to the TracerLab FXFN synthesis module is fixed at 40 psi and this was used to transfer the crude reaction mixture. 3 x silica Sep-Paks removed any unreacted dibromomethane (b.p. = 97 °C) and aceteonitrile (b.p. = 82 °C) that, despite their higher boiling points, may otherwise have distilled over. Non-volatile components, including kryptofix-2.2.2 and potassium carbonate, remained in the reaction vessel. In contrast, the volatility of [18F]fluorobromomethane (b.p. = 9 °C) allowed pseudo-distillation over to valve-17 where it was trapped on the C18 cartridge and concomitantly reacted with DMAE (400 μL) to generate [18F]fluoromethylcholine. After 10 mins of distillation, the C18 Sep-Pak cartridge was washed with ethanol to elute the [18F]fluoromethylcholine as well as unreacted [18F]fluorobromomethane and DMAE. As [18F]fluoromethylcholine is positively charged it was trapped on the CM-Plus cation exchange resin, whilst the uncharged precursor species were washed to waste. The CM was then washed with Sterile Water for Injection, USP to remove any residual solvents and by-products. Finally, [18F]fluoromethylcholine was eluted from the CM-Plus Sep-Pak into the collection flask with sterile saline (3 mL). The dose was passed through a sterile filter into a sterile dose vial and released for quality control testing (see Section 3). Typical non-decay corrected yields of [18F]fluoromethylcholine using this optimized production method were 5%, (n = 7). Doses were free of residual solvents, kryptofix-2.2.2 and dibromomethane, but were found to contain residual DMAE (100-1000 μg/mL). The radiochemical yield of fluorocholine, and levels of residual DMAE, were similar to that reported by Kryza and co-workers,[15] and proved acceptable for our current preclinical demands. Nevertheless, optimization of this chemistry, particularly exploiting recent improvements reported by Slaets and colleagues,[70] is ongoing in our laboratory.
2.4.3 Production of [18F]SFB
Introduction
Labeling of large bioactive molecules such as peptides, proteins and antibodies with fluorine-18 typically requires prior formation of a smaller labeled prosthetic group. A number of strategies have been reported which allow such labeling including alkylation, acylation, amidation and radio-click chemistry. Of these approaches, acylation with N-succinimidyl-4-[18F]fluorobenzoate ([18F]SFB) is the workhorse, routinely providing fluorine-18 labeled bioactive molecules in high radiochemical yield. Until recently however, production of [18F]SFB was not trivial and required a three-step radiochemical synthesis which used multiple reaction vessels. Typically this is done manually,[71] but the process has also been automated,[16, 72] although successful automation does require access to synthesis modules possessing multiple reaction vessels. A breakthrough came when Kabalka and co-workers reported a one-pot synthesis of [18F]SFB requiring only Sep-Pak purification.[73] We recently reported a fully automated synthesis of [18F]SFB, adapted from Kabalka’s original report and exploiting the spectacular chemistry originally reported by Haka and co-workers,[74] using a Tracerlab FXFN synthesis module.[11] Concurrent with our efforts in this area, Tang and co-workers reported a related automated synthesis of [18F]SFB, also employing a Tracerlab FXFN.[22]
Synthesis Procedures
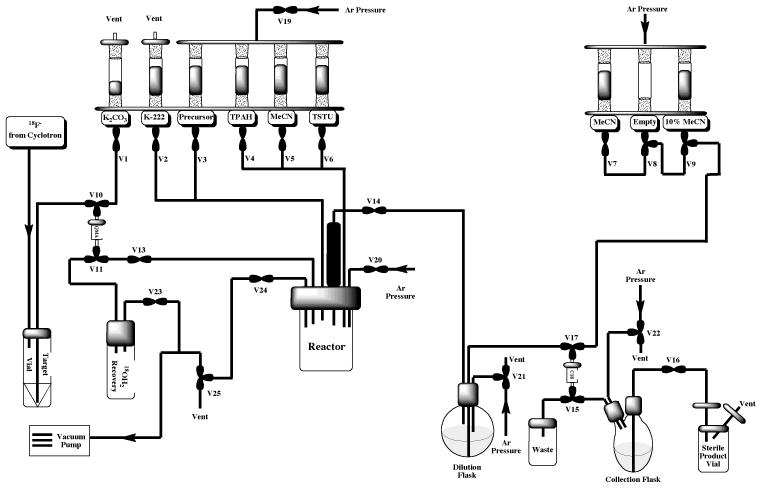
To prepare [18F]SFB, the Tracerlab was configured as shown in Figure 12 and vials were loaded as follows: Vial 1: potassium carbonate (3.5 mg in 0.5 mL water); Vial 2: kryptofix-2.2.2 (15 mg in 1 mL MeCN); Vial 3: 4-(ethoxycarbonyl)-N,N,N- trimethylbenzenaminium triflate precursor (5 mg in 0.5 mL DMSO); Vial 4: TPAH (20 μL in 0.5 mL MeCN); Vial 5: acetonitrile (1 mL); Vial 6: TSTU (10 mg in 0.6 mL MeCN); Vial 7: MeCN (2 mL); Vial 9: 10% MeCN in water (10 mL); dilution flask: 5 mL of 5% acetic acid and 15 mL water.
Figure 12.
Modified Tracerlab FXFN Configuration for [18F]SFB Production
Following drying of the fluoride using the general procedure, the trimethylbenzeneaminium triflate precursor (5 mg in 0.5 mL DMSO) was added to the reactor and the reaction vessel was heated to 90 °C for 10 min to provide [18F]ethyl 4-fluorobenzoate. After this time, tetrapropylammonium hydroxide (TPAH, 20 μL in 0.5 mL MeCN) was added to saponify the ester group. Heating at 120 °C for 3 min provides [18F]4-fluorobenzoic acid as the corresponding TPA salt. After saponification, O-(N-succinimidyl)-1,1,3,3-tetramethyluronium tetrafluoroborate TSTU, 10 mg in 0.6 mL MeCN) was added and the reaction vessel was heated at (90 °C for 5 min to provide [18F]SFB. The crude reaction mixture was then cooled down (40 °C) and transferred to the round-bottom dilution flask (pre-charged with 10 mL of 1.5% acetic acid) and the resulting solution was transferred through a Waters C18 Plus Sep-Pak. The C18 Sep-Pak was then washed with 10% MeCN (10 mL) to elute off unwanted hydrophilic impurities included unreacted fluoride-18. Following washing, [18F]SFB was eluted off into the collection vial with neat MeCN (2 mL). Typical yields of [18F]SFB using this method were 38% (non-decay corrected, n = 20) and radiochemical purity is always >95%. The [18F]SFB can be used as the obtained solution in MeCN or, alternatively, can be evaporated to dryness (heat gun) and re-dissolved in a solvent more appropriate for the subsequent coupling reaction (e.g. DMSO).
2.5 Synthesis Module Cleaning
The radiopharmaceuticals described in Sections 2.2 – 2.4 can be prepared on a single Tracerlab FXFN. Changing between module configurations is straightforward if the luer-lock fittings described in Section 2.1 are incorporated into the Tracerlab. However, module reconfiguration does raise the issue of how best to clean and dry the module between syntheses, especially syntheses using different module set-ups. In order to accomplish this reliably, the Tracerlab is returned to its basic configuration (Figure 3), after completion of a given synthesis, and then Clean, Clean-Disinfect, and Dry cycles are performed as outlined below. Note that the round-bottomed dilution flask and product collection vial are removed, washed and oven-dried separately between syntheses and after completion of the cleaning cycles. The reaction vessel should be checked afterwards to ensure cleanliness and can also be further cleaned and oven-dried manually if required. It is recommended that the reactor be cleaned in this fashion at least once a week.
2.5.1 Clean Cycle
Vials 1, 4, 5, 6, 7 and 9 are charged with sterile water. The water from vials 1, 4, 5 and 6 is washed into the reactor and then out, through the HPLC load loop, to waste. Similarly, the water from vials 7 and 9 is washed through the lines connecting the vials to V-17 and subsequently to waste. This ensures that the main lines of the synthesis module all get washed with sterile water.
2.5.2 Clean-Disinfect Cycle
Vials 1 – 6 are charged with 70% ethanol in sterile water. The 70% ethanol from all of the vials is washed into the reactor and then out, through the HPLC load loop, to waste. Moreover, 70% ethanol is pumped through the HPLC column and associated lines. During this cycle, the product collection vial is also washed with 70% ethanol to disinfect it. These steps ensure that the main lines of the synthesis module are sterilized during the clean and disinfect cycle, and that the HPLC column is stored under 70% ethanol between runs.
2.5.3 Dry Cycle
Vials 1 – 6 are charged with acetone. The acetone from all of the vials is washed into the reactor and the reactor is then heated to 80 °C for 5 min. After this time, the reactor is cooled and the acetone is pushed out, through the HPLC load loop, to waste. Subsequently, all of the vials and lines are dried with both argon pressure and vacuum over 20 min, to ensure that all water and acetone are removed from the synthesis module.
3. Quality Control Procedures
Quality control of radiopharmaceuticals prepared at the University of Michigan PET Center for clinical use is carried out according to the U.S. Pharmacopeia [USP-32, NF-27, 2009] as detailed below. After successfully meeting all release criteria, doses are released to physicians for clinical use.
Visual Inspection
Doses are visually examined and must be clear, colorless and free of particulate matter.
Dose pH
The pH of the doses is analyzed by applying a small amount of the dose to colorpHast® pH 2.0 – 9.0 non-bleeding pH-indicator strips and determined by visual comparison to the scale provided.
Chemical Purity and Radiochemical Purity / Identity
Chemical and radiochemical purity/identity are analyzed using a Shimadzu VP-Series HPLC equipped with a Bioscan FC3300 radioactivity detector and either a conductivity detector ([18F]NaF; [18F]fluorocholine) or a UV detector ([18F]FLT, [18F]FAZA, [18F]MPPF, [18F]FEOBV, [18F]SFB) using conditions described below. Whilst radiochemical purity for doses must be >95%, there are currently no chemical purity requirements for release of radiopharmaceuticals in clinical research. However, we calculate chemical purity by normalizing to a specific activity of 2 Ci / μmol. Doses are typically 100% chemically pure. Radiochemical identity is confirmed and quantified by calculating the relative retention time (RRT = [retention time of radiochemical peak] / [retention time of unlabelled reference standard peak]).
[18F]FLT
Condition A: Column: Phenomonex Luna C18(2) 5μ, 150 × 2.0 mm; mobile phase: 5% MeCN / 20 mM NH4OAc, pH: 4.5; flow rate: 0.5 mL / min; oven: 40 °C; UV: 254 nm, RT = 6.5 min; Condition B: Column: Phenomonex Synergi Hydro-RP, 150 × 4.6 mm; mobile phase: 10% EtOH in water; flow rate: 1.0 mL / min; UV: 254 nm, RT = 7.8 min [19F]FLT non-radioactive fluoride-19 reference standard was purchased from ABX Advanced Biochemicals.
[18F]FAZA
Column: Phenomonex Luna C8(2) 5μ, 100 × 2.0 mm; 5% acetonitrile : 95% 20 mM aqueous ammonium acetate, pH 4.5; flow rate: 0.5 mL/min, UV = 254 nm, RT = 6.2 min. [19F]FAZA reference standard was purchased from Prof. Friedrich Hammerschmidt (Universität Wien, Austria) and Prof. Hans-Jürgen Machulla (Steinbeis Transfer Center Radiopharmacy, Germany).
[18F]MPPF
Column: Phenomonex Luna C8(2) 5μ, 100 × 2.0 mm; mobile phase: 35% MeOH / 20 mM NH4OAc, pH: 4.5; flow rate: 0.8 mL / min; oven: 40 °C; UV: 254 nm, RT = 5.6 min. [19F]MPPF non-radioactive reference standard was purchased from ABX Advanced Biochemicals.
[18F]FEOBV
Column: Phenomenex Gemini C18 5μ, 50 × 2.0 mm; mobile phase: 40% acetonitrile, 60% water, 0.2% aqueous diethylamine; flow rate: 0.8 mL / min; oven: 40 °C; UV: 260 nm, RT = 4.6 – 4.8 min. [19F]FEOBV non-radioactive reference standard was prepared in house.
[18F]Sodium Fluoride
Column: Phenomonex Rezex RHM-monosaccharide (hydrogen form), 300 × 7.8 mm; mobile phase: 0.0015 M aqueous sulfuric acid; flow rate: 0.8 mL / min, RT = 8.8 min. Sodium fluoride (99.99%) was purchased from Sigma Aldrich and used as the non-radioactive fluoride-19 reference standard.
[18F]Fluorocholine
Column: Waters IC-Pak Cation M/D Column, 150 × 3.9 mm; mobile phase: 5 mM aqueous hydrochloric acid, flow rate: 1.0 mL / min; conductivity detector: negative polarity, RT = 7.0 min. [19F]Fluoromethyl choline non-radioactive reference standard was purchased from ABX Advanced Biochemicals.
[18F]SFB
Column: Chromolith RP18, 100x4.6mm (Merck, Germany); Mobile Phase: 0.1%TFA in 50% MeOH, flow rate: 0.8 mL / min; UV: 254 nm, RT: 3.0 min. Non-radioactive [19F]SFB was used as the reference standard.
Radionuclidic Identity
Radionuclidic identity is confirmed by measuring the half-life of radiopharmaceutical doses and comparing it to the known half-life of fluorine-18 (109.77 min). Activities are measured using a Capintec CRC®-15R Radioisotope Dose Calibrator and half-life is calculated using equation (1). Calculated half-life must be 105 – 115 min.
| (1) |
Radionuclidic Purity
Radionuclidic purity is analyzed by gamma-ray spectrometry and doses from the University of Michigan are allowed to decay, and then sent out to a CRO (Dade Moeller and Associates, Gaithersburg, MD, USA) to test for the presence of long-lived radioactive contaminants.
Sterile Filter Integrity Test
Sterile filters from doses (with needle still attached) are connected to a nitrogen supply via a regulator. The needle is then submerged in water and the nitrogen pressure gradually increased. If the pressure is raised above the filter acceptance pressure (typically 50 psi) without seeing a stream of bubbles, the filter is considered intact.
Bacterial Endotoxins
Endotoxin content in radiopharmaceutical doses is analyzed by a Charles River Laboratories EndoSafe® Portable Testing System and according to the US Pharmacopeia. Doses must contain <175 Endotoxin Units (EU).
Sterility
Culture tubes of fluid thioglycolate media (FTM) and soybean casein digest agar media (SCDM) are inoculated with samples of [18F]-labeled radiopharmaceutical doses and incubated (along with positive and negative controls) for 14 days. FTM is used to test for anaerobes, aerobes and microaerophiles whilst SCDM is used to test for non-fastidious and fastidious microorganisms. Culture tubes are visually inspected on the 3rd, 8th and 14th days of the test period and compared to the positive and negative standards. Positive standards must show growth (turbidity) on the plates and dose / negative controls must have no culture growth after 14 days to be indicative of sterility.
4. Conclusions
In conclusion, the Tracerlab FXFN has proven a versatile and efficient automated synthesis module in our hands. The results disclosed herein demonstrate that through simple incorporation of luer lock fittings, the synthesis module can be configured (and reconfigured) in a straightforward manner to allow production of clinical doses of many different radiopharmaceuticals. As proof-of-concept, the production of each radiopharmaceutical discussed in this paper uses a different state-of-the-art radiochemical technique. Each synthesis has been fully automated and all radiopharmaceutical doses for clinical use meet and exceed established quality control criteria.
Supplementary Material
Table 1.
Representative Quality Control Data
| QC Test | Release Criteria | FLT | FAZA | MPPF | FEOBV | NaF | Fluorocholined | SFBd | |
|---|---|---|---|---|---|---|---|---|---|
| Method A |
Method B |
||||||||
| n | N/A | 5 | 25 | 25 | 28 | 10 | 10 | 7 | 20 |
| Starting Activity (mCi) | N/A | 500 | 1550 | 1550 | 1550 | 1550 | 120 | 1500 | 1000 |
| Mean Yield (mCi) @ EOS | N/A | 115 | 65 | 75 | 136 | 146 | 115 | 75 | 380 |
| % Yield (Non-decay corrected) | N/A | 23% | 4.2% | 4.8% | 8.8% | 9.4% | 95% | 5% | 38% |
| Specific Activity (Ci/μmol) | N/A | 17329 | 4548 | 5225 | 8075 | 4557 | N/A | >1000e | >1000e |
| Appearance | Clear, colorless, free of particulates |
Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| pH | 4.5 – 8.0 | 6.0 | 6.5 | 6.5 | 5.0 | 5.0 | 5.0 | 5.5 | N/A |
| Residual Kryptofix | < 50 μg / mL | < 50 | < 10 | < 10 | < 10 | < 10 | N/A | < 10 | N/A |
| Radiochemical Purity | > 95% by HPLC | 100% | 99.5% | 99.5% | 99.0% | 98.5% | 99.4% | 99.7% | 100% |
| Chemical Puritya | N/A | 100% | 75% | 100% | 94% | 100% | 100% | N/A | N/A |
| Radiochemical Identity | RRTb = 0.9 – 1.1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | N/A |
| Radionuclidic Identity | T1/2 = 105 – 115 min | 108 | 108 | 108 | 108 | 110 | 107 | 106 | N/A |
| Radionuclidic Purity | >99% | >99.9% | >99.9% | >99.9% | >99.9% | >99.9% | >99.9% | N/A | N/A |
| Residual Solvent Analysis | MeCN (<410 μg/mL) | 1.0 | 47.2 | 79.02 | 1.4 | 66 | N/A | 0 | N/A |
| DMSO (<5000 μg/mL) | N/A | 298 | 252.4 | 1.1 | 16 | N/A | |||
| Acetone (<5000 μg/mL) | 7.0 | 9.9 | 2.0 | 1.5 | 4.5 | 10 | |||
| THF (<5000 μg/mL) | N/A | N/A | N/A | 34.8 | N/A | N/A | |||
| MeOH (<3000 μg/mL) | N/A | N/A | N/A | 19.0 | N/A | N/A | |||
| Filter Integrity Test | >40 psi | >50 | >50 | >50 | >40 | >50 | >50 | >50 | N/A |
| Bacterial Endotoxins | <17.5 EUc / mL | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | N/A |
| Sterility | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | N/A |
chemical purity normalized to 2 Ci / μmol
RRT = Relative retention time.
EU = Endotoxin units.
[18F]fluorocholine and [18F]SFB are not subject to full QC testing as presently they are only made for pre-clinical application.
For the pre-clinical work described in this study, no efforts were made to optimize specific activity of [18F]SFB.
Acknowledgements
The University of Michigan Cyclotron and Radiochemistry Group gratefully acknowledges the Office of Biological Research (BER) of the Office of Science (SC), U. S. Department of Energy (DE-FG02-08ER64645) and the National Institutes of Health (NIH NS15655) for financial support of this research, and Siemens Molecular Imaging and Biomarker Research for donation of a test sample of the Boc-Boc-Nosyl FLT precursor.
References
- [1].Wuest F, Berndt M, Kniess T. Ernst Schering Res. Found. Workshop; 2007; pp. 182–213. [DOI] [PubMed] [Google Scholar]
- [2].Scott PJH. Angew. Chem. Int. Ed. 2009;48:6001–6004. doi: 10.1002/anie.200901481. [DOI] [PubMed] [Google Scholar]
- [3].Miller PW, Long NJ, Vilar R, Gee AD. Angew. Chem. Int. Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- [4].Ametamey SM, Honer M, Schubiger PA. Chem. Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- [5].Antoni G, Långström B. In: Positron Emission Tomograpy. Bailey DL, Townsend DW, Valk PE, Maisey MN, editors. Springer; London: 2003. pp. 237–250. [Google Scholar]
- [6].Cai L, Lu S, Pike VW. Eur. J. Org. Chem. 2008:2853–2873. [Google Scholar]
- [7].Dolle F, Roeda D, Kuhnast B, Lasne MC. Fluorine and Health. 2008:3–65. [Google Scholar]
- [8].Schirrmacher R, Wangler C, Schirrmacher E. Mini-Rev. Org. Chem. 2007;4:317–329. [Google Scholar]
- [9].Kilbourn MR, Shao X. In: Fluorine in Medicinal Chemistry and Chemical Biology. Ojima I, editor. Wiley-Blackwell; 2009. pp. 361–388. [Google Scholar]
- [10].Långström B, Itsenko O, Rahman O, Label J. Compd. Radiopharm. 2007;50:794–810. [Google Scholar]
- [11].Scott PJH, Shao X. J. Label. Compd. Radiopharm. 2010;53 DOI:10.1002/jlcr.1785. [Google Scholar]
- [12].Shao X, Kilbourn MR. Appl. Radiat. Isot. 2009;67:602–605. doi: 10.1016/j.apradiso.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [13].Hockley BG, Scott PJH. Appl. Radiat. Isot. 2010;68:117–119. doi: 10.1016/j.apradiso.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mori T, Kasamatsu S, Mosdzianowski C, Welch MJ, Yonekura Y, Fujibayashi Y. Nucl. Med. Biol. 2006;33:281–286. doi: 10.1016/j.nucmedbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [15].Kryza D, Tadino V, Filannino MA, Villeret G, Lemoucheux L. Nucl. Med. Biol. 2008;35:255–260. doi: 10.1016/j.nucmedbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- [16].Mäding P, Füchtner F, Wüst F. Appl. Radiat. Isot. 2005;63:329–332. doi: 10.1016/j.apradiso.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [17].Teng B, Wang S, Fu Z, Dang Y, Wu Z, Liu L. Appl. Radiat. Isot. 2006;64:187–193. doi: 10.1016/j.apradiso.2005.07.017. [DOI] [PubMed] [Google Scholar]
- [18].Oh SJ, Chi DY, Mosdzianowski C, Kil HS, Ryu JS, Moon DH. Appl. Radiat. Isot. 2007;65:676–681. doi: 10.1016/j.apradiso.2006.06.016. [DOI] [PubMed] [Google Scholar]
- [19].Chin FT, Namavari M, Levi J, Subbarayan M, Ray P, Chen X, Gambhir SS. Mol. Imaging Biol. 2008;10:82–91. doi: 10.1007/s11307-007-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wilson AA, Garcia A, Houle S, Vasdev N, Label J. Compd. Radiopharm. 2009;52:490–492. [Google Scholar]
- [21].Lodi F, Trespidi S, Pierro DD, Marengo M, Farsad M, Fanti S, Franchi R, Boschi S. Appl. Radiat. Isot. 2007;65:691–695. doi: 10.1016/j.apradiso.2006.10.011. [DOI] [PubMed] [Google Scholar]
- [22].Tang G, Tang X, Wang X. J. Label. Compd. Radiopharm. 2010;53:543–547. [Google Scholar]
- [23].Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Nat. Med. 1998;4:1344–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- [24].Been LB, Suurmeijer AJH, Cobben DCP, Jager PL, Hoekstra HJ, Elsinga PH. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:1659–1672. doi: 10.1007/s00259-004-1687-6. [DOI] [PubMed] [Google Scholar]
- [25].Walsh JC, Padgett HC. U. S. Patent Appl. 2005131224. 2005:11.
- [26].Lee SJ, Oh SJ, Chi DY, Lee BS, Ryu JS, Moon DH. J. Label. Compd. Radiopharm. 2008;51:80–82. [Google Scholar]
- [27].Martin SJ, Eisenbarth JA, Wagner-Utermann U, Mier W, Henze M, Pritzkow H, Haberkorn U, Eisenhut M. Nucl. Med. Biol. 2002;29:263–273. doi: 10.1016/s0969-8051(01)00289-x. [DOI] [PubMed] [Google Scholar]
- [28].Machulla HJ, Blocher A, Kuntzsch M, Piert M, Wei R, Grierson JR. J. Radioanal. Nucl. Chem. 2000;243:843–846. [Google Scholar]
- [29].Wodarski C, Eisenbarth J, Weber K, Henze M, Haberkorn U, Eisenhut M. J. Label. Compd. Radiopharm. 2000;43:1211–1218. [Google Scholar]
- [30].Kumar P, Wiebe LI, Asikoglu M, Tandon M, McEwan AJB. Appl. Rad. Isotop. 2002;57:697–703. doi: 10.1016/s0969-8043(02)00185-9. [DOI] [PubMed] [Google Scholar]
- [31].Reischl G, Ehrlichmann W, Bieg C, Solbach C, Kumar P, Wiebe LI, Machulla HJ. Appl. Rad. Isotop. 2005;62:897–901. doi: 10.1016/j.apradiso.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [32].Reischl G, Dorow DS, Cullinane C, Katsifis A, Roselt P, Binns D, Hicks RJ. J. Pharm. Pharm. Sci. 2007;10:203–211. [PubMed] [Google Scholar]
- [33].Grosu A-L, Souvatzoglou M, Roeper B, Dobritz M, Wiedenmann N, Jacob V, Wester H-J, Reischl G, Machulla H-J, Schwaiger M, Molls M, Piert M. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:541–551. doi: 10.1016/j.ijrobp.2007.05.079. [DOI] [PubMed] [Google Scholar]
- [34].Beck R, Roeper B, Carlsen JM, Huisman MC, Lebschi JA, Andratschke N, Picchio M, Souvatzoglou M, Machulla H-J, Piert M. J. Nucl. Med. 2007;48:973–980. doi: 10.2967/jnumed.106.038570. [DOI] [PubMed] [Google Scholar]
- [35].Piert M, Machulla HJ, Picchio M, Reischl G, Ziegler S, Kumar P, Wester HJ, Beck R, McEwan AJB, Wiebe LI, Schwaiger M. J. Nucl. Med. 2005;46:106–113. [PubMed] [Google Scholar]
- [36].Pike VW, Halldin C, Wikstrom H. Prog. Med. Chem. 2001;38:189–247. doi: 10.1016/s0079-6468(08)70094-8. [DOI] [PubMed] [Google Scholar]
- [37].Krasikova RN, Truong P, Halldin C. J. Label. Compd. Radiopharm. 2003;46(Suppl. 1):S244. [Google Scholar]
- [38].Maiti DK, Chakraborty PK, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Appl. Radiat. Isot. 2005;62:721–727. doi: 10.1016/j.apradiso.2004.10.005. [DOI] [PubMed] [Google Scholar]
- [39].McCarron JA, Turton DR, Pike VW, Poole KG. J. Label. Compd. Radiopharm. 1996;38:941–953. [Google Scholar]
- [40].Wadsak W, Mien LK, Ettlinger DE, Lanzenberger RR, Haeusler D, Dudczak R, Kletter K, Mitterhauser M. Radiochim. Acta. 2007;95:417–422. [Google Scholar]
- [41].Krasikova RN, Andersson J, Truong P, Nag S, Shchukin EV, Halldin C. Appl. Radiat. Isot. 2009;67:73–78. doi: 10.1016/j.apradiso.2008.07.008. [DOI] [PubMed] [Google Scholar]
- [42].Aznavour N, Zimmer L. Neuropharmacology. 2007;52:695–707. doi: 10.1016/j.neuropharm.2006.09.023. [DOI] [PubMed] [Google Scholar]
- [43].Zhuang ZP, Kung MP, Kung HF. J. Med. Chem. 1994;37:1406–1407. doi: 10.1021/jm00036a003. [DOI] [PubMed] [Google Scholar]
- [44].Shiue CY, Shiue GG, Mozley PD, Kung MP, Zuang ZP, Kim HJ, Kung HF. Synapse. 1997;25:147–154. doi: 10.1002/(SICI)1098-2396(199702)25:2<147::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [45].Le Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, Hassoun W, Leviel V, Mekhsian P, Weissmann D, Pujol JF, Luxen A, Comar D. Nucl. Med. Biol. 1998;25:343–350. doi: 10.1016/s0969-8051(97)00229-1. [DOI] [PubMed] [Google Scholar]
- [46].Bohnen NI, Frey KA. Mol. Imag. Biol. 2007;9:243–257. doi: 10.1007/s11307-007-0083-6. [DOI] [PubMed] [Google Scholar]
- [47].Candy JM, Perry RH, Perry EK, Irving D, Blessed G, Fairbairn AF, Tomlinson BE. J. Neurol. Sci. 1983;59:277–289. doi: 10.1016/0022-510x(83)90045-x. [DOI] [PubMed] [Google Scholar]
- [48].Whitehouse PJ, Hedreen JC, White CL, Price DL. Ann. Neurol. 1986;13:243–248. doi: 10.1002/ana.410130304. [DOI] [PubMed] [Google Scholar]
- [49].Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Proc. Natl. Acad. Sci. USA. 1987;84:5976–5980. doi: 10.1073/pnas.84.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ. Neurobiol. Aging. 2000;21:11–17. doi: 10.1016/s0197-4580(00)00090-7. [DOI] [PubMed] [Google Scholar]
- [51].Baskin DS, Browning JL, Pirozzolo FJ, Korporaal S, Baskin JA, Appel SH. Arch. Neurol. 1999;56:1121–1123. doi: 10.1001/archneur.56.9.1121. [DOI] [PubMed] [Google Scholar]
- [52].Altar CA, Marien MR. Synapse. 1988;2:486–493. doi: 10.1002/syn.890020504. [DOI] [PubMed] [Google Scholar]
- [53].Rogers GA, Parsons SM, Anderson DC, Nilsson LM, Bahr BA, Kornreich WD, Kaufman R, Jacobs RS, Kirtman B. J. Med. Chem. 1989;32:1217–1230. doi: 10.1021/jm00126a013. [DOI] [PubMed] [Google Scholar]
- [54].Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. J. Comp. Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- [55].Kuhl DE, Koeppe RA, Fessler JA, Minoshima S, Ackermann RJ, Carey JE, Gildersleeve DL, Frey KA, Wieland DM. J. Nucl. Med. 1994;35:405–410. [PubMed] [Google Scholar]
- [56].Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, Wieland DM, Koeppe RA. Ann. Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- [57].Mulholland GK, Wieland DM, Kilbourn MR, Frey KA, Sherman PS, Carey JE, Kuhl DE. Synapse. 1998;30:263–274. doi: 10.1002/(SICI)1098-2396(199811)30:3<263::AID-SYN4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [58].Kilbourn MR, Hockley B, Lee L, Sherman P, Quesada C, Frey KA, Koeppe RA. Nucl. Med. Biol. 2009;36:489–493. doi: 10.1016/j.nucmedbio.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gould P. Nature. 2009;460:312–313. doi: 10.1038/460312a. [DOI] [PubMed] [Google Scholar]
- [60].Voith M. Chem. Eng. News. 2009;87:8. [Google Scholar]
- [61].Schirrmeister H, Rentschler M, Kotzerke J, Diederichs CG, Reske SN. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 1998;168:451–456. doi: 10.1055/s-2007-1015161. [DOI] [PubMed] [Google Scholar]
- [62].Weber DA, Keyes JW, Jr., Landman S, Wilson GA. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1974;121:184–190. doi: 10.2214/ajr.121.1.184. [DOI] [PubMed] [Google Scholar]
- [63].Nandy SK, Ragan MGR, Soni PS, Rangarajan V. BARC Newsletter. 2007;281:16–23. [Google Scholar]
- [64].Dennis EA, Vance DE, editors. Methods in Enzymology. Volume 209: Phospholipid Biosynthesis. Elsevier; 1992. p. 584. [DOI] [PubMed] [Google Scholar]
- [65].Hara T, Kosaka N, Kishi H. J. Nucl. Med. 1998;39:990–995. [PubMed] [Google Scholar]
- [66].Hara T, Kosaka N, Kondo T, Kishi H, Kobori O. J. Nucl. Med. 1997;38(Suppl):250. [Google Scholar]
- [67].DeGrado TR, Coleman RE, Wang S, Baldwin SW, Orr MD, Robertson CN, Polascik TJ, Price DT. Cancer Res. 2001;61:110–117. [PubMed] [Google Scholar]
- [68].DeGrado TR, Baldwin SW, Wang S, Orr MD, Liao RP, Friedman HS, Reiman R, Price DT, Coleman RE. J. Nucl. Med. 2001;42:1805–1814. [PubMed] [Google Scholar]
- [69].Iwata R, Pascali C, Bogni A, Furumoto S, Terasaki K, Yanai K. Appl. Radiat. Isot. 2002;57:347–352. doi: 10.1016/s0969-8043(02)00123-9. [DOI] [PubMed] [Google Scholar]
- [70].Slaets D, De Bruyne S, Dumolyn C, Moerman L, Mertens K, De Vos F. Eur. J. Nuc. Med. Mol. Imaging. 2010;37:2136–2145. doi: 10.1007/s00259-010-1508-z. [DOI] [PubMed] [Google Scholar]
- [71].Vaidyanathan G, Zalutsky MR. Nat. Prot. 2006;1:1655–1661. doi: 10.1038/nprot.2006.264. [DOI] [PubMed] [Google Scholar]
- [72].Marik J, Sutcliffe JL. Appl. Radiat. Isot. 2007;65:199–203. doi: 10.1016/j.apradiso.2006.06.007. [DOI] [PubMed] [Google Scholar]
- [73].Tang G, Zeng W, Yu M, Kabalka G. J. Label. Compd. Radiopharm. 2008;51:68–71. [Google Scholar]
- [74].Haka MS, Kilbourn MR, Watkins GL. J. Label. Compd. Radiopharm. 1989;27:823–833. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.