Abstract
BACKGROUND
Although infertility is a serious concern in survivors of pediatric cancers, little is known about the influence of the degree of sexual maturation at the time of irradiation on spermatogenic recovery after treatment. Thus, we address this question in a non-human primate model, the rhesus monkey (Macaca mulatta).
METHODS
Two pubertal (testis size 3 and 6.5 ml, no sperm in ejaculate) and four prepubertal (testis size 1 ml, no sperm in ejaculate) macaques were submitted to a single fraction of testicular irradiation (10 Gy). Unilateral autologous transfer of cryopreserved testis cells was performed 2 months after irradiation. Testicular volume, histology and semen parameters were analyzed to assess irradiation effects and testicular recovery.
RESULTS
Irradiation provoked acute testis involution only in the two pubertal monkeys. Subsequently, testis sizes recovered and sperm was present in the ejaculates. Longitudinal outgrowth of seminiferous tubules continued, and, in testes without autologous cell transfer, 4–22% of tubular cross sections showed spermatogenesis 2 years after irradiation. In contrast, the four prepubertal monkeys showed neither a detectable involution as direct response to irradiation, nor a detectable growth of seminiferous tubules later. However, two of these animals showed spermarche 2 years after irradiation, and 8–12% of tubules presented spermatogenesis. One prepubertally irradiated monkey presented fast growth of one testis after cell transfer, and showed spermarche 1 year after irradiation. The infused testis had spermatogenesis in 70% of the tubules. The contralateral testis remained smaller.
CONCLUSION
We conclude that irradiation before puberty has a severe detrimental effect on outgrowth of seminiferous tubules. But, within the seminiferous epithelium, spermatogenetic recovery occurs at a low rate with no detectable relation to the maturity of the epithelium at irradiation. We also show that autologous testis cell transplantation can enhance spermatogenesis, but only in isolated cases.
Keywords: testis, irradiation, puberty, rhesus monkey, germ cell transfer
Introduction
In the human testis, the exact mechanism of spermatogonial recovery after irradiation has not been conclusively elucidated but it appears to involve the activation of surviving spermatogonial stem cells. The speed of recovery is dependent on the dose and fractionation of irradiation therapy (Rowley et al., 1974; Hahn et al., 1982; Schlatt et al., 2009). In men recolonization of surviving spermatogonia can first be detected 6 months after a dose of 0.2 Gy (Rowley et al., 1974), 9–18 months after a dose of 1 Gy and >4 years after a dose of 10 Gy (Rowley et al., 1974; Anserini et al., 2002). Observations in adult monkeys revealed that testicular irradiation with doses of 0.5–4 Gy leads to an immediate decline of the population of Apale spermatogonia while that of Adark spermatogonia remains initially unchanged (van Alphen et al., 1988a). Following irradiation, the previously quiescent Adark spermatogonia start to proliferate, which, at higher doses of radiation, is followed by a decrease in the number of both Apale and Adark spermatogonia. Analysis of spermatogenesis in adult monkeys who had been exposed to testicular irradiation with doses of 4–8.5 Gy during the juvenile period revealed that recovery can occur focally in a few seminiferous tubules up to 3–4 years after irradiation and that the final testicular size remains reduced (de Rooij et al., 2002). Significant morphological changes were also detected in the somatic environment, particularly in Sertoli cells.
Pediatric patients undergoing total body irradiation for marrow ablation therapy prior to hematopoietic stem cell transplantation receive testicular irradiation doses of 10–12 Gy. Very little is known with respect to the acute effects of radiation on prepubertal and pubertal human testes. The best and closest model organisms to obtain clinically relevant data are non-human primates. Macaques show close anatomical and endocrinological similarities to men and have therefore been used in previous approaches to explore irradiation defects on the testis (van Alphen et al., 1988a,b; de Rooij et al., 2002). Here we monitored the testicular recovery after a single-fraction testicular irradiation of 10 Gy. Two of the irradiated animals were early pubertal and four were prepubertal at irradiation. Unilaterally, one testis in each monkey was injected with autologous testicular cells, which had been cryopreserved prior to irradiation. We were able to determine the differential effects of irradiation on outgrowth of seminiferous tubules according to pubertal stage at the time of irradiation, while spermatogenetic recovery occurred at a low scale with no detectable relation to the maturity of the epithelium at irradiation.
Materials and Methods
Animals
Six male rhesus monkeys at the ages of 30–49 months (Macaca mulatta) and derived from the institutional breeding facilities were used for the study. All animal experiments were approved by and performed under the guidance of the Animal Care and Use Committee at the University of Pittsburgh, School of Medicine.
Testicular biopsy and testicular single-cell preparation
Testicular size of animals was recorded monthly for 4 months before testicular irradiation. One of the testes in each animal was biopsied under general anesthesia using isoflurane 6 weeks before testicular irradiation. In one monkey (#3199), unilateral orchidectomy was performed. The collected testicular tissue was dissected into small fragments (∼1 mm3). Minced tissue was analyzed using phase-contrast microscopy to determine whether spermatozoa were present in the tissues, and a sample was also fixed in Bouin solution (see below). Most of the material was processed to obtain a testicular single-cell solution, which was cryopreserved as described earlier (Hermann et al., 2007). Briefly, testis tissue was digested using 1 mg/ml collagenase I (Sigma, St. Louis, MO, USA; No. C2674) and 5 mg/ml DNase (15 U/ml; Roche Applied Science, Indianapolis, IN, USA; No. 104132) in Dulbecco's Minimum Essential Medium (DMEM, 4.5 g glucose/ml; Mediatech Inc., No. 10-013-CV) mixed 1:1 with Ham's F12 (Mediatech Inc.; No. 10-080-CV), supplemented with 1% non-essential amino acids (BioWhittaker Walkersville, MD, USA; No. 13-114E), 100 IU/ml Penicillin and 100 mg/ml Streptomycin (Mediatech Inc.; No. 30-002-CI). The cell suspension was maintained at 37°C during digestion (15–20 min). The fragments of seminiferous tubules were obtained by sedimentation at unit gravity for 5 min and were then digested into a single-cell suspension using 1 mg/ml collagenase I, 5 lg/ml DNase and 1 mg/ml hyaluronidase (Sigma, No. H-3506). The cell suspension was cleared by straining through a mesh. Cells were then removed from the digestion medium by centrifugation and washing with fresh medium. For cryopreservation, the pellet was resuspended with DMEM high glucose (4 g/l) containing 10% fetal calf serum, non-essential amino acids and antibiotics. The suspensions were then placed on ice. Within 15 min, dimethylsulfoxide (DMSO) was added dropwise to the suspension to a final concentration of 1.4 M. The cells were then transferred into cryovials and submitted to a slow freezing protocol (Jahnukainen et al., 2007). In addition, small fragments of tissues (1 mm3) were cryopreserved, employing the same cryopreservation media and similar procedures as stated above, for future grafting procedures not reported here.
Irradiation procedure
Local irradiation of the scrotal area with both testes in the irradiation field was performed at UPMC Presbyterian Hospital. Animals were shipped from the animal facility to the hospital. They were sedated using ketamine and transported to the irradiation vault in transport cages. Prior to irradiation, the length, width and thickness of both testes of each monkey were measured accurately using calipers to determine the target volume. The lengths and widths of the testes varied from 14.2 to 25.4 mm and 6.8 to 16 mm respectively; average thickness of the testes was 12 mm. During irradiation the testes were placed on a 30 cm × 30 cm × 2.5 cm (length × width × thickness) bolus material. A Superflab (Radiation Products Design, Inc., USA) phantom, reproducing the shape and size of the testes and the bolus material on which they were placed, was constructed. CT scan of this phantom was performed and transferred to an ADAC Pinnacle (v 7.0 g) treatment planning system (Phillips Medical System, Andover, MA, USA). Treatment plans were generated such that they provided a uniform dose distribution to the entire target volume. A dose of 10 Gy, at a dose rate of 2.3 Gy/min, was delivered to the 99% isodose line which covered 100% of the target volume which was defined as the total volume of the two testes; 6 MV photon beams from a Varian Clinac 2100 linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) were used for irradiation. All monkeys were placed in supine position on the treatment table and irradiated using an anterior–posterior field of size of 6 cm × 6 cm, except for monkeys #3212 and #3083, in which cases field sizes of 6 cm × 9 cm and 7 cm × 6 cm were used, respectively. Also, for the purpose of delivering uniform dose to both testes, as calculated by the treatment planning system, monkeys were irradiated by placing 1.0 cm thick and 30 cm square Superflab bolus material above the entire irradiation area. The isocenter of the beams was placed in the middle of the irradiation volume.
Testicular cell transplantation
Vials with cryopreserved cell suspensions were rapidly thawed in a water bath at 37°C. Ice cold DMEM was slowly added to dilute the cryomedia ∼10-fold. Cells were kept on ice for a maximum of 1 h until spun down by centrifugation immediately prior to germ cell infusion. Then the pellet was resuspended in 100 µl of DMEM taken up into the bottom of a 1 ml syringe which was filled with an additional 500 µl of medium, and 150–400 µl of cell suspension and medium were flushed autologously into one testis of each anesthetized (Ketamine) monkey. The contralateral testis received an infusion of DMEM. The injection was guided to reach the rete testis of the monkeys but was performed without ultrasound guidance. Instead the injection was performed through the scrotum into the central area of each testis. Injections into the rather large area of the rete testis in immature monkeys were judged by an obviously lower backpressure and higher resistance of the rete testis tissue compared with the surrounding tubular tissue. Infusions were terminated when the testis had reached a critical hardening due to the infusion of the DMEM. The physical consistence of the testicular parenchyma differs from the consistence of the rete testis, so that the operator notices when the needle is placed in the rete testis. The method was further validated by performing sham injections with DMEM and Trypan Blue into excised immature primate testes. It was possible to reproducibly and reliably fill the rete testis and adjacent testicular tubules with indicator dye (Trypan Blue) during those sham injections.
Follow-up after testicular irradiation
Testicular size was recorded monthly up to the end of experiment. Animals were electroejaculated three times prior to irradiation using a rectal probe. Monthly electroejaculations were reinitiated 10 months after irradiation and were continued up to the end of experiment. Semen samples were analyzed microscopically and sperm concentration and motility were determined. In cases where no sperm were found, the ejaculate was spun down and the presence of sperm was analyzed in the pellet. Bilateral testicular biopsies were performed 18 months after irradiation and orchiectomies were performed 2 years after irradiation. Testicular tissue was fixed for 18–24 h in Bouin's solution, transferred for storage into 70% ethanol and embedded in paraffin for sectioning at 4 µm. All tissue sections were stained with periodic acid-Schiff's reagent (PAS)/Gill's hematoxylin and examined with oil immersion under the light microscope.
Histology and immunohistochemistry
For immunohistochemistry, Bouin-fixed testicular tissue was embedded in paraffin, sectioned to 4 μm thickness and stained as indicated below. The antibodies employed were anti-VASA (Abcam, Cambridge, FL, USA, cat. no. ab13840; dil. 1:100), anti-OCT3/4 (H-134, Santa Cruz, Heidelberg, Germany, cat. no. sc-9081; dil. 1:100), anti-AP-2γ (Santa Cruz, cat. no. sc-12762; dil. 1:50), anti-c-KIT (C-19, Santa Cruz, cat. no. sc-168; dil. 1:100) and MAGE-A (gift from Dr Giulio Spagnoli, University of Basel, Switzerland) with the appropriate biotinylated secondary antibodies: goat anti-rabbit (Sigma, cat. no. B7389, Steinheim, Germany) and goat anti-mouse (Sigma, cat. no. B7264, Steinheim, Germany). Briefly, sections were dewaxed in xylene, rehydrated in graded alcohols and washed in tap water. All slides underwent antigen retrieval by heating for 40 min in a steam cooker in 0.01 M citrate buffer (pH 6.0) as described previously (Norton et al., 1994). Sections were treated with 3% (v/v) H2O2 in distilled water, rinsed with distilled water and washed in Tris-buffered saline (TBS, 0.05 M Tris and 0.85% (w/v) NaCl, pH 7.6). Sections were blocked from non-specific binding with 25% goat serum diluted in TBS containing 0.5% (w/v) bovine serum albumin (BSA) (Sigma, cat. no. A9647) for 30 min. For the staining with AP-2γ, the goat serum was diluted at 5% in TBS containing 0.1% (w/v) BSA. The slides were incubated overnight with the primary antibody at 4°C in a humidified chamber. Sections were washed three times for 5 min with TBS and incubated 1h with the biotinylated secondary antibody diluted 1:100 in goat serum (5% v/v) at 4°C in the humidified chamber. This was followed by two further 5 min washes in TBS and incubation for 30 min with Streptavidin–horse-radish peroxidase at 1:100 (Sigma, cat. no. S5512), diluted in TBS. For a chromogen, 3,3′-diaminobenzidine (DAB; Sigma, cat. no. D4168) was employed following the manufacturer's recommendations. Sections were counterstained with hematoxylin, dehydrated in graded alcohols and immersed in xylene before mounting with Merckoglass (Merck, Nottingham, England). For each experiment a negative control was included, in which the primary antibody had been omitted. Images were captured using an Axioskop connected to an Axiocam (Carl Zeiss, Inc., Oberkochen, Germany).
Spermatogenetic recovery was defined by light microscopic observation of seminiferous tubules. The fertility index (percentage of tubules containing spermatogonia), most advanced germ cell type and percentage of tubules with the most advanced germ cell type were recorded in 50 tubular cross sections selected for analysis by random systematic sampling. To obtain the absolute cord volume, a point-counting analysis was applied in which the percentage of the testicular area occupied by cords was calculated from a total of 42 points per field. The cord size was calculated from independent measurements of the cord diameter in nearly round seminiferous tubular cross sections (n = 20 per monkey). The length of the cords was estimated by using the formula for the height of a cylinder: h = v/(π × r2), where ‘h' represents the length of the cord, ‘v' the volume of the cords and ‘r' the radius of the cord section. For analysis of immunostaining in germ cells, the cells were recognized by their uniform round nuclear shape. The light microscopic determinations were conducted by one observer (K.J.). Single data points represent the results from a single monkey. Data in the table are presented as a mean.
Results
Testicular histology before irradiation
Histological analysis before irradiation revealed meiotic germ cells in the testis of 36- and 49-month-old monkeys. These pubertal animals had round spermatids and spermatozoa, respectively, as the most advanced germ cell type. The other four monkeys aged 30–48 months showed prepubertal testicular histology. Two of them had B-spermatogonia and two others had preleptotene spermatocytes as the most advanced germ cell type. Electroejaculation before irradiation showed no spermatozoa in semen samples of all monkeys. However, in the 49-month-old monkey, motile intratesticular spermatozoa were detected when fresh minced testicular tissue was observed using phase-contrast microscopy. Morphological data are shown in Table I and Fig. 3.
Table I.
Basic morphometric data of testicular tissue of monkeys that received 10 Gy testicular irradiation at the prepubertal period (n = 4) and during pubertal initiation of spermatogenesis (n = 2).
| Age at irrad (mo) | Side | Type of injection | Pre irradiation |
18 months after irradiation |
24 months after irradation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tubular length (m) | FI (%) | Most advanced germ cell type | Proportion of tubules with most advanced germ cell type (%) | Tubular length (m) | FI (%) | Most advanced germ cell type | Proportion of tubules with most advanced germ cell type (%) | Tubular length (m) | FI (%) | Most advanced germ cell type | Proportion of tubules with most advanced germ cell type (%) | ||||
| Pubertals | |||||||||||||||
| #3083 | 36 | Left | Medium | - | - | - | - | 3.09 | 22 | Sperm | 12 | 4.24 | 22 | Sperm | 22 |
| Right | Cells | 1.62 | 100 | Rounds | 6 | 3.09 | 14 | Sperm | 8 | 3.86 | 24 | Sperm | 22 | ||
| #3212 | 49 | Left | Medium | - | - | - | - | 5.05 | 6 | Sperm | 4 | 5.22 | 24 | Sperm | 4 |
| Right | Cells | 2.52 | 100 | Sperm | 33 | 5.09 | 2 | Sperm | 2 | 5.52 | 15 | Sperm | 11 | ||
| Prepubertals | |||||||||||||||
| #3117 | 30 | Left | Cells | 1.26 | 83 | B-spg | 31 | 0.35 | 0 | SCO | 0.20 | 0 | SCO | ||
| Right | Medium | - | - | - | - | 0.35 | 0 | SCO | 0.08 | 0 | SCO | ||||
| #3082 | 37 | Left | Cells | - | - | - | - | 1.65 | 0 | SCO | 1.24 | 0 | SCO | ||
| Right | Medium | 1.35 | 93 | Prelept | 5 | 1.17 | 0 | SCO | 1.13 | 18 | Rounds | 12 | |||
| #3199 | 34 | Left | Medium | 2.29 | 91 | Prelept | 2 | - | - | - | - | - | - | - | - |
| Right | Cells | - | - | - | - | 1.80 | 20 | Sperm | 6 | 1.91 | 24 | Sperm | 8 | ||
| #3213 | 48 | Left | Medium | 1.12 | 100 | B-spg | 33 | 1.80 | 5 | Sperm | 2 | Nd | Nd | Nd | Nd |
| Right | Cells | - | - | - | - | 1.91 | 100 | Sperm | 70 | 4.01 | 8 | Sperm | 8 | ||
FI = fertility index, rounds = round spermatids, sperm = spermatozoa, spg = spermatogonia, prelept = preleptotene spermatocytes, SCO = Sertoli cell only, Nd = not available.
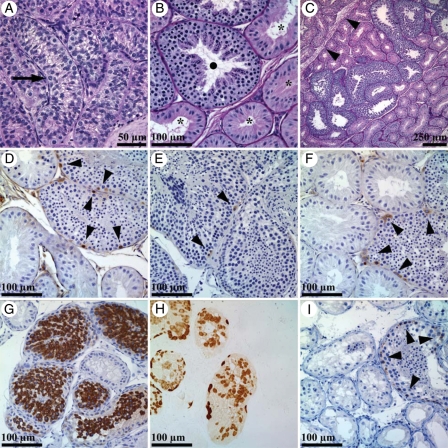
Figure 3.
Representative light micrographs of prepubertal monkey testicular tissue before (3a) and 2 years after irradiation with a dose of 10 Gy as a single fraction (3b–i). Before irradiation, only B-spermatogonia (3a, arrow) were detected and spermatogenesis was not initiated. After irradiation, recovering seminiferous tubules showed full spermatogenesis (3b, •) while non-recovering tubules showed Sertoli cell only pattern (3b, *). Spermatogenetic recovery was shown to be localized in a single testicular lobulus separated by fibrotic septa (3c, arrowheads). Seminiferous tubules with recovering spermatogenesis showed normal expression of germ cell markers NANOG (3d, arrowheads) PGP9,5 (e, arrowheads), GFRalpha1 (3f, arrowheads), VASA (3g) MAGE-A (3h) and C-kit (3i, arrowheads), as visualized by brown DAB chromogen.
Testicular size and sperm samples after irradiation
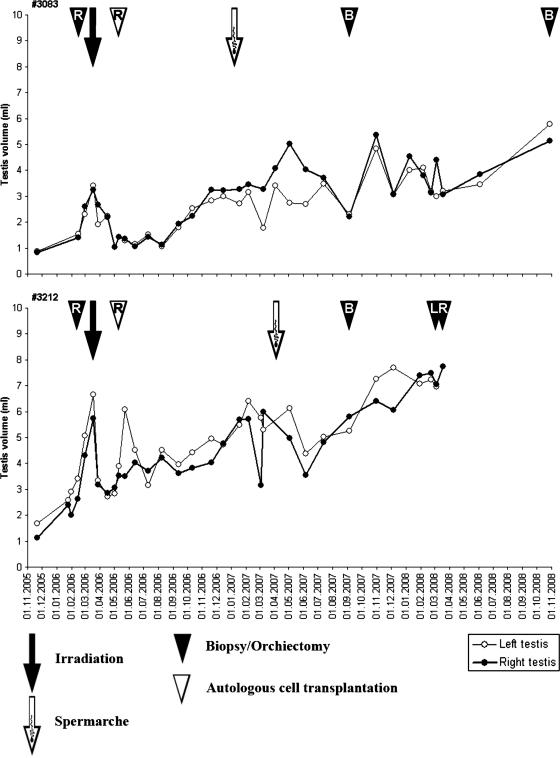
Changes in testicular size for respective monkeys are depicted in Figs 1 and 2. Fast involution of testicular size was detected for the two pubertal monkeys until 2 months after irradiation when a long recovery period with steadily increasing testicular sizes was observed. The pre-irradiation level of testicular size (3–6 ml) was reached at ∼1 year post-irradiation. Concurrently, the first spermatozoa were detected in semen samples (Fig. 1). The pubertal monkeys had spermarche 10 and 13 months after irradiation. They showed a low sperm concentration of 0.02–8.6 million cells/ml with 30–60% motility during the remainder of the follow-up period. Testicular growth of the pubertal animals continued during the 2 years follow-up period and reached the size of 5 and 7 ml. No difference in the size was detected between transplanted and non-transplanted testes.
Figure 1.
Testicular size of two pubertally irradiated monkeys. The closed arrow depicts the time of irradiation (10 Gy) and the open arrow the time of spermarche. The closed arrowhead indicates the time of testicular biopsy/orchiectomy and the open arrowhead the time of injection of autologous cryopreserved testis cells (L = left testis, R = right testis, B = bilateral).
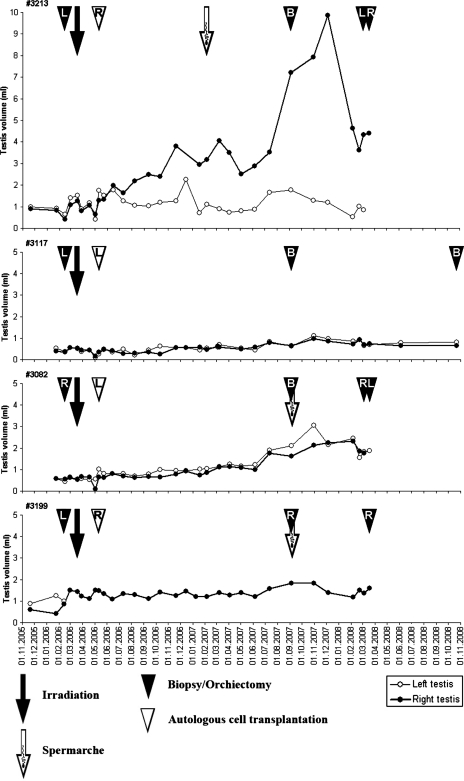
Figure 2.
Testicular size of four prepubertally irradiated monkeys. The closed arrow depicts the time of irradiation (10 Gy) and the open arrow the time of spermarche. The closed arrowheads indicate the time of testicular biopsy/orchiectomy and the open arrowhead the time of injection of autologous cryopreserved testis cells (L = left testis, R = right testis, B = bilateral).
Testicular irradiation in prepubertal monkeys caused no acute effects in testicular size and it remained at pre-irradiation level for 1.5 years. Only a modest increase in testicular size was detected 16 months after irradiation in two monkeys (#3082 and #3199) and it was followed by spermarche 3 months later. A low sperm concentration of 0.04–0.1 million cells/ml with no motility was detected. A testicular size of 1.5–2 ml was reached for both animals with no difference between transplanted or non-transplanted testes. The testicular size remained at 0.5 ml for one prepubertal monkey (#3117) and no spermarche was detected (Fig. 2).
For one prepubertally irradiated monkey (#3213), the pattern of testicular growth differed significantly (Fig. 2). The testis injected with testicular cells started to grow soon after transplantation and reached a size of 10 ml ∼1.5 years after transplantation. Spermarche was detected 10 months after testicular cell infusion and 11 months after irradiation. Sperm concentrations for this monkey increased steadily and reached 49 million cells/ml with 11% motility 24 months after irradiation. The contralateral non-transplanted testis showed poor growth and remained at a volume of 1.5 ml.
Testicular histology after irradiation
During the first 1.5 years after irradiation, tubular length doubled in testes of the two monkeys irradiated during puberty and this growth continued up to the end of the experiment. This was in contrast to prepubertally irradiated monkeys where no or very little extension of seminiferous tubules was observed after irradiation. However the testis of monkey #3213 which showed significant testicular growth after germ cell infusion showed a tubular extension comparable to the two monkey testes irradiated during puberty (#3082 and #3199, Table I).
Recovery of spermatogenesis, as detected by the presence of spermatogonial markers (Fig. 3), was seen in 2–12% of tubular cross sections 1.5 years after irradiation in the two pubertally irradiated monkeys and in one out of four prepubertally irradiated monkeys. After two years, 4–22% of tubular cross sections of the two pubertally irradiated and 8–12% of the seminiferous tubules in the testis of the three prepubertally irradiated monkeys had ongoing spermatogenesis. Tubules with ongoing spermatogenesis were focally arranged and separated from Sertoli cell only (SCO) tubules by fibrotic septae (Fig. 3c). The testes of one prepubertal monkey showed SCO.
Discussion
The present study reveals that testicular recovery after irradiation-induced damage differs in prepubertal and pubertal non-human primates. Irradiation before initiation of pubertal testis growth had a severely more detrimental effect on pubertal outgrowth of seminiferous tubules compared with irradiation of testes which had started pubertal development. Interestingly, prior to pubertal onset, Sertoli cells were more radiosensitive than those after initiation of spermatogenesis. These data indicate that signals responsible for the terminal differentiation of the primate Sertoli cells at puberty affect their radiosensitivity. Germ cell differentiation starts at puberty immediately after intense expansion of the Sertoli cell population rather than in association with a slow and progressive increase in Sertoli cell numbers (Marshall and Plant, 1996). Therefore, the high mitotic activity of Sertoli cells in the prepubertal testis may be responsible for the increased sensitivity to irradiation. The high sensitivity to irradiation before puberty could be related to other factors such as variable efficiencies of DNA repair mechanisms (Masuyama et al., 2005; Nakajima et al., 2006).
Detailed histological analysis showed that irradiation with 10 Gy almost eradicated the potential of the testis to initiate germ cell differentiation. Recovery of spermatogenesis occurred at a low rate with no detectable relation to the maturity of the epithelium at irradiation. The recovery index of the tubular cross sections doubled during the second year of follow-up, and <20% of tubular cross sections had ongoing spermatogenesis irrespective of testicular size. We therefore conclude that the effects of irradiation on monkey spermatogonia are similar in prepubertal and pubertal primate testis and that the depletion of testicular stem cells is not related to an age-dependent difference in sensitivity of spermatogonia to irradiation. These data let us conclude that neither the stem cells nor their somatic niches show an age-related difference in the sensitivity to irradiation. This conclusion is not supported by previous studies in rodents, where significantly increased spermatogonial toxicity is associated with cytotoxic drug and irradiation treatment in the early postnatal days (Erickson and Blend, 1976; Velez de la Calle et al., 1988; Hou et al., 2005). It has been speculated that more pronounced sensitivity of germ cells shortly after birth may also be due to the presence of particularly sensitive stem cell niches.
In contrast to the reasonably well studied effects of irradiation on the adult testis, only one experimental study has been performed previously to explore irradiation exposure to immature monkeys and the long-term effect into adulthood (de Rooij et al., 2002). This study showed that single or fractionated irradiation with doses of 4–8.5 Gy leads to a dose-dependent increase of seminiferous tubules which are fully depleted of germ cells. A complete SCO-situation was only observed at the highest or at fractionated doses and lower doses induced a mild to severe focal SCO-pattern. No significant progression of the repopulation was observed >3 years after irradiation. This study also reports a depletion of Sertoli cells at higher doses of irradiation, leading to lower testis weights in adulthood. The authors conclude that irradiation evoked a depletion of spermatogonia and Sertoli cells in immature monkeys. These findings are strikingly similar to our present findings, which indicate a dose-dependent depletion of spermatogonia which is similar in all age groups and also a depletion of Sertoli cells at higher doses of irradiation. Unfortunately, the pubertal status of the animals at irradiation in the study of de Rooij et al. was not analyzed.
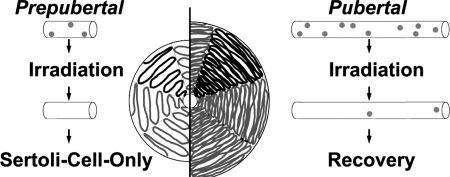
The present observation that recovery of primate spermatogenesis occurred within single testicular lobules is new. Recovery of human and monkey spermatogenesis from irradiation damage is known to be patchy. The present analysis of longitudinally sectioned testicular samples reveals that this patchy recovery reflects the recovery of spermatogenesis in isolated seminiferous tubules of single testicular lobules (Fig. 3). This observation can be explained by the anatomical organization of the primate testis. While rodent testes contain few and long seminiferous tubules extending through the entire testis and connected at both ends to the rather small and superficial rete testis, the primate testis contains many loops of seminiferous tubule separated by fibrotic septa. Each lobule contains several individual seminiferous tubules which are anatomically connected only via the rather large and central rete testis. Surviving spermatogonial stem cells are able to repopulate only the respective testicular lobule while tubules devoted to stem cells remain SCO. Since migration of spermatogonia between seminiferous tubules via the rete testis appears impossible, few stem cells in mouse testes are able to recolonize a significant proportion of the germinal epithelium, and the same number of stem cells in a primate testis can only colonize a small proportion of seminiferous tubules. The resulting patchy arrangement of spermatogenesis after focal testicular recovery and the consequences of a less prominent outgrowth of seminiferous tubules in prepubertally irradiated monkeys are indicated in Fig. 4.
Figure 4.
Schematic drawing showing the prepubertal (left side of panel) and pubertal/adult (right side of panel) organization of the seminiferous tubules in the rhesus monkey, and the potential effect of gonadal irradiation in the testis at these two stages of ontogenetic development. Note that the seminiferous tubules are always organized within strictly separated lobules, and that these tubules open only into the centrally located rete testis. Thus, spermatogonia cannot migrate from one tubule (=lobule) into the adjacent tubule. If e.g. irradiation leads to the complete loss of spermatogonia in one tubule, spermatogenesis in this tubule cannot recover, leading to the common ‘all-or-nothing' appearance of testicular tissue in primates after irradiation or chemotherapy. Also note that due to the much shorter tubules, and thus to the much lower absolute number of spermatogonia in the prepubertal tissue, smaller volume of testis will show recovered spermatogenesis while the proportion of recovery remains the same in both age groups.
Our attempt to perform autologous germ cell transplantation was associated with enhanced spermatogenetic recovery in only one prepubertally irradiated monkey (#3213). All other monkeys showed no difference between the infused and non-infused site. Spermatogenic recovery was detected in 70% of seminiferous tubules in the testis of monkey #3213 and these seminiferous tubules showed a 100% recovery index. The infused side showed no patchy spermatogenesis, suggesting that the rete testis injection had successfully flushed spermatogonial stem cells into all individual testicular lobules. While autologous testicular cell transplantation was able to enhance testicular recovery only in this isolated testis, this observation confirms the principle that an injection of cryopreserved spermatogenetic stem cells via the rete testis can enhance the spermatogenetic recovery after irradiation by allowing recolonization in the entire intratubular compartment. This finding supports our previous study in adult monkeys showing that autologous transplantation following irradiation occurred in only two out of five monkeys but had a profound effect on recovery if the procedure was successful (Schlatt et al., 2002). This finding indicates that the transfer procedure might be a highly critical step and needs to be optimized to achieve better success rates before germ cell transplantation can be considered a clinical tool.
In conclusion, irradiation before spermarche severely disrupts the pubertal outgrowth of seminiferous tubules in the primate testis. Recovery of germ cell development occurs at a low rate irrespective of the developmental status of the monkey at the time of irradiation. Our observations suggest that the stem cell niches and spermatogenetic stem cells show no maturation-related difference in radiosensitivity. Recovery of spermatogenesis in single testicular lobules suggests that spermatogenetic stem cells in primate testis are only capable of recolonizing their respective loop of seminiferous tubule but not of migrating across the rete testis. We assume that these observations can partly explain the slow and highly focal recovery of primate spermatogenesis after irradiation. Further clinical studies on testicular recovery need to be conducted to explore if similar maturation-dependent effects can also be detected in the human testis after irradiation.
Authors' roles
K.J. was involved in designing the sample evaluation, performed the histological analysis of the tissues, and wrote the manuscript. J.E. was involved in the irradiation procedure and some of the surgical interventions, designed the immunohistochemical studies, performed their evaluation, and was involved in writing the manuscript. M.A.Q. designed and performed the targeted testis irradiation procedures, and critically reviewed the manuscript. M.S.H. designed and performed the targeted testis irradiation procedures and critically reviewed the manuscript. M.W.E. designed and performed the targeted testis irradiation procedures and critically reviewed the manuscript. S.H. was involved in the irradiation procedure, assisted in all surgical interventions and sample collections, performed the histological analysis of some tissue samples and critically reviewed the manuscript. M.N. performed the immunohistochemical stainings and their evaluation and was involved in writing the manuscript. S.S. designed the study and performed all surgical interventions and sample collections. He was involved in the irradiation treatment, and compiled the manuscript.
Funding
This study was supported by grants from NIH-grants (1RO1 01050617-01; 2U54HD008610, project 1), the German Federal Ministry of Education and Research (BMBF, Grant 01GN 0809), the Swedish Barncancerfonden, the Finnish Cancer Society, the Helsingin Sanomat Centennial Foundation, the Finnish Pediatric Research Foundation and the Nona and Kullervo Väre Foundation and the Lance Armstrong Foundation.
Acknowledgements
We thank Taija Leinonen and Jutta Salzig for skillful technical assistance.
References
- Anserini P, Chiodi S, Spinelli S, Costa M, Conte N, Copello F, Bacigalupo A. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30:447–451. doi: 10.1038/sj.bmt.1703651. doi:10.1038/sj.bmt.1703651. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, van de Kant HJ, Dol R, Wagemaker G, van Buul PP, van Duijn-Goedhart A, de Jong FH, Broerse JJ. Long-term effects of irradiation before adulthood on reproductive function in the male rhesus monkey. Biol Reprod. 2002;66:486–494. doi: 10.1095/biolreprod66.2.486. doi:10.1095/biolreprod66.2.486. [DOI] [PubMed] [Google Scholar]
- Erickson BH, Blend MJ. Response of the Sertoli cell and stem germ cell to 60Co gamma-radiation (dose and dose rate) in testes of immature rats. Biol Reprod. 1976;14:641–650. doi: 10.1095/biolreprod14.5.641. doi:10.1095/biolreprod14.5.641. [DOI] [PubMed] [Google Scholar]
- Hahn EW, Feingold SM, Simpson L, Batata M. Recovery from aspermia induced by low-dose radiation in seminoma patients. Cancer. 1982;50:337–340. doi: 10.1002/1097-0142(19820715)50:2<337::aid-cncr2820500229>3.0.co;2-6. doi:10.1002/1097-0142(19820715)50:2<337::AID-CNCR2820500229>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. doi:10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Chrysis D, Nurmio M, Parvinen M, Eksborg S, Soder O, Jahnukainen K. Doxorubicin induces apoptosis in germ line stem cells in the immature rat testis and amifostine cannot protect against this cytotoxicity. Cancer Res. 2005;65:9999–10005. doi: 10.1158/0008-5472.CAN-05-2004. doi:10.1158/0008-5472.CAN-05-2004. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod. 2007;22:1060–1067. doi: 10.1093/humrep/del471. doi:10.1093/humrep/del471. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54:1192–1199. doi: 10.1095/biolreprod54.6.1192. doi:10.1095/biolreprod54.6.1192. [DOI] [PubMed] [Google Scholar]
- Masuyama S, Tateishi S, Yomogida K, Nishimune Y, Suzuki K, Sakuraba Y, Inoue H, Ogawa M, Yamaizumi M. Regulated expression and dynamic changes in subnuclear localization of mammalian Rad18 under normal and genotoxic conditions. Genes Cells. 2005;10:753–762. doi: 10.1111/j.1365-2443.2005.00874.x. doi:10.1111/j.1365-2443.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Lan L, Kanno S, Usami N, Kobayashi K, Mori M, Shiomi T, Yasui A. Replication-dependent and -independent responses of RAD18 to DNA damage in human cells. J Biol Chem. 2006;281:34687–34695. doi: 10.1074/jbc.M605545200. doi:10.1074/jbc.M605545200. [DOI] [PubMed] [Google Scholar]
- Norton AJ, Jordan S, Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994;173:371–379. doi: 10.1002/path.1711730413. doi:10.2307/3574084. [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59:665–678. doi:10.2307/3574084. [PubMed] [Google Scholar]
- Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17:55–62. doi: 10.1093/humrep/17.1.55. doi:10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Ehmcke J, Jahnukainen K. Testicular stem cells for fertility preservation: preclinical studies on male germ cell transplantation and testicular grafting. Pediatr Blood Cancer. 2009;53:274–280. doi: 10.1002/pbc.22002. doi:10.1002/pbc.22002. [DOI] [PubMed] [Google Scholar]
- van Alphen MM, van de Kant HJ, de Rooij DG. Depletion of the spermatogonia from the seminiferous epithelium of the rhesus monkey after X irradiation. Radiat Res. 1988a;113:473–486. doi:10.2307/3577244. [PubMed] [Google Scholar]
- van Alphen MM, van de Kant HJ, de Rooij DG. Repopulation of the seminiferous epithelium of the rhesus monkey after X irradiation. Radiat Res. 1988b;113:487–500. doi:10.2307/3577245. [PubMed] [Google Scholar]
- Velez de la Calle JF, Soufir JC, Chodorge F, Boisseau C, Kercret H, Jegou B. Reproductive effects of the anti-cancer drug procarbazine in male rats at different ages. J Reprod Fertil. 1988;84:51–61. doi: 10.1530/jrf.0.0840051. doi:10.1530/jrf.0.0840051. [DOI] [PubMed] [Google Scholar]