Abstract
BACKGROUND
Intact frozen-thawed embryos have a greater potential than damaged embryos to establish successful pregnancies. This study aimed to determine whether elevated concentrations of sucrose during freezing would increase the proportion of patients with ≥50% of embryos intact after thawing (primary outcome), and improve clinical outcome.
METHODS
In a two arm, parallel group, pragmatic trial, IVF/ICSI couples were randomized prospectively to have their supernumerary embryos frozen in a medium containing 0.1 M sucrose (control; n = 99) or 0.3 M sucrose (intervention; n = 102).
RESULTS
More control (74/99) than intervention (63/102) couples had at least one embryo thawed (P = 0.07). Significantly more (P = 0.005) intervention (53/63) than control (45/74) couples had ≥50% of embryos intact. Freezing in a medium containing 0.3 M sucrose increased by 3.4-fold [95% confidence interval (CI) (1.45, 7.82)] the likelihood of a couple having ≥50% of their embryos intact. In the fresh cycle, live birth rate per transfer was similar in the control (35/95) and intervention (36/93) groups (P = 0.91). More control (19/63) than intervention (9/59) couples had a live birth after frozen embryo transfer (P = 0.08). When fresh and frozen cycles were combined, fewer intervention (n = 102) than control (n = 99) couples had at least one live birth (42 versus 53%). The difference in cumulative live birth rate was not significant [hazard ratio = 0.75, 95% CI (0.49, 1.13); P = 0.17].
CONCLUSIONS
Increasing the concentration of sucrose in the freezing medium improves embryo survival, but this is not reflected by increased cumulative birth rates.
Clinical Trials Registration number: ISRCTN93314892.
Keywords: freezing, embryo, sucrose, prospective RCT, cumulative live birth
Introduction
Embryo freezing has been an integral part of assisted reproduction for at least two decades, despite the knowledge that many embryos are damaged in the process, resulting in reduced pregnancy and delivery rates (Van den Abeel et al., 1997; Guerif et al., 2002; de Mouzon et al., 2010). The key advantage of cryopreserving supernumerary embryos is to maximize the chance of a live birth from a single cycle of ovarian stimulation while limiting the risk of multiple birth. The increasing use of elective single embryo transfer (eSET) as a means of minimizing iatrogenic multiples in IVF has re-focused attention on embryo cryopreservation as a means of maintaining cumulative birth rates (Tiitinen et al., 2001; Thurin et al., 2004; Veleva et al., 2006, 2009). However, critical to the acceptance of eSET is the ability to access robust cryopreservation methods with reliably high rates of embryo survival and subsequent live birth.
Conventionally, embryos are considered to have survived freezing and thawing when at least 50% of blastomeres remain intact (e.g. Lassalle et al., 1985; Mohr et al., 1985; Testart et al., 1987; Van Steirteghem et al., 1987). However, blastomere loss is associated with reduced developmental potential of the embryos in vitro (Archer et al., 2003), and reduced rates of implantation and pregnancy (Van den Abbeel et al., 1997; Burns et al., 1999; Edgar et al., 2000; Guerif et al., 2002; El-Toukhy et al., 2003; Pal et al., 2004) as well as reduced rates of live birth (Van den Abbeel et al., 1997; Guerif et al., 2002). Retrospective analyses of large data sets suggest that frozen embryos surviving with all blastomeres intact have an implantation potential similar to that of fresh embryos at the same developmental stage (Edgar et al., 2000). Typically, only about 50% of embryos survive freezing without loss of blastomeres (Mandelbaum et al., 1988; Edgar et al., 2000; Archer et al., 2003; Kattera and Chen, 2005; Balaban et al., 2008). Thus, there is a clear and urgent need to improve methods of cryopreservation that increase the proportion of embryos surviving freezing with all blastomeres intact.
Pronucleate and cleavage stage embryos are frozen almost universally in a medium containing 1,2-propanediol (PrOH; ∼1.5 M) and sucrose (0.1 M), according to protocols based on the method developed 26 years ago for mouse embryos (Renard and Babinet, 1984) and modified for human embryos a year later (Lassalle et al., 1985). Lassalle et al. (1985) froze embryos in solutions with or without 0.1 M sucrose, but had insufficient evidence to comment on the value of adding sucrose. Testart et al. (1986) froze embryos in medium with sucrose (0.1 M) and reported high pregnancy rates, while Mandelbaum et al. (1988) found that embryo survival was improved when sucrose (0.1 M) was added to the medium. With notable exceptions (Van den Abbeel et al., 1988; Van der Elst et al., 1995), freezing in a solution of 1.5 M PrOH and 0.1 M sucrose has been largely unchallenged until recently (Jericho et al., 2003; Edgar et al., 2009).
Human oocytes are particularly sensitive to freezing, and rates of post-thaw survival were unacceptably low for many years. Raising the concentration of sucrose in the freezing medium 2- and 3-fold from 0.1 M increased oocyte survival from 31 to 60% and 80% (Fabbri et al., 2001). Similarly, the survival of biopsied (Jericho et al., 2003; Zheng et al., 2005) and non-biopsied (Edgar et al., 2009) embryos was improved significantly after freezing in a medium containing 0.2 M sucrose.
The aim of this pragmatic RCT was to compare the survival of supernumerary pronucleate and cleavage stage embryos frozen in a solution of PrOH with either the conventional concentration (0.1 M) or an elevated concentration (0.3 M) of sucrose. The primary end-point was the proportion of couples with at least 50% of embryos fully intact after thawing. Cumulative live birth after the transfer of embryos in the oocyte collection cycle and in one or more thawing cycles was a secondary end-point.
Materials and Methods
Participants and study design
The study was a two arm, parallel group, pragmatic RCT. Couples were recruited from one major teaching hospital in the northeast of Scotland. All couples attending the Assisted Reproduction Unit (ARU), University of Aberdeen, for IVF or ICSI treatment between September 2003 and December 2006 were considered for inclusion. Exclusion criteria were (i) women with no supernumerary embryos for freezing in two previous treatment cycles, (ii) women ≥38 years of age with one previous cycle without freezing, (iii) couples using donor gametes and (iv) couples recruited to any other ongoing clinical trial. The trial was approved by the Grampian Research Ethics Committee and the Human Fertilization and Embryology Authority of the UK. Written informed consent was obtained from all couples who participated.
Stimulation, oocyte collection, fertilization and embryo culture
Standard protocols for ovarian stimulation in IVF were followed (Bhattacharya et al., 2001; Haggarty et al., 2006). Follicles were aspirated transvaginally under ultrasound guidance at 36–38 h post hCG injection. Oocyte–cumulus complexes (OCCs) were handled on heated stages in medium (Medicult Universal Medium until May 2005, thereafter Cook's Fertilization Medium) warmed to 37°C. For IVF the OCCs were mixed with sperm 40–41 h post hCG. For ICSI the cumulus cells were removed enzymatically and sperm injected at 40–42 h post hCG.
Oocytes were examined for the presence of pronuclei and polar bodies 18–20 h post insemination (p.i.). Normally fertilized eggs with two pronuclei and two polar bodies were either cultured under oil in 30 µl drops of medium (Medicult Universal Medium until May 2005, Cook's Cleavage Medium thereafter) at 37°C in an atmosphere of 5–6% CO2 in air, or frozen immediately. The cultured embryos were examined microscopically ∼3 h before transfer on Day 2 or Day 3 p.i.; the final selection for transfer and freezing was made just before embryo transfer.
Freezing and thawing
Freezing and thawing protocols were identical for embryos in the control (conventional concentration of sucrose) and intervention (increased concentration of sucrose) groups, but the freezing and thawing solutions differed. Medicult freezing solution no. 10264010 (1.5 M PrOH + 0.1 M sucrose) and thawing solution no 10984010 (0.2 M sucrose) were used for control embryos and freezing solution no. 10485010 (1.4 M PrOH + 0.3 M sucrose) and thawing solution no 10494010 (0.3 M sucrose) for intervention embryos.
Embryos were prepared for freezing at room temperature (∼22°C). They were washed briefly (1–2 min) in Dulbecco's phosphate-buffered saline (PBS) supplemented with human serum albumin (HSA) and synthetic serum replacement (PBS + HSA + SSR: control embryos) or PBS + HSA + alpha- and beta-globulins (PBS + HSA + G: intervention embryos), and then transferred into a similar buffer + PrOH for 12 min and finally into the freezing medium (buffer + PrOH + sucrose) for 5–10 min before cooling. Embryos were frozen singly in sealed straws. Cooling commenced at 2°C per minute to −7°C, the sample was seeded, and 10 min later cooling was resumed at 0.3°C per minute to −30°C and then at 10°C per minute to −150°C before the straws were transferred into liquid nitrogen for storage at −196°C.
For thawing, the straw was held in air at room temperature for 40 s and then immersed in water at 25°C until the ice disappeared. Embryos were transferred sequentially at 5 min intervals at room temperature through aliquots of buffer containing sucrose (control: PBS + HSA + SSR + 0.2 M sucrose; intervention: PBS + HSA + G + 0.3 M sucrose) and decreasing concentrations of PrOH (control: 1.0 M, 0.5 M; intervention: 0.9 M, 0.4 M) and finally into buffer without sucrose. The morphology of the embryo was recorded and then it was cultured under oil in a 30 µl drop of medium (Medicult Universal or Cook's Cleavage Medium) at 37°C in an atmosphere of 5–6% CO2 in air until transfer.
Embryos were frozen after the fresh embryo transfer on Day 2 or Day 3 p.i., except for couples in which the woman was at risk of developing ovarian hyperstimulation syndrome (OHSS). In the latter cases, no embryos were transferred in the fresh cycle and instead, all embryos were frozen at the pronucleate stage on Day 1 and/or after cleavage on Day 2. Embryos were thawed on the day before the intended transfer and then cultured overnight (pronucleate and Day 2 embryos), or thawed and cultured for up to 6 h on the day of transfer (Day 2 and Day 3 embryos). Unless the couple wished otherwise, embryos were thawed until two survived for transfer with at least one being fully intact. When embryos failed to resume mitosis during overnight culture, additional embryos were thawed for immediate transfer. Embryos were transferred in either a natural or a stimulated cycle.
Embryo scoring
Only those embryos with an appropriate number of evenly cleaved blastomeres for the day p.i. (2–6 blastomeres on Day 2; 4–8 + on Day 3) and ≤25% of fragmented cytoplasm were considered suitable for freezing. As a measure of embryo quality at the time of freezing, we categorized the embryos as having the optimal number of cells for the day p.i. (4 cells on Day 2; 8 cells on Day 3), fewer cells than optimal (2–3 cells on Day 2; 4–7 cells on Day 3), or more cells than optimal. Embryos frozen on Day 1 had both pronuclei still visible. Thawed embryos were scored immediately after thawing and before transfer.
Randomization and outcomes
Couples were randomized after embryos had been selected for fresh transfer and freezing. Consenting couples with at least two embryos for freezing were randomized to control or intervention by the embryologist performing cryopreservation, who opened sequentially numbered, sealed, opaque envelopes. The allocation sequence was generated independently of the study team using simple randomization. Couples were told which treatment they had received only if they asked for the information. The embryologist freezing and thawing the embryos was aware of the treatment in order to select the correct solutions, but when circumstances allowed, a second embryologist scored post-thaw survival.
The primary outcome of the study was the proportion of couples with ≥50% of embryos surviving freezing and thawing with all the blastomeres intact. The secondary outcomes included cumulative live birth, stillbirth, clinical pregnancy, miscarriage and ectopic pregnancy. Cumulative live birth per couple was defined as the birth of one or more live babies from the fresh and all frozen cycles arising from a single oocyte retrieval. Clinical pregnancies were identified by the presence of at least one gestational sac with a fetal heart beat at ultrasound scan 5 weeks after embryo transfer. The outcome of all thawing cycles up to November 2009 have been analysed, including the cycles in which embryos were thawed for transfer and also those in which the couple did not wish to have embryos transferred, but consented to have them thawed and their morphology assessed before they were discarded.
Statistical analysis
In order to detect a difference of 25% (i.e. 50–75%) in the proportion of couples with at least 50% of their embryos fully intact after thawing with 90% power at 5% significance, 85 couples were required in each randomized arm. We aimed to recruit 100 couples in each group to allow for the couples who may not return for embryo thawing.
The primary outcome, the proportion of couples with at least 50% of embryos fully intact after thawing, was analysed by a χ2 test. To adjust for any chance imbalance in prognostic factors (couple diagnosis, parity and female age) and for the varying number of embryos thawed, logistic regression was applied. The odds ratio (OR) and 95% confidence interval (CI) have been reported. Only couples with at least one embryo thawed could be included in the analysis of the primary outcome. The secondary outcome was cumulative live birth following a fresh and one or more frozen cycles, i.e. the proportion of couples randomized with at least one live birth. OHSS patients who did not have a transfer in the fresh cycle were included in the analysis. All couples randomized were included in the analysis following an intention to treat approach. Cox regression was applied to compare the time to first live birth, with adjustment for female age, diagnosis and parity. The hazard ratio (HR) and 95% CI have been reported.
The Mann–Whitney U-test was applied to quantitative variables following a non-normal distribution and the χ2 test to categorical variables. The log rank test was used to compare time to first thaw between the groups. Embryo outcomes were analysed using techniques appropriate for the non-independence of embryos within couples. Random effects logistic regression was applied to compare categorical outcomes across the two randomized groups. Data were analysed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA; version 17) and STATA (StataCorp, College Station, TX, USA; Release 8). P < 0.05 was considered statistically significant.
Results
Baseline characteristics
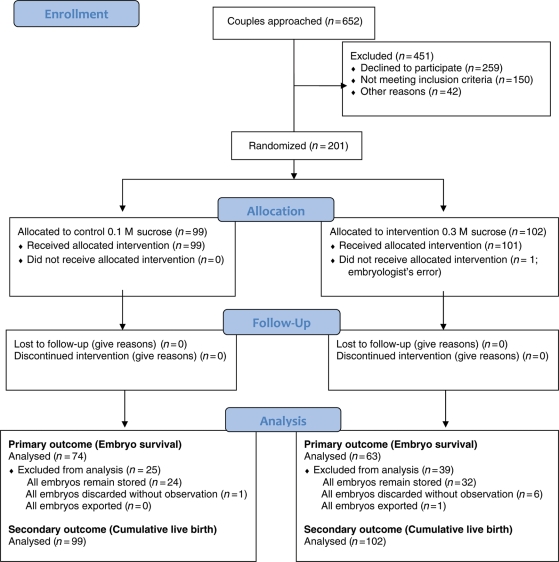
A total of 201 couples were recruited between September 2003 and December 2006 (Fig. 1). Couples in the two groups had similar baseline characteristics (Table I) although the intervention group had more couples with male factor infertility. The median number of oocytes collected, fertilized and available for freezing was similar across the groups, as was the quality of embryos at freezing (Table I).
Figure 1.
Progress of participants through the RCT of conventional versus increased concentration sucrose in freezing and thawing solutions for human embryos.
Table I.
Baseline characteristics of couples in RCT of conventional versus increased concentration sucrose in freezing and thawing solutions for human embryos.
| Embryos frozen in a medium containing |
||
|---|---|---|
| 0.1 M sucrose (control) | 0.3 M sucrose (intervention) | |
| Number of couples (n) | 99 | 102 |
| Female age in years; mean (SD) | 34.3 (3.7) | 34.3 (3.8) |
| Number of parous women (%) | 11 (11%) | 4 (4%) |
| Couple's diagnosis (%): | ||
| Male factor only | 21 (21 %) | 35 (34%) |
| Female factor only | 67 (68%) | 55 (54%) |
| Both | 11 (11%) | 12 (12%) |
| Treatment (%): | ||
| IVF | 73 (74%) | 72 (71%) |
| ICSI | 21 (21%) | 28 (27%) |
| IVF and ICSI | 5 (5%) | 2 (2%) |
| Median (IQRa) number of oocytes per patient: | ||
| Collected | 11 (8, 14) | 10 (8, 13) |
| Inseminated | 10 (8, 14) | 9 (7, 12) |
| Fertilized normallyb | 7 (5, 10) | 7 (5, 9.25) |
| Median (IQR) number of embryos frozen per patient | 4 (3, 6) | 4 (3, 6) |
| Quality score at freezing: | ||
| Median (IQR) proportion of embryos with optimal number of cells at freezing | 50 (20, 80) | 50 (23.75, 75) |
aInterquartile range.
bTwo pronuclei and two polar bodies.
Outcome of thawing
A total of 137/201 (68%) couples had at least one embryo thawed; more couples in the control (74/99; 75%) than intervention (63/102; 62%) arm had embryos thawed (P = 0.07) (Table II). The rate at which couples returned for their first thaw was similar across the two groups (P = 0.14). The number of thawing cycles did not differ between groups, but more embryos were thawed per couple in the control arm (P = 0.06) (Table II).
Table II.
Outcome of thawing frozen embryos for couples wishing an autologous transfer and for those couples consenting to the embryos being observed and then discarded.
| Embryos frozen in a medium containing |
P-value | ||
|---|---|---|---|
| 0.1 M sucrose (control) | 0.3 M sucrose (intervention) | ||
| Number of couples randomized | n = 99 | n = 102 | |
| Number (%) of couples with ≥1 embryo thawed | 74 (75%) | 63 (62%) | 0.07 |
| Median (IQR) number of embryos thawed | 3 (0, 4) | 2 (0, 4) | 0.06 |
| Median (IQR) number of thawing cycles | 1.(0, 1) | 1 (0, 1) | 0.23 |
| Number of thawing cycles | |||
| 0 | 25 (25%) | 39 (38%) | |
| 1 | 51 (52%) | 39 (38%) | |
| 2 | 15 (15%) | 13 (13%) | |
| 3 | 5 (5%) | 8 (8%) | |
| 4–6 | 3 (3%) | 3 (3%) | |
| Total number of couples with embryo(s) thawed and morphology recordeda | n = 74 | n = 63 | |
| Number (%) of couples with ≥50% embryos fully intact over all thawing cycles | 45/74 (61%) | 53/63 (84%) | 0.005b |
| Number of couples having embryos thawed with intention of autologous transferc | n = 67 | n = 59 | |
| Median (IQR) number of frozen embryos transferredd | 2 (2, 3) | 2 (2, 4) | 0.18 |
| Median (IQR) proportion of embryos thawed that were transferred | 0.67 (0.5, 0.8) | 0.71 (0.5, 1.0) | 0.02 |
aIncludes couples intending embryo transfer and those who allowed the embryos to be thawed and scored before being discarded.
bLogistic regression with adjustment for number of embryos thawed, female age, parity (yes/no), couple diagnosis (male only, female only, male and female).
cOne couple had embryos thawed for autologous transfer and also two embryos thawed for donation, these two embryos have not been included in further analysis.
dFresh embryos transferred in mixed transfers are not included.
Embryo survival
A greater proportion of couples had at least 50% of their embryos fully intact after thawing in the intervention group (53/63; 84%), compared with the control group (45/74, 61%; P = 0.005). Freezing embryos in a medium containing 0.3 M sucrose (intervention) increased the likelihood of a couple having at least 50% of their embryos fully intact after thawing by 3.4-fold [95% CI (1.45, 7.82); P = 0.005; adjusted for number of embryos thawed, female age, parity and couple diagnosis]—a significant improvement compared with control couples whose embryos were frozen in medium with a conventional concentration of sucrose (Table II).
In couples who had embryos thawed with the intention of autologous transfer, a significantly greater proportion of the embryos thawed were transferred in the intervention arm (P = 0.02).
Clinical outcomes
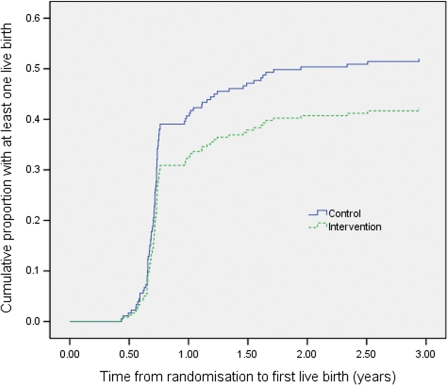
When fresh and frozen cycles are combined, fewer intervention couples had at least one live birth [43/102 (42%) versus 52/99 (53%); Table III]. Although this difference did not achieve significance, couples in the intervention arm were less likely to have at least one live birth [HR = 0.75, 95% CI (0.49, 1.13); P = 0.17] (Fig. 2).
Table III.
Clinical outcome.
| Embryos frozen in a medium containing |
P-value | ||
|---|---|---|---|
| 0.1 M sucrose (control) | 0.3 M sucrose (intervention) | ||
| Number of couples randomized | 99 | 102 | |
| Cumulative live birthsa | 52/99 (53%) | 43/102 (42%) | 0.17b |
| Outcome of embryo transfer in fresh cycle | |||
| Number of couples having embryo transfer | 95 | 93 | |
| Number of couples with live births | 35/95 (37%) | 36/93 (39%) | 0.91 |
| Number of babies born | |||
| Singleton | 21 | 23 | |
| Twin | 13c | 13d | |
| Triplet | 1 | 0 | |
| Number of: | |||
| Clinical pregnancies | 37 | 39 | |
| Miscarriages | 2 | 1 | |
| Terminations | 0 | 1 | |
| Ectopic pregnancies | 0 | 1 | |
| Stillbirths | 1 | 1 | |
| Neonatal deaths | 1 | 0 | |
| Outcome of embryo transfer over all frozen cycles | |||
| Number of couples having ≥1 frozen embryo transfer | 63 | 59 | |
| Number of couples with at least one live birth | 19/63 (30%) | 9/59 (15%) | 0.08 |
| Number of live births per embryo transfere | 21/89 (24%) | 10/87 (11%) | |
| Number of babies born over all frozen embryo transfers | |||
| Singleton | 16 | 8 | |
| Twin | 5 | 2 | |
| Number of: | |||
| Clinical pregnancies | 30 | 17 | |
| Miscarriages | 7 | 5 | |
| Terminations | 1 | 1 | |
| Ectopic Pregnancies | 0 | 0 | |
| Stillbirths | 1 | 1 | |
| Neonatal deaths | 0 | 0 | |
aCumulative live birth per couple is the birth of one or more live babies from the fresh and all frozen embryo transfer cycles arising from a single oocyte collection.
bCox regression adjusted for female age, female parity and couple diagnosis.
cIncludes two twin deliveries with one live birth and one stillbirth or neonatal death.
dIncludes one twin delivery with one live birth and one stillbirth.
eThree couples (two control and one intervention) had live births in two frozen transfer cycles.
Figure 2.
Time to first live birth in groups allocated to control or intervention. For couples with more than one pregnancy leading to live birth, the time to event is defined by the number of years between randomization and the date of the first live birth; subsequent live births are not included; couples with no live birth were censored at the date the database was closed or at the date all their embryos were discarded or exported.
Embryo transfer in the oocyte collection (fresh) cycle
Thirteen (6%) couples (four control; nine intervention) had no embryos transferred in the oocyte collection cycle because the woman was at risk of OHSS. A total of 188 couples had one or two embryos transferred on Day 2 or Day 3 p.i., with the majority (93%) of transfers on Day 2 (88 control; 87 intervention). The distribution of transfers on Day 2 or Day 3 did not differ between the groups. The outcome of the transfers is shown in detail in Table III. Live birth rate per transfer was 35/95 (37%) in the control and 36/93 (39%) in the intervention group (P = 0.91). The incidence of multiple birth, ectopic pregnancy, termination, miscarriage, stillbirth and neonatal death was similar in both groups.
Transfer of frozen-thawed embryos
Of the 67 control and 59 intervention couples who had embryos thawed with the intention of autologous transfer, four control couples had no surviving embryos and thus no transfer. The remaining 63 control and 59 intervention couples had embryos transferred [median number (interquartile range: IQR): control 2 (2,3); intervention 2 (2,4)] in a similar number of transfers (89 versus 87, respectively). Most couples (44 control; 37 intervention) had just one cycle of thawing and transfer, but 19 control and 22 intervention couples had two or more cycles. Two of the control and five of the intervention couples had a 'mixed' transfer, i.e. one frozen and one non-frozen embryo transferred in cycles in which insufficient fresh embryos were available. For six couples, the clinical outcome from the frozen embryo is unambiguous: five failed to establish a pregnancy and one couple had dizygotic twins. All are included in the analysis. The seventh couple (intervention group) had a singleton birth that has been included in the analysis according to the intention to treat principle.
The distribution of baseline characteristics of the couples who received frozen embryo transfer in the two groups was similar to the distribution displayed for all randomized patients (Table I). Although we did not consider, a priori, the type of endometrial preparation for embryo transfer to be an important prognostic factor, there was—by chance—a slight excess of transfers in a natural cycle in the control arm (36/63 versus 28/59).
More control couples than intervention couples had a live birth after frozen embryo transfer [19/63 (30%) versus 9/59 (15%)] and this difference was of borderline significance (P = 0.08) (Table III). There is a similar disparity when the number of live births per embryo transfer is calculated [21/89 (24%) versus 10/87 (11%)]. The incidence of multiple birth, ectopic pregnancy, miscarriage, termination, stillbirth and neonatal death was similar in both groups.
Survival of the frozen embryos
Overall, 60% (586/972) of embryos were thawed and all but three were recovered and scored (Table IV). Significantly more embryos in the intervention group (196/267; 73%) than the control group (180/316; 57%) were fully intact [OR (95% CI) 2.9 (1.6, 5.4); P < 0.001]. When the less stringent definition of survival commonly used by embryologists was applied, i.e. embryos with ≥50% of cells intact, survival remained elevated in the intervention group [OR (95% CI) 2.4 (1.3, 4.2); P = 0.003].
Table IV.
Survival of embryos after freezing and thawing.
| Embryos frozen in a medium containing |
|||
|---|---|---|---|
| 0.1 M sucrose (control) | 0.3 M sucrose (intervention) | OR (95% CI); P | |
| Number of embryos frozen | 472 | 500 | |
| Total number of embryos (%) thaweda | 317 (67%)b | 269 (54%)c | |
| Number of embryos (%) recovered | 316 (67%) | 267 (53%) | |
| Number of embryos (%) fully intact | 180/316 (57%) | 196/267 (73%) | 2.9 (1.6, 5.4); <0.001d |
| Number of embryos (%) with ≥50% of blastomeres intact | 252/316 (80%) | 239/267 (90%) | 2.4 (1.3, 4.2); 0.003d |
OR, odds ratio; CI, confidence interval.
aIncludes embryos thawed for couples intending embryo transfer and those for couples who allowed observation of their embryos before they were discarded.
bFifteen embryos frozen on Day 1, 290 on Day 2 and 12 on Day 3 post insemination.
cTwenty three embryos frozen on Day 1, 224 on Day 2 and 22 on Day 3 post insemination.
dRandom effect logistic model includes random effect for couple and treatment effect is adjusted for day of embryo freezing.
Discussion
The results of this trial show that an increase in the concentration of sucrose in the freezing medium, from 0.1 to 0.3 M, was associated with higher rates of embryo survival, resulting in 23% (53/63 versus 45/74) more couples having at least 50% of their embryos fully intact after thawing. This improved survival was not reflected in an increase in the number of live births or in the cumulative live birth rate after the embryos were transferred.
This is the first trial examining the effect of changes in the sucrose content of embryo-freezing medium in which couples were allocated to treatment prospectively using a rigorous method of randomization. Furthermore, the couple (not the embryo) was the unit of primary analysis, reflecting the experimental design. Studies in which the unit of analysis (embryo) does not match the unit of allocation (couple) will lead to spuriously low P-values. Previously, Jericho et al. (2003) and Edgar et al. (2009) reported increased blastomere survival and a greater proportion of embryos surviving with all blastomeres intact, when biopsied and non-biopsied embryos were frozen in solutions with an elevated sucrose concentration (0.2 versus 0.1 M). The biopsied embryo data were analysed retrospectively (Jericho et al., 2003), while Edgar et al. (2009) randomized patients to treatment prospectively, but each freezing treatment was carried out in a different clinic. In both studies, the embryo was the unit of analysis although the patient was assigned to treatment. The present study suffered a slight loss of power because only 137 of the anticipated 170 couples had their embryos thawed. Nonetheless, the results presented provide the strongest evidence to date that the concentration of sucrose in the freezing medium is critical for blastomere survival. In future studies, a greater allowance should be made for the high proportion of couples who fail to return for embryo thawing.
Data from retrospective studies suggest that the transfer of embryos that survive freezing with all blastomeres intact increases the likelihood of live birth (Van den Abbeel et al., 1997; Guerif et al., 2002). It was hypothesized that increasing the proportion of intervention couples with at least 50% of thawed embryos intact would result in higher cumulative live birth rates. Since the present study was not powered for the secondary outcome of cumulative live birth, there is insufficient evidence to conclude that one solution is superior to the other. However, the data suggest that the true effect of freezing embryos in a medium containing 0.3 M sucrose is unlikely to be greater than a 1.13-fold increase in the rate of live birth compared with control, while treatment using 0.3 M sucrose could potentially reduce the live birth rate considerably. So, while we must be cautious in our interpretation because of the small sample size, our results suggest that freezing embryos in a medium containing 0.3 M sucrose is unlikely to have any beneficial effect on clinical outcome and may in fact be detrimental when compared with our standard practice of freezing in a medium containing 0.1 M sucrose. A retrospective comparison of clinical data led Borini et al. (2006) to question the clinical efficiency of freezing human oocytes in solutions similar to those in the intervention arm of this study, despite a significant increase in oocyte survival and high rates of fertilization. Thus evidence is accumulating to suggest that it is unwise to rely on surrogate end-points, such as embryo survival, when assessing changes in cryopreservation strategy. Future cryopreservation studies should be adequately powered to detect clinically worthwhile differences in cumulative live birth.
It is possible that the live birth rate after frozen embryo transfer was influenced by the clinical strategy of thawing sufficient embryos to obtain the number required for transfer, with at least one embryo fully intact. In a pragmatic trial where the intention is to mirror normal clinical practice, it is inevitable that there will be conflict with sound experimental design. However, it seems unlikely that the outcome in this study was biased since the live birth rate after frozen transfer amongst control couples was almost double that of the intervention couples. The embryo selection policy would have been expected to minimize differences between the two groups.
Similarly, the excess of frozen embryo transfers in natural cycles in the control arm of the trial (57 versus 48%), could have potentially influenced the clinical outcome. Transfer in natural or stimulated cycles was carried out entirely according to clinical protocols and thus this disparity occurred purely by chance. To the best of our knowledge, there is no conclusive evidence (Ghobara and Vanderkerchove, 2008; Hill et al., 2010) to suggest that transfer in a natural cycle is more favourable than transfer in a stimulated cycle. The incidence of live birth after frozen embryo transfer in the control group was double that in the intervention arm (Table III) and thus, although this relatively small imbalance could either reduce or increase the difference between live birth rate in the two arms, it is very unlikely that it would result in the intervention treatment being superior to control.
The requirement to use commercially available freezing and thawing solutions made it impossible to change the concentration of sucrose in the freezing medium without introducing confounding factors. The slight reduction in PrOH concentration in the intervention group (from 1.5 to 1.4 M) plainly had no deleterious effect on embryo survival. The protein content of the two freezing media also differed, but—since others have associated sucrose concentration with oocyte (Fabbri et al., 2001) and embryo (Jericho et al., 2003; Zheng et al., 2005; Edgar et al., 2009) survival—it seems likely that the increased survival reported here in the intervention arm can be attributed to the elevated sucrose. The inclusion of similar concentrations of sucrose in both freezing and diluent solutions in the intervention arm may not be optimal (Jericho et al., 2003; Bianchi et al., 2007; Edgar et al., 2009), but nonetheless embryo survival was plainly superior to that of controls.
Only 31% of the couples approached (201/652; Fig. 1), took part in the trial but it is unlikely that this introduced bias in our study population. Not unexpectedly, 40% of couples declined to take part in research that might directly affect the outcome of their treatment. Subsequently almost 50% (192/393) of those who gave informed consent were excluded, mainly because they did not meet the inclusion criterion of having at least two embryos suitable for freezing—a proportion similar to that among all couples having treatment in the ARU. A potential weakness of the trial concerns the lower proportion of couples returning for frozen embryo transfer in the 0.3 M sucrose group. There is no reason to believe that the freezing solution influenced a couple's decision to return, especially because similar levels of live birth occurred in the fresh cycle and couples were blinded to treatment allocation unless they asked for the information. Although it is likely that this difference has occurred by chance, there is a small possibility that bias has been introduced. However, the difference in embryo survival is so striking that it is unlikely that any bias introduced by the differential return rate would change the overall result.
Recently many clinics have begun to vitrify embryos as an alternative to freezing, mainly because of the higher rates of survival obtained (Rama Raju et al., 2005; Balaban et al., 2008). However the clinical superiority of vitrification over freezing has not been demonstrated in RCTs (Kolibianakis et al., 2009). Vitrification has risks associated with the use of unsealed containers and the storage of ultra-small samples (Bielanski and Vajta, 2009; Wood, 2011) and it is too early to be certain that it carries no risks for any children born. The low rates of embryo survival so often reported after freezing in solutions containing PrOH and 0.1 M sucrose are overcome by increasing the concentration of sucrose as demonstrated here and elsewhere (Jericho et al., 2003; Edgar et al., 2009). Although we were unable to show an improvement in clinical outcome after transfer of embryos frozen in 0.3 M sucrose, Edgar et al. (2009) reported that freezing in a medium containing 0.2 M sucrose increased the rate of implantation per embryo thawed and thus the efficiency of the freezing programme. It is plain that the outcome of freezing can be improved and it would be prudent to retain an open mind as to the superiority of any cryopreservation approach until randomized controlled trials with live birth as a primary outcome are reported.
Conclusion
This trial shows that increasing the concentration of sucrose from 0.1 to 0.3 M in the freezing medium improves survival in early stage human embryos and significantly increases the likelihood of a couple having at least one fully intact frozen-thawed embryo for transfer. It provides no evidence that the increased embryo survival translates into increased cumulative birth rates for the couples and has highlighted the need for robust clinical outcomes in laboratory trials.
Authors’ roles
M.J.W. contributed to the conception and design of the trial and interpretation of the results, wrote the first draft of the article, and critically reviewed and approved the final version; J.M. analysed and interpreted the results, contributed to writing the article, and critically reviewed and approved the final version; K.H. was involved in initial statistical analysis and interpretation of data, critical review of the manuscript and approval of the final version. E.F., T.M., A.S. and L.B. contributed to the acquisition of data, critical review of the manuscript and approval of the final version. S.B. contributed to the conception and design, critically reviewed the manuscript and approved the final version.
Funding
The trial was funded by The Wellcome Trust (067469). Medicult (Surrey, UK) provided the intervention freezing solution for half of all participants in the trial. Funding to pay the Open Access publication charges for this article was provided by The Wellcome Trust.
Acknowledgements
The authors are grateful to Adriana Bos-Mikich, Anne Duncan, Moira Laing and Valerie Peddie for their assistance in obtaining consent from couples and with embryo cryopreservation. We are indebted to the data management team for setting up the database.
References
- Archer J, Gook DA, Edgar DH. Blastocyst formation and cell numbers in human frozen-thawed embryos following extended culture. Hum Reprod. 2003;18:1669–1673. doi: 10.1093/humrep/deg319. [DOI] [PubMed] [Google Scholar]
- Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, Gardner DK. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23:1976–1982. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, Braude P, Kennedy R, Rutherford A, Hartshorne G, et al. Conventional in-vitro fertilization versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357:2075–2079. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]
- Bianchi V, Coticchio G, Distratis V, Di Giusto N, Flamigni C, Borini A. Differential sucrose concentration during dehydration (0.2 mol/l) and rehydration (0.3 mol/l) increases the implantation rate of frozen human oocytes. Reprod BioMed Online. 2007;14:64–71. doi: 10.1016/s1472-6483(10)60765-1. [DOI] [PubMed] [Google Scholar]
- Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24:2457–2467. doi: 10.1093/humrep/dep117. [DOI] [PubMed] [Google Scholar]
- Borini A, Sciajno R, Bianchi V, Sereni E, Flamigni C, Coticchio G. Clinical outcome of oocyte cryopreservation after slow cooling with a protocol utilizing a high sucrose concentration. Hum Reprod. 2006;21:512–517. doi: 10.1093/humrep/dei346. [DOI] [PubMed] [Google Scholar]
- Burns WN, Gaudet TW, Martin MB, Leal YR, Schoen H, Carlton AE, Schenken RS. Survival of cryopreservation and thawing with all blastomeres intact identifies multicell embryos with superior frozen embryo transfer outcome. Fertil Steril. 1999;72:527–532. doi: 10.1016/s0015-0282(99)00280-0. [DOI] [PubMed] [Google Scholar]
- de Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V, Kupka M, Nygren KG, Nyboe Andersen A The European IVF-monitoring (EIM) Consortium, for the European Society of Human Reproduction and Embryology (ESHRE) Assisted reproductive technology in Europe 2006: results generated from European registers by ESHRE. Hum Reprod. 2010 doi: 10.1093/humrep/des255. doi:10.1093/humrep/deq124. [DOI] [PubMed] [Google Scholar]
- Edgar DH, Bourne H, Speirs AL, McBain JC. A quantitative analysis of the impact of cryopreservation on the implantation potential of human early cleavage stage embryos. Hum Reprod. 2000;15:175–179. doi: 10.1093/humrep/15.1.175. [DOI] [PubMed] [Google Scholar]
- Edgar DH, Karani J, Gook DA. Increasing dehydration of human cleavage-stage embryos prior to slow cooling significantly increases cryosurvival. Reprod BioMed Online. 2009;19:521–525. doi: 10.1016/j.rbmo.2009.06.002. [DOI] [PubMed] [Google Scholar]
- El-Toukhy T, Khalaf Y, Al-Darazi K, Andritsos V, Taylor A, Braude P. Effect of blastomere loss on the outcome of frozen embryo replacement cycles. Fertil Steril. 2003;79:1106–1111. doi: 10.1016/s0015-0282(03)00072-4. [DOI] [PubMed] [Google Scholar]
- Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16:411–416. doi: 10.1093/humrep/16.3.411. [DOI] [PubMed] [Google Scholar]
- Ghobara T, Vanderkerchove P. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database of Syst Rev. 2008;1:003414. doi: 10.1002/14651858.CD003414.pub2. [DOI] [PubMed] [Google Scholar]
- Guerif F, Bidault R, Cadoret V, Couet M-L, Lansac J, Royere D. Parameters guiding selection of best embryos for transfer after cryopreservation: a reappraisal. Hum Reprod. 2002;17:1321–1326. doi: 10.1093/humrep/17.5.1321. [DOI] [PubMed] [Google Scholar]
- Haggarty P, Wood M, Ferguson E, Hoad G, Srikantharajah A, Milne E, Hamilton M, Bhattacharya S. Fatty acid metabolism in human preimplantation embryos. Hum Reprod. 2006;21:766–773. doi: 10.1093/humrep/dei385. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Miller KA, Frattarelli JL. A GnRH agonist and exogenous hormone stimulation protocol has a higher live-birth rate than a natural endogenous hormone protocol for frozen-thawed blastocyst-stage embryo transfer cycles: an analysis of 1391 cycles. Fertil Steril. 2010;93:416–422. doi: 10.1016/j.fertnstert.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Jericho H, Wilton L, Gook DA, Edgar DH. A modified cryopreservation method increases the survival of human biopsied cleavage stage embryos. Hum Reprod. 2003;18:568–571. doi: 10.1093/humrep/deg106. [DOI] [PubMed] [Google Scholar]
- Kattera S, Chen C. A modified embryo cryopreservation method increases post-thaw survival with a concomitant increase in implantation. Fertil Steril. 2005;84:1498–1504. doi: 10.1016/j.fertnstert.2005.04.054. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Curr Opin Obstet Gynecol. 2009;21:270–274. doi: 10.1097/GCO.0b013e3283297dd6. [DOI] [PubMed] [Google Scholar]
- Lassalle B, Testart J, Renard J-P. Human embryo features that influence the success of cryopreservation with the use of 1,2 propanediol. Fertil Steril. 1985;44:645–651. doi: 10.1016/s0015-0282(16)48981-8. [DOI] [PubMed] [Google Scholar]
- Mandelbaum J, Junca AM, Plachot M, Alnot MO, Salat-Baroux J, Alvarez S, Tibi C, Cohen J, Debache C, Tesquier L. Cryopreservation of human embryos and oocytes. Hum Reprod. 1988;3:117–119. doi: 10.1093/oxfordjournals.humrep.a136642. [DOI] [PubMed] [Google Scholar]
- Mohr LR, Trounson A, Freeman L. Deep-freezing and transfer of human embryos. J In Vitro Fertil Embryo Transfer. 1985;2:1–10. doi: 10.1007/BF01130825. [DOI] [PubMed] [Google Scholar]
- Pal L, Kovacs P, Witt B, Jindal S, Santoro N, Barad D. Postthaw blastomere survival is predictive of the success of frozen-thawed embryo transfer cycles. Fertil Steril. 2004;82:821–826. doi: 10.1016/j.fertnstert.2004.02.136. [DOI] [PubMed] [Google Scholar]
- Rama Raju GA, Haranath GB, Krishna KM, Prakash GJ, Madan K. Vitrification of human 8-cell embryos, a modified protocol for better pregnancy rates. Reprod BioMed Online. 2005;11:434–437. doi: 10.1016/s1472-6483(10)61135-2. [DOI] [PubMed] [Google Scholar]
- Renard JP, Babinet C. High survival of mouse embryos after rapid freezing and thawing inside plastic straws with 1,2 propanediol as cryoprotectant. J Exp Zool. 1984;230:443–448. doi: 10.1002/jez.1402300313. [DOI] [PubMed] [Google Scholar]
- Testart J, Lassalle B, Belaisch-Allart J, Hazout A, Forman R, Rainhorn JD, Frydman R. High pregnancy rate after early human embryo freezing. Fertil Steril. 1986;46:268–272. doi: 10.1016/s0015-0282(16)49524-5. [DOI] [PubMed] [Google Scholar]
- Testart J, Lassalle B, Forman R, Gazengel A, Belaisch-Allart J, Hazout A, Rainhorn JD, Frydman R. Factors influencing the success rate of human embryo freezing in an in vitro fertilization and embryo transfer program. Fertil Steril. 1987;48:107–112. doi: 10.1016/s0015-0282(16)59298-x. [DOI] [PubMed] [Google Scholar]
- Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, Bergh C. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–2402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- Tiitinen A, Halttunen M, Härkki P, Vuoristo P, Hyden-Granskog C. Elective single embryo transfer: the value of cryopreservation. Hum Reprod. 2001;16:1140–1144. doi: 10.1093/humrep/16.6.1140. [DOI] [PubMed] [Google Scholar]
- Van den Abbeel E, Van der Elst J, Van Waesberghe L, Camus M, Devroey P, Khan I, Smitz J, Staessen C, Wisanto A, Van Steirteghem A. Hyperstimulation: the need for cryopreservation of embryos. Hum Reprod. 1988;3(Suppl. 2):53–57. doi: 10.1093/humrep/3.suppl_2.53. [DOI] [PubMed] [Google Scholar]
- Van den Abbeel E, Camus M, Van Waesberghe L, Devroey P, Van Steirteghem AC. Viability of partially damaged human embryos after cryopreservation. Hum Reprod. 1997;12:2006–2010. doi: 10.1093/humrep/12.9.2006. [DOI] [PubMed] [Google Scholar]
- Van der Elst J, Camus M, Van den Abbeel E, Maes R, Devroey P, Van Steirteghem AC. Prospective randomized study on the cryopreservation of human embryos with dimethylsulfoxide or 1,2-propanediol protocols. Fertil Steril. 1995;63:92–100. [PubMed] [Google Scholar]
- Van Steirteghem AC, Van den Abbeel E, Camus M, Van Waesberghe L, Braeckmans P, Khan I, Nijs M, Smitz J, Staessen C, Wisanto A, et al. Cryopreservation of human embryos obtained after gamete intra-Fallopian transfer and/or in-vitro fertilization. Hum Reprod. 1987;2:593–598. doi: 10.1093/oxfordjournals.humrep.a136595. [DOI] [PubMed] [Google Scholar]
- Veleva Z, Vilska S, Hydén-Granskog C, Tiitinen A, Tapanainen JS, Martikainen H. Elective single embryo transfer in women aged 36–39 years. Hum Reprod. 2006;21:2098–2102. doi: 10.1093/humrep/del137. [DOI] [PubMed] [Google Scholar]
- Veleva Z, Karinen P, Tomás C, Tapanainen JS, Martikainen H. Elective single embryo transfer with cryopreservation improves the outcome and diminishes the costs of IVF/ICSI. Hum Reprod. 2009;24:1632–1639. doi: 10.1093/humrep/dep042. [DOI] [PubMed] [Google Scholar]
- Wood M. Vitrification of oocytes. Obstet Gynaecol. 2011 in press. [Google Scholar]
- Zheng WT, Zhuang GL, Zhou CQ, Fang C, Ou JP, Li T, Zhang MF, Liang XY. Comparison of the survival of human biopsied embryos after cryopreservation with four different methods using non-transferable embryos. Hum Reprod. 2005;20:1615–1618. doi: 10.1093/humrep/deh808. [DOI] [PubMed] [Google Scholar]