Abstract
BACKGROUND
One injection of corifollitropin alfa replaces the first seven daily FSH injections in controlled ovarian stimulation (COS) cycles. Repeated treatment with therapeutic proteins may cause immune responses or hypersensitivity reactions. We assessed the immunogenicity and safety of corifollitropin alfa treatment in up to three COS cycles.
METHODS
In this multicentre, phase III uncontrolled trial, patients (>60 kg) started treatment with one injection of 150 µg corifollitropin alfa on cycle Day 2 or 3 of menses and 0.25 mg ganielix on stimulation Day 5 or 6. Primary outcome measures were antibody formation against corifollitropin alfa (using highly sensitive radioimmunoprecipitation assay), hypersensitivity reactions, local tolerance and adverse events (AEs).
RESULTS
First, second and third COS cycles were started by 682, 375 and 198 patients, respectively. No clinically relevant immunogenicity or drug-related hypersensitivity was observed. For 192 patients undergoing their third cycle a post-treatment blood sample was negative in the anti-corifollitropin antibody assay, resulting in an upper limit of the one-sided 95% confidence interval (CI) of 1.5%. Most frequent AEs were procedural pain (17.7%, 95% CI: 14.9–20.8%), headache (9.1%, 95% CI: 7.0–11.5%) and pelvic pain (7.6%, 95% CI: 5.7–9.9%).
Cumulative ongoing pregnancy rate after three cycles, including frozen-thawed embryo transfer cycles and spontaneous pregnancies, was 61% (95% CI: 56–65%) after censoring for patients who discontinued.
CONCLUSIONS
Treatment with corifollitropin alfa can safely and effectively initiate and sustain ovarian stimulation during the first 7 days of COS in normal responder patients undergoing up to three treatment cycles, without concerns of immunogenicity.
The trial was registered under ClinicalTrials.gov identifier NCT00696878.
Keywords: corifollitropin alfa, immunogenicity, drug safety, controlled ovarian stimulation, pregnancy rate
Introduction
Corifollitropin alfa is a recombinant fusion protein consisting of the α-subunit of human FSH and a hybrid β-subunit composed of the sequence of the β-subunit of human FSH and the carboxy-terminal peptide of the β-subunit of hCG. Compared with recombinant (r) FSH, corifollitropin alfa has a prolonged half-life and slower absorption to peak serum levels (Fares et al., 1992; LaPolt et al., 1992; Duijkers et al., 2002; Fauser et al., 2009). It has been demonstrated that corifollitropin alfa can initiate and sustain follicular growth for 1 week (Duijkers et al., 2002), so that a single injection can replace the first seven daily injections of gonadotrophin in each ovarian stimulation treatment cycle prior to assisted reproduction. In view of the inverse relationship between exposure and body weight, based on the results of the phase II dose-finding study in combination with modelling and simulation, 100 and 150 μg corifollitropin alfa and a 60 kg body weight cut-off were selected to result in similar exposure and therefore similar ovarian response for all body weight groups (De Greef et al., 2010).
Delivering pharmacological doses of a therapeutic (fusion) protein may raise concerns of inducing an immune response or hypersensitivity reaction (Schellekens, 2002). To monitor the potential immunogenicity of corifollitropin alfa, a testing strategy was designed in line with Mire-Sluis et al. (2004) to evaluate all patients exposed to corifollitropin alfa in up to three treatment cycles. The theoretical probability of corifollitropin alfa being immunogenic (Schellekens, 2002) in humans is estimated to be low based on the molecular structure, purity and formulation (Fauser et al., 2009). Also, it is injected only once per treatment cycle with the majority of patients requiring no more than three to four treatment cycles. To date, up to four injections of 15 μg corifollitropin alfa in hypogonadotrophic, hypogonadal men (Bouloux et al., 2001) or a single injection of 100 or 150 μg corifollitropin alfa in more than 1000 patients has not induced any hypersensitivity reaction or an immune response (Devroey et al., 2009; The corifollitropin alfa Ensure study group, 2010).
The primary objective of the Trust trial was to assess the immunogenicity of repeated exposure to 150 μg corifollitropin alfa in a standard GnRH antagonist protocol in normal responder patients weighing >60 kg with a normal BMI (18–29 kg/m2) and a regular menstrual cycle undergoing up to three cycles of controlled ovarian stimulation (COS) with corifollitropin alfa for IVF and/or ICSI. In addition, the overall safety and efficacy of the new corifollitropin alfa regimen used during sequential cycles was evaluated.
Materials and Methods
The Trust trial was a multicentre, open-label, uncontrolled clinical trial carried out in 30 centres in Australia, Europe (Denmark, France, Germany, Hungary, Italy, The Netherlands, Norway and Sweden) and South America (Argentina, Brazil and Chile) between September 2006 and May 2009.
The study was conducted in accordance with principles of good clinical practice and was approved by the appropriate institutional review boards and regulatory agencies. Written informed consent was provided by all subjects.
Study population
Patients aged 18–39 years with a body weight of >60 kg, a BMI of 18–29 kg/m2, menstrual cycle length within 24–35 days range, access to ejaculatory sperm and an indication for COS for infertility using IVF or ICSI were eligible to enrol in the study.
Patients were excluded from the study if one or more of the following conditions were present: a history of, or any current (treated), endocrine abnormality; clinically relevant abnormal laboratory values or chronic disease; or relevant ovarian or tubal pathology that could interfere with ovarian stimulation. Patients were also excluded if they had a prior history of ovarian hyper-response or ovarian hyperstimulation syndrome (OHSS) (>30 follicles ≥11 mm), polycystic ovary syndrome or >20 basal antral follicles on ultrasound (<11 mm, both ovaries combined). Other exclusion criteria included a previously low ovarian response to FSH or hMG treatment (i.e. cycle cancelled due to inadequate ovarian response or three or less oocytes obtained), FSH or LH levels >12 IU/L in the early follicular phase, more than three unsuccessful IVF cycles since the last ongoing pregnancy or abnormal karyotype in the subject or her partner.
Study design
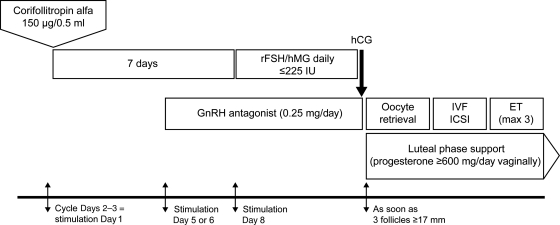
The trial was designed to assess the safety, including the local and general tolerance, of corifollitropin alfa in healthy female partners of infertile couples undergoing COS for IVF or ICSI. For each subject, the trial period covered one to three stimulated treatment cycles and no more than six frozen-thawed embryo transfer (FTET) cycles from the first two treatment cycles. The study design per cycle is summarized in Fig. 1.
Figure 1.
Treatment scheme during the first stimulation cycle. In case patients did not become pregnant, the same treatment was applied in the second and third stimulation cycle. Between two treatment cycles patients could have replacement of cryopreserved oocytes or embryos obtained in a previous treatment cycle. ET, embryo transfer; rFSH, recombinant FSH.
All subjects started their COS cycle on menstrual cycle Day 2 or 3 (stimulation Day 1). Subjects started stimulation with a single s.c. injection of 150 µg (0.5 ml) corifollitropin alfa (Elonva®, N.V. Organon, The Netherlands). Injections were carried out by the patient herself (44.6%) or by a medically qualified person (55.4%). From stimulation Day 8 onwards, treatment was continued with a daily s.c. dose of FSH (follitropin alfa, follitropin beta or menotropins) until the day of hCG administration (FSH administration on the day of hCG administration was optional). The maximum FSH dose for continuing treatment was 225 IU but this dose could be reduced as appropriate. For normal responders, the recommended daily dose of FSH was 150 IU. The investigator was allowed to withhold FSH administration for a maximum of 3 days (coasting) up to and including the day of hCG administration. If the ovarian response was too high in the opinion of the investigator, the investigator was allowed to cancel the cycle at any time. However, if there was a risk of OHSS, defined as >30 follicles ≥11 mm on transvaginal ultrasound, hCG was withheld and the treatment cycle was cancelled per protocol. The maximum total duration of stimulation was 19 days.
To prevent premature LH surges, a GnRH antagonist (0.25 mg; ganirelix or cetrorelix acetate) was administered once daily starting on stimulation Day 5 or 6 up to and including the day of hCG. Either urinary hCG (10000 IU or 5000 IU in case of a high ovarian response) or rhCG (250 μg) was administered to induce final oocyte maturation as soon as three follicles ≥17 mm were observed by ultrasound. Investigators were allowed to delay hCG administration for 1 day when preferred for practical reasons.
Approximately 34–36 h after hCG administration, oocytes were retrieved and standard IVF or ICSI was performed. At embryo transfer, 3 or 5 days after oocyte retrieval, a maximum of three embryos were transferred. To support implantation and early pregnancy, progesterone (≥600 mg/day vaginally) was started on the day of oocyte retrieval and continued for 5–6 weeks or up to menses or a negative pregnancy test performed at least 14 days after embryo transfer. Cryopreservation of human embryos was, as per local embryo protection law, not allowed in Chile, Germany and Italy: in these countries super numerous two pronuclei oocytes instead of embryos were frozen.
Assessments
Before the start of COS, subjects underwent an hCG test to exclude pregnancy, a clinical examination and ultrasound scan to assess the number of antral follicles, and each subject gave a blood sample for laboratory assessments. On stimulation Day 1, 30 min after injection of corifollitropin alfa, subjects underwent a clinical examination during which medical staff assessed injection-site pain, itching, swelling and redness.
Subjects returned to the clinic on stimulation Day 5 or 6 (before the first administration of the GnRH antagonist) and Day 8 for assessment of the size and number of follicles and hormone concentrations, and then at least every other day up to and including the day of rhCG administration for assessment of the size and number of follicles.
Immunogenicity
Immunogenicity was determined by monitoring the development of potential anti-corifollitropin alfa antibodies. A pretreatment and post-treatment sample was obtained from each subject after each treatment cycle, 2 weeks after embryo transfer or 2–3 weeks after cycle discontinuation. To monitor the potential immunogenicity of corifollitropin alfa, a testing strategy was designed to monitor all patients after each exposure to the drug up to three treatment cycles. For that purpose, a highly sensitive anti-corifollitropin alfa antibody assay was designed and validated according to the white paper of Mire-Sluis et al. (2004). The screening assay was a validated, sensitive radioimmunoprecipitation assay able to detect any immune response, regardless of titre, affinity or class of immunoglobulins (sensitivity 1.37 ng antibody/ml serum). A patient-population-specific floating cut-off point was established in serum samples from over 300 IVF patients collected at various time points of the menstrual cycle. If a post-treatment sample was above the pre-defined cut-off point, pretreatment and post-treatment samples were compared to determine whether the response could be drug induced. Post-treatment samples, which had a statistically higher assay response than pretreatment samples as determined by a paired t-test and in which binding affinity was depletable with corifollitropin alfa, were reported and further evaluated to assess the titre and isotypes, specificity (cross-reactivity with rFSH, rLH or hCG) and their neutralizing potential in an in vitro bioassay.
Adverse events
Adverse events (AEs) and serious AEs (SAEs) were assessed whenever they occurred. AEs were defined as any unfavourable sign, symptom or disease that occurred during the study period. Moderate or severe local tolerance reactions up to 24 h after any injection of corifollitropin alfa, OHSS, ectopic pregnancy and miscarriage were always considered at least an AE. OHSS was graded as mild, moderate or severe, according to the OHSS guidelines (WHO Scientific Group, 1973). If the same patient was reported with more than one grade of OHSS, only the highest severity of OHSS was included.
SAEs were defined as AEs that were life-threatening, required (prolonged) hospitalization or resulted in persistent or significant disability or incapacity.
End-points
The primary outcome measures were antibody formation against corifollitropin alfa, hypersensitivity reactions, local tolerance and occurrence of AEs or SAEs, including the incidence of OHSS.
The secondary outcome measures were efficacy, determined for each COS cycle, including the number of cumulus–oocyte complexes (COC) retrieved, the number and quality of embryos obtained and transferred, the ongoing pregnancy rates and the cumulative pregnancy rate.
Statistical analysis
A sample size of 600 subjects was planned, with the anticipation that 50% of patients starting each cycle would start the subsequent cycle, thus giving 300 subjects starting Cycle 2 and 150 starting Cycle 3. If no immunogenicity to corifollitropin alfa was observed with 150 subjects undergoing three treatment cycles, this allowed for an upper limit of the one-sided 95% confidence interval (CI) of 2%, the target set for this study.
The cumulative ongoing pregnancy rate was calculated using the Kaplan–Meier approach (Kaplan and Meier, 1958) and included all ongoing pregnancies from the treatment regimen, including fresh cycles, frozen-thawed cycles and spontaneous pregnancies (Vail and Gardener, 2003). Patients who discontinued treatment without becoming pregnant after Cycles 1 or 2 or after FTET cycles were censored assuming that patients who did not return for a subsequent IVF cycle would have had the same chance of an ongoing pregnancy as patients who continued treatment.
Results
Patient characteristics and disposition
A total of 682 patients were included in the trial, 117 in Australia, 304 in Europe and 261 in South America. The mean (SD) age, body weight and BMI were 32.9 (3.6) years, 67.0 (6.5) kg and 24.2 (2.4) kg/m2, respectively. The most frequently reported cause of infertility was male factor (59.4%) followed by tubal factor (24.2%) and unexplained infertility (19.2%). In total 392 subjects (57.5%) suffered from primary infertility and 290 subjects (42.5%) presented with secondary infertility. The mean (SD) duration of infertility was 3.8 (3.0) years.
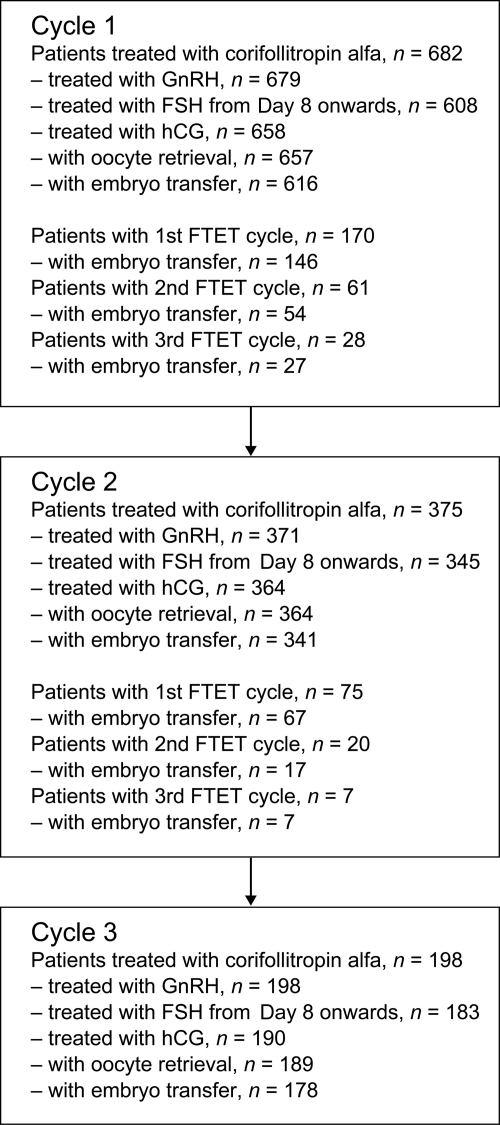
Of the 682 patients who started their first COS treatment cycle with corifollitropin alfa, 375 patients continued with a second cycle and 198 patients started their third treatment cycle. Fig. 2 shows the numbers of patients in each cycle continuing treatment at each stage of the protocol.
Figure 2.
Flow of patients through the treatment cycles. FTET, frozen-thawed embryo transfer.
The cancellation rate per cycle (i.e. patients who started treatment but did not have embryo transfer in that cycle) was 9.7, 9.3 and 10.1% for Cycles 1, 2 and 3, respectively. The overall reasons for cycle discontinuation are shown in Table I. Reasons for trial discontinuation (not undergoing embryo transfer in Cycle 3) were AEs or SAEs (1.2%, n = 8, pregnancy (including spontaneous pregnancy) after treatment Cycles 1 or 2 or after an FTET cycle (44.6%, n = 304), withdrawal of consent (12.8%, n = 87), termination of the trial (4.7%, n = 32), non-SAE events in Cycle 3 (2.6%, n = 18) and other reasons (8.1%, n = 55). Among the most frequent other reasons to discontinue the trial were too high an ovarian response in the previous treatment cycle(s) (2.9%, n = 20) and too low an ovarian response (1.2%, n = 8).
Table I.
Patients discontinued from treatment cycles, i.e. those who received corifollitropin alfa treatment but did not undergo embryo transfer.
| Treatment cycle |
|||
|---|---|---|---|
| Cycle 1 (N = 682) | Cycle 2 (N = 375) | Cycle 3 (N = 198) | |
| Patients with cycle discontinuation, n (%) | 66 (9.7) | 35 (9.3) | 20 (10.1) |
| AE or SAE, n (%) | 6 (0.9) | 3 (0.8) | 2 (1.0) |
| Insufficient ovarian response, n (%) | 8 (1.2) | 5 (1.3) | 7 (3.5) |
| Risk of OHSS, n (%) | 7 (1.0) | 1 (0.3) | 0 (0) |
| Too high ovarian responsea, n (%) | 9 (1.3) | 6 (1.6) | 1 (0.5) |
| Insufficient number and quality of oocytes retrieved, n (%) | 6 (0.9) | 5 (1.3) | 1 (0.5) |
| No or abnormal fertilization, n (%) | 18 (2.6) | 9 (2.4) | 3 (1.5) |
| Insufficient number and quality of embryos for transfer, n (%) | 6 (0.9) | 3 (0.8) | 5 (2.5) |
| Other reasons, n (%) | 6 (0.9) | 3 (0.8) | 1 (0.5) |
AE, adverse event; OHSS, ovarian hyperstimulation syndrome; SAE, serious adverse event.
aIn the view of the investigator.
Safety end-points
Immunogenicity
Post-treatment serum samples for assessment in the anti-corifollitropin alfa antibody assay were available for 681 out of 682 (99.9%), 372 out of 375 (99.2%) and 192 out of 198 (97.0%) patients who underwent one, two and three COS cycles, respectively. All post-treatment samples were reported negative, with the exception of one post-treatment sample taken after Cycle 2. This sample appeared to have a statistically significant increased binding (P < 0.05) in the antibody assay that was depletable by corifollitropin alfa and rFSH, but not by rLH or hCG. However, the binding of the post-treatment sample was so low (titre 2) that isotyping was technically impossible. The post-treatment sample was without neutralizing activity, thus it did not interfere with the bioactivity of corifollitropin alfa or rFSH. For this subject no AEs were reported in either Cycle 1 or 2 and an additional blood sample taken 6 months after the previous sample tested negative. In view of the very low titre and the absence of neutralizing activity, of moderate or severe local tolerance reactions and of any AEs, the test result was judged as not clinically relevant. The subject, who discontinued from the trial after Cycle 2, became pregnant thereafter in a treatment cycle with daily FSH. The upper limit of the one-sided 95% CI for the incidence of immunogenicity for subjects with three treatment cycles was 1.5%.
Hypersensitivity
No drug-related hypersensitivity reactions, including skin rash, urticaria, hypotension, allergic asthma, chest tightness, bronchospasm, dyspnoea and wheezing, were reported following injection of corifollitropin alfa.
Local tolerance at the injection site
Local tolerance reactions were mild, mainly redness and occurred in 2.5, 4.3 and 2.5% of patients in Cycles 1, 2, and 3, respectively. There were no moderate or severe injection-site reactions (Table II).
Table II.
Incidence of subjects with at least one (serious) adverse event, with OHSS or with mild local tolerance reaction.
| Cycle 1 (N = 682) | Cycle 2 (N = 375) | Cycle 3 (N = 198) | |
|---|---|---|---|
| Subjects with AEs, % (95% CI) | 46.8 (43.0–50.6) | 35.2 (30.4–40.3) | 31.3 (24.9–38.3) |
| Procedural pain | 14.2 (11.7–17.1) | 11.2 (8.2–14.8) | 10.1 (6.3–15.2) |
| Headache | 5.6 (4.0–7.6) | 5.3 (3.3–8.1) | 5.6 (2.8–9.7) |
| Pelvic pain | 4.8 (3.4–6.7) | 3.7 (2.1–6.2) | 2.5 (0.8–5.8) |
| Subjects with SAEs, % (95% CI) | 3.4 (2.1–5.0) | 1.6 (0.6–3.4) | 1.5 (0.3–4.4) |
| OHSS, any grade, % (95% CI) | 3.5 (2.3–5.2) | 1.9 (0.8–3.8) | 0 |
| Mild | 1.8 | 0.8 | |
| Moderate | 0.9 | 0.5 | |
| Severe | 0.9 | 0.5 | |
| Local tolerance | |||
| Mild, % (95% CI) | 2.5 (1.5–4.0) | 4.3 (2.5–6.8) | 2.5 (0.8–5.8) |
CI, confidence interval.
Adverse events
In Cycles 1, 2 and 3, 46.8, 35.2 and 31.3% of patients, respectively, had at least one AE (Table II). Overall, including all treatment cycles, the most common reported AEs were procedural pain related to oocyte retrieval (17.7%, 95% CI: 14.9–20.8%), headache (9.1%, 95% CI: 7.0–11.5%) and pelvic pain (7.6%, 95% CI: 5.7–9.9%).The incidences of these AEs per treatment cycle are presented in Table II. In Cycles 1, 2 and 3, 11.1, 3.7 and 2.0% of AEs were considered to be related to treatment.
AEs of severe intensity were uncommon, occurring in 2.5, 1.3 and 0.5% of patients in Cycles 1, 2 and 3, respectively. A total of 63 SAEs were reported in 47 patients (6.9%) overall, and occurred in 3.4, 1.6 and 1.5% of patients, respectively according to COS treatment Cycles 1, 2 or 3. Among the reported SAEs were eight ectopic pregnancies, two ruptured ectopic pregnancies, one heterotopic pregnancy, three missed abortions, two spontaneous abortions and two imminent abortions. In total, 15 SAEs were considered to be related to trial medication, including all 10 occurrences of OHSS. In addition, one subject in Cycle 1 with OHSS experienced a recoverable pulmonary embolism.
Incidence of OHSS
OHSS was reported in 24 patients (3.5%) in their first cycle of COS and in seven patients (1.9%) in the second cycle and did not occur during the third treatment cycle (Table II). One subject experienced OHSS in Cycle 1 and Cycle 2. It should be noted that 25 subjects discontinued the trial after the first (n = 16) or after the second (n = 9) cycle because of too high an ovarian response or signs or symptoms of OHSS. In total 15 cases of OHSS were considered mild, eight cases were considered moderate and eight cases were reported as severe OHSS. In the first cycle, eight patients were hospitalized, one with mild, one with moderate and six with severe OHSS. In the second cycle, two patients with OHSS were hospitalized, one with mild and one with severe OHSS. One subject who experienced severe OHSS in the second cycle received ambulant treatment in the fertility clinic and was not hospitalized.
Efficacy outcomes
In the first, second and third treatment cycles, corifollitropin alfa was self-administered by 39.9, 48.8 and 52.5% of the patients, respectively. The hCG criteria were reached on stimulation Day 8 or before Day 8 for 122 subjects (17.9%) in Cycle 1, for 60 subjects (16.0%) in Cycle 2 and for 36 subjects (18.2%) in Cycle 3. The median dose of rFSH/hMG was 150 IU per day in all three treatment cycles and the total median dose was 400, 450 and 450 IU in Cycles 1, 2 and 3, respectively. The majority of patients (>85%) finished their first, second or third cycle with rFSH and the minority (≤0.6%) used hMG. The duration of stimulation (10 days) was consistent from Cycles 1 to 3 (Table III).
Table III.
Amount of recombinant FSH/hMG and hCG administered and numbers of cumulus–oocyte complexes retrieved, embryos obtained and embryos transferred.
| Treatment cycle |
|||
|---|---|---|---|
| Cycle 1 (N = 682) | Cycle 2 (N = 375) | Cycle 3 (N = 198) | |
| Total dose of rFSH/hMG administered from Day 8 (IU)a | 400 (0, 2100) | 450 (0, 1950) | 450 (0, 2250) |
| Total duration of stimulation (days)a | 10 (7, 18) | 10 (6, 19) | 10 (7, 18) |
| Dose of hCG administered, n (%) | |||
| None | 24 (3.5) | 11 (2.9) | 8 (4.0) |
| 5000 IU (urinary) | 60 (8.8) | 25 (6.7) | 10 (5.1) |
| 10 000 IU (urinary) | 439 (64.4) | 245 (65.3) | 132 (66.7) |
| 250 μg (recombinant) | 159 (23.3) | 94 (25.1) | 48 (24.2) |
| Number of cumulus–oocyte complexes per started cycleb | 11.9 (7.2) | 11.5 (6.8) | 11.3 (7.6) |
| Number of embryos obtained on Day 3c | 6.4 (4.5) | 6.5 (4.4) | 6.6 (4.8) |
| Number of good quality embryos obtained on Day 3c | 3.2 (3.1) | 2.9 (2.8) | 2.8 (2.7) |
| Number of embryos transferredc | 1.9 (0.7) | 2.1 (0.7) | 2.2 (0.7) |
| Number of good quality embryos transferredd | 1.4 (0.9) | 1.5 (1.0) | 1.6 (1.0) |
aMedian (min, max) and restricted to patients with hCG injection.
bMean (SD); per started cycle.
cRestricted to subjects with IVF and/or ICSI.
dRestricted to subjects with embryo transfer.
In total 50.6, 57.1 and 62.6% of patients in Cycles 1, 2 and 3, respectively, started the GnRH antagonist on stimulation Day 5 and 47.4, 41.3 and 37.4% in Cycles 1, 2 and 3, respectively, started on Day 6. The majority of patients received ganirelix (79.6, 82.7 and 96% in Cycles 1, 2 and 3, respectively) and a minority cetrorelix (19.6, 15.5 and 3.5% in Cycles 1, 2 and 3, respectively). The amount of urinary or rhCG administered in each cycle to trigger final oocyte maturation is given in Table III. The number of COCs retrieved and embryos obtained and transferred were similar for the three treatment cycles (Table III).
Pregnancy rates
Table IV shows the pregnancy rates for patients who started ovarian stimulation with corifollitropin alfa, which was consistent over Cycles 1, 2 and 3. The ongoing pregnancy rate per started cycle was 22.7% in the first cycle, 20.5% in the second cycle and 20.7% in the third cycle. However, the pregnancy rates varied widely among the 30 participating sites: the 10th and 90th percentiles for the ongoing pregnancy rate per site in Cycle 1 were 11.6 and 43.7%, respectively. Most ongoing pregnancies were singletons (81.9–87.8% in each cycle). Of the 46 multiple pregnancies, 28 occurred in Cycle 1, 13 in Cycle 2 and 5 in Cycle 3. In Cycle 1 there were 26 twin and 2 triple pregnancies; in Cycle 2 there were 12 twin pregnancies and 1 triple pregnancy; and in Cycle 3 there were 5 twin pregnancies. The miscarriage rate per clinical pregnancy was 12.8, 12.5 and 14.6% for Cycles 1, 2 and 3, respectively.
Table IV.
For fresh cycles only: pregnancy rates per started cycle and implantation rates per embryo transfer.
| Treatment cycle |
|||
|---|---|---|---|
| Cycle 1 (N = 682) | Cycle 2 (N = 375) | Cycle 3 (N = 198) | |
| Biochemical pregnancy (%)a | 31.4 | 28.5 | 27.8 |
| Clinical pregnancy (%)b | 26.2 | 23.5 | 24.2 |
| Vital pregnancy (%)c | 23.0 | 21.3 | 21.2 |
| Ongoing pregnancy (%)d | 22.7 | 20.5 | 20.7 |
| Singletons | 81.9 | 83.1 | 87.8 |
| Twins | 16.8 | 16.9 | 12.2 |
| Triplets | 1.3 | 1.3 | 0 |
| Implantation rate, % (ne) | 21.2 (n = 616) | 16.6 (n = 340) | 16.3 (n = 178) |
aBiochemical pregnancy: pregnancy proven by a biochemical pregnancy test or with ultrasound showing at least one gestational sac.
bClinical pregnancy: presence of at least one gestational sac as assessed by ultrasound.
cVital pregnancy: presence of at least one fetus with heart activity as assessed by ultrasound.
dOngoing pregnancy: presence of at least one fetus with heart activity at least 10 weeks after embryo transfer or live birth.
ePatients with embryo transfer.
In Cycle 1, coasting was applied in 15 patients with hCG injection and 5 out of these 15 patients had an ongoing pregnancy (33.3%). In Cycle 2, coasting was applied for 9 patients with hCG injection and 1 patient became pregnant (11.1%). In Cycle 3, coasting was applied for 1 patient who did not become pregnant.
The cumulative ongoing pregnancy rate after three COS cycles including in-between FTET cycles and spontaneous pregnancies was 51%; after censoring for patients who discontinued treatment, the rate was 61% (Table V).
Table V.
Cumulative ongoing pregnancy rate: pregnancies after treatment, FTET cycles and spontaneous pregnancies.
| Ongoing pregnancies | Cumulative incidence | 95% Confidence intervala | |
|---|---|---|---|
| Cycle 1 | 155 | 0.23 | 0.20–0.26 |
| Spontaneous pregnancies after cycle 1 | 11 | 0.25 | 0.22–0.28 |
| FTET cycles between Cycle 1 and Cycle 2 | 37 | 0.31 | 0.27–0.35 |
| Spontaneous pregnancies after the FTET cycles | 4 | 0.32 | 0.28–0.35 |
| Cycle 2 | 76b | 0.45 | 0.42–0.50 |
| Spontaneous pregnancies after Cycle 2 | 8 | 0.47 | 0.43–0.52 |
| FTET cycles between Cycle 2 and Cycle 3 | 11 | 0.50 | 0.46–0.54 |
| Spontaneous pregnancies after the FTET cycles | 3 | 0.51 | 0.47–0.55 |
| Cycle 3 | 40c | 0.61 | 0.56–0.65 |
FTET, frozen-thawed embryo transfer.
aLimits of the 95% CI for the cumulative incidence rate.
bOne woman registered ongoing pregnancy twice, once in a FTET cycle between treatment Cycles 1 and 2 and once in Cycle 2; she was not counted as pregnant in Cycle 2 since per protocol she discontinued the trial without being treated in another cycle.
cOne woman registered pregnancy in Cycles 2 and 3; she was only counted as pregnant in Cycle 2 since per protocol she discontinued the trial after her pregnancy in Cycle 2.
Discussion
In the current trial, exposure to a single injection of corifollitropin alfa in up to three repeated COS cycles using a standard GnRH antagonist protocol in 682 patients weighing >60 kg with normal BMI and a regular menstrual cycle was safe and well-tolerated without concerns of immunogenicity. The antibody assay used was a highly sensitive assay in which a relatively small difference between binding affinity of the pretreatment and post-treatment sample could lead to a statistically significant increased binding to corifollitropin alfa. To assess whether an elevated assay response was indicative of the presence of corifollitropin alfa-specific antibodies or whether it concerned a non-specific interaction, an immunodepletion assay was also performed. The post-treatment sample of one patient in her second treatment cycle was depletable with corifollitropin alfa and rFSH. However, this sample was negative in the neutralizing activity assay, and thus the antibodies did not interfere with the FSH bioactivity of either gonadotrophin preparation.
There is in general limited information on immune responses to therapeutic gonadotrophins in women undergoing treatment for infertility. To date, two reviews have reported the absence of antibody response to exogenous urinary or rFSH (Koren et al., 2002; Wadhwa et al., 2003). The incidence of antibody formation against hCG has been reported in hypogonadotrophic hypogonadal males who received long-term treatment with urinary products resulting in loss of efficacy of the drug (reported incidence of antibody formation: 0–40%), but so far no antibodies have been reported in humans using rhCG (Moudgal et al., 1997). The presence of circulating immunoglobulins cross-reacting with endogenous FSH has been reported (Meyer et al., 1990; Gobert et al., 2001; Haller et al., 2005; Haller et al., 2007); however it is not stated whether these antibodies lowered the endogenous FSH levels by neutralization. Anti-FSH antibodies may be naturally occurring antibodies associated with peripheral FSH concentrations and produced in higher levels in infertile women (Haller et al., 2007). Case reports have also been published on the presence of (transient) anti-hCG antibodies in infertile women with recurrent pregnancy loss (Pala et al., 1988; Amato et al., 2002).
In addition to the lack of antibody immune responses against corifollitropin alfa in this trial, there were also no treatment-related hypersensitivity reactions; injection-site reactions were only mild in nature. Lack of immunogenicity with repeated (n = 3) cycles of corifollitropin alfa treatment is consistent with the previous study of Bouloux et al. (2001). This is also consistent with the findings of the Engage trial, in which there were no drug-related immune responses to corifollitropin alfa and no moderate or severe reactions at the injection site (Devroey et al., 2009). In the current study, mild local tolerance reactions were observed in fewer than 5% of subjects in each cycle. This is similar to the reported 30 min local tolerance reactions in the Engage trial when reactions were also only mild, 6.1% in the corifollitropin alfa arm and 6.1% in the rFSH arm (Devroey et al., 2009).
In the current trial, the overall incidence of OHSS in the first cycle was 3.5 versus 7.0% in the Engage trial (Devroey et al., 2009) and the incidence of moderate or severe cases of OHSS was 1.8 versus 3.9% in the Engage trial. The lower incidence of OHSS in the trial reported here is likely to be related to the patient population included, who were slightly older (32.9 years in the current study versus 31.5 years in Engage) and who had a lower basal antral follicle count (10.9 in the current study and 12.3 in Engage) (Devroey et al., 2009). In addition, the current trial was conducted more closely in line with current medical practice; only 43.5% of the patients received FSH/hMG on the day of hCG for triggering final oocyte maturation and 23.3% of patients received 250 μg rhCG (∼6500 IU) instead of 10000 IU urinary hCG. Furthermore, in patients who required additional FSH injections from Day 8 onwards, a lower dose than 200 IU was more frequently given to complete ovarian stimulation. The nature and incidence of reported SAEs other than OHSS (ectopic pregnancies, procedural pain, pelvic pain, pelvic discomfort and headache) were similar to those reported in both the corifollitropin alfa and rFSH arms of the Engage trial (Devroey et al., 2009).
Concerning efficacy variables, the mean number of COCs retrieved per attempt was similar (11.9–11.3) across the three treatment cycles, and the mean number of embryos (6.4–6.6) and good-quality embryos (3.2–2.8) obtained was also consistent across the three treatment cycles. The ongoing pregnancy rate per started cycle was comparable between the three cycles which is in line with consistent pregnancy rates across cycles in patients receiving their first six cycles of IVF treatment (Malizia et al., 2009). Since patients included were not allowed more than three IVF cycles prior to the trial, none of the patients had more than six treatment cycles.
The mean ongoing pregnancy rate in cycle 1 was lower than has been reported previously for patients undergoing a similar COS treatment regimen with corifollitropin alfa [22.7% in the current study compared with 38.9% in the Engage trial (Devroey et al., 2009)]. However, there was a considerable range of pregnancy rates among the different participating sites with some sites providing high ongoing pregnancy rates of over 40%. In contrast to the Engage trial, the current trial did not include any IVF units within the USA, the latter often having higher success rates than the rest of the world (Baker et al., 2010) but did include IVF clinics in countries such as Italy, Germany and Chile with restrictions in terms of embryo selection for transfer and cryopreservation of embryos/zygotes. This implied that in these clinics, maximally three zygotes were kept in culture to develop into embryos and that these embryos were transferred, regardless of their quality. In addition to different IVF units contributing to the current trial outcome, the patient population was slightly older than that in the Engage trial, with a lower ovarian reserve, as mentioned before. Nevertheless, half of the patients were pregnant after undergoing one, two or three treatment cycles without any unexpected AEs, with very few cases of OHSS and with an excellent local and general tolerance.
In conclusion, the results of this trial suggest that repeated treatment cycles with a single injection of 150 μg corifollitropin alfa can be safely and effectively applied in potential normal responder patients undergoing COS prior to IVF or ICSI, without concerns for immunogenicity.
Authors’ roles
R.N., F.Z. and B.S. are investigators who contributed to the acquisition and interpretation of data in this manuscript to which they provided intellectual content. M.M. was responsible for the statistical analysis and J.E., E.H. and B.M. were responsible for the design of the study, interpretation of data and drafting of this article. B.M. and R.N. wrote the manuscript.
Conflict of interest
R.N. has served as chairperson on the Medical Advisory Board of Schering-Plough in Australia and Asia-Pacific and has received speaker honoraria from Schering-Plough. F.Z. declares no conflicts of interest. B.S. has served on the Advisory Board for Schering-Plough. J.E., E.H., M.M. and B.M. are employees of MSD.
Funding
Financial support for this study was provided by Merck, Sharp and Dohme & Co. Medical writing and editorial assistance was provided by P. Milner, PhD, of PAREXEL, UK. This assistance was funded by Merck, Sharp and Dohme & Co. Funding to pay the Open Access publication charges for this article was provided by Merck, Sharp and Dohme & Co.
Acknowledgements
The authors would like to thank Uschi Rose, Frank van Aarle, Bart Hendriks, Peter van Zandvoort, Gijs Verheijden and Edwin van de Heuvel, MSD, Oss, The Netherlands, for the design and testing for potential immunogenicity to corifollitropin alfa in this trial.
Appendix: Trust investigators
Argentina: Papier, CEGyR Buenos Aires; Vilela, IFER Buenos Aires; Blaquier, FERTILAB Riobamba, Buenos Aires; Ruhlmann, San Isidro Medicina, San Isidro; Botti, PROAR, Rosario; Pasqualini, Halitus, Buenos Aires. Australia: Rombauts, Monash IVF, Clayton, Victoria; Hale, Melbourne IVF, East Melbourne, Victoria; Watkins, Tasmania IVF, Hobart; Norman, Repromed, Dulwich, SA and University of Adelaide; Illingworth, IVF Australia, Westmead, NSW. Brazil: Petracco, Hospital de Sao Lucas, Porto Alegre-RS; Ferriani, Hospital das Clinicas de Faculdade de Medicina de Ribeirao Preto, Ribeirao Preto. Chile: Devoto, Instituto de Investigacion Materno Infantil (IDIMI), Santiago; Zegers-Hochschild and Camus, Clinica las Condes, Santiago. Germany: Schultze-Mosgau, Universitätklinikum Lübeck, Lübeck; Dieterle, Kinderwunschcentrum Dortmund, Dortmund; Fiedler, Kinderwunsch Centrum München, München. Denmark: Nyboe Andersen, Rigshospitalet, København Ø. France: Salle, Groupement Hospitalier Est Hopital Femme Mere Infant PMA, Bron; Hazout, Clinique de la Muette, Paris. Hungary: Konc, St Janos Hospital and Outpatient Institute, Budapest. Italy: La Sala, Arcispedale S. Maria Nuova, Reggio Emilia; De Placido, Azienda Universitaria Policlinico Federico II, Napoli. Netherlands: Laven, Erasmus Medisch Centrum, Rotterdam; Lambalk, Academisch Ziekenhuis Vrije Universteit Amsterdam, Amsterdam; Jansen, Reinier de Graaf Groep, Voorburg; Cohlen, Isala Klinieken (locatie Sophia), Zwolle. Norway: Tanbo, Rikshospitalet HF, Oslo. Sweden: Wramsby, IVF Kliniken St. Görans, Stockholm.
References
- Amato F, Warnes GM, Kirby CA, Norman RJ. Infertility caused by hCG autoantibody. J Clin Endocrinol Metab. 2002;87:993–997. doi: 10.1210/jcem.87.3.8334. [DOI] [PubMed] [Google Scholar]
- Baker VL, Jones CE, Cometti B, Hoehler F, Salle B, Urbancsek J, Soules MR. Factors affecting success rates in two concurrent clinical IVF trials: an examination of potential explanations for the difference in pregnancy rates between the United States and Europe. Fertil Steril. 2010;94:1287–1291. doi: 10.1016/j.fertnstert.2009.07.1673. [DOI] [PubMed] [Google Scholar]
- Bouloux PM, Handelsman DJ, Jockenhöl F, Nieschlag E, Rabinovici J, Frasa WL, de Bie JJ, Voortman G, Itskovitz-Eldor J FSH-CTP study group. First human exposure to FSH-CTP in hypogonadotrophic hypogonal males. Hum Reprod. 2001;16:1592–1597. doi: 10.1093/humrep/16.8.1592. [DOI] [PubMed] [Google Scholar]
- De Greef R, Zandvliet A, de Haan A, IJzerman-Boon P, Marintcheva-Petrova M, Mannaerts B. Dose selection of corifollitropin alfa by modeling and simulation in controlled ovarian stimulation. Clin Pharmacol Ther. 2010;88:79–87. doi: 10.1038/clpt.2010.54. [DOI] [PubMed] [Google Scholar]
- Devroey P, Boostanfar R, Koper NP, Mannaerts BM, Ijzerman-Boon PC, Fauser BC on behalf of the ENGAGE Investigators. A double-blind, non-inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Hum Reprod. 2009;24:3063–3072. doi: 10.1093/humrep/dep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijkers IJM, Klipping C, Boerrigter PJ, Machielsen CSM, de Bie JJ, Voortman G. Single dose pharmacokinetics and effects on follicular growth and serum hormones of a long-acting recombinant FSH preparation (FSH-CTP) in healthy pituitary-suppressed females. Hum Reprod. 2002;17:1987–1993. doi: 10.1093/humrep/17.8.1987. [DOI] [PubMed] [Google Scholar]
- Fares FA, Suganuma N, Nishimori K, LaPolt PS, Hsueh AJ, Boime I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin beta subunit to the follitropin beta subunit. Proc Natl Acad Sci USA. 1992;89:4304–4308. doi: 10.1073/pnas.89.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BCJM, Mannaerts BMJL, Devroey P, Leader A, Boime I, Baird DT. Advances in recombinant DNA technology: corifollitropin alfa, a hybrid molecule with sustained follicle-stimulating activity and reduced injection frequency. Hum Reprod Update. 2009;15:309–321. doi: 10.1093/humupd/dmn065. [DOI] [PubMed] [Google Scholar]
- Gobert B, Jolivet-Reynaud C, Dalbon P, Barbarino-Monnier P, Faure GC, Jolivet M, Bene MC. An immunoreactive peptide of the FSH involved in autoimmune infertility. Biochem Biophys Res Comm. 2001;289:819–824. doi: 10.1006/bbrc.2001.6059. [DOI] [PubMed] [Google Scholar]
- Haller K, Mathieu C, Rull K, Matt K, Bene MC, Uibo R. IgG, IgA and IgM antibodies against FSH: serological markers of pathogenic autoimmunity or of normal immunoregulation? Am J Reprod Immunol. 2005;54:262–269. doi: 10.1111/j.1600-0897.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- Haller K, Salumets A, Grigorova M, Talja I, Salur L, Bene MC, Laan M, Uibo R. Putative predictors of antibodies against follicle-stimulating hormone in female fertility: a study based on in vitro fertilization patients. Am J Reprod Immunol. 2007;57:193–200. doi: 10.1111/j.1600-0897.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Koren E, Zuckermann LA, Mire-Sluis AR. Immune responses to therapeutic proteins in humans—clinical significance, assessment and prediction. Curr Pharm Biotechnol. 2002;3:349–360. doi: 10.2174/1389201023378175. [DOI] [PubMed] [Google Scholar]
- LaPolt PS, Nishimori K, Fares FA, Perlas E, Boime I, Hsueh AJ. Enhanced stimulation of follicle maturation and ovulatory potential by long acting follicle-stimulating hormone agonists with extended carboxyl-terminal peptides. Endocrinology. 1992;131:2514–2520. doi: 10.1210/endo.131.6.1446593. [DOI] [PubMed] [Google Scholar]
- Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- Meyer WR, Lavy G, Alan H, DeCherney AH, Visintin I, Economy K, Luborsky JL. Evidence of gonadal and gonadotropin antibodies in women with a suboptimal ovarian response. Obstet Gynecol. 1990;75:795–798. [PubMed] [Google Scholar]
- Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, Parish T, Scott G, Shankar G, Shores E, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289:1–16. doi: 10.1016/j.jim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Moudgal NR, Murthy GS, Prasanna-Kumar KM, Martin F, Suresh R, Medhamurthy R, Patil S, Sehgal S, Saxena BN. Responsiveness of human male volunteers to immunization with ovine follicle stimulation hormone vaccine: result of a pilot study. Hum Reprod. 1997;12:457–463. doi: 10.1093/humrep/12.3.457. [DOI] [PubMed] [Google Scholar]
- Pala A, Coghi I, Spampinato G, Di Gregorio R, Strom R, Carenza L. Immunochemical and biological characteristics of a human autoantibody to human chorionic gonadotropin and luteinizing hormone. J Clin Endocrinol Metab. 1988;67:1317–1321. doi: 10.1210/jcem-67-6-1317. [DOI] [PubMed] [Google Scholar]
- Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1:457–462. doi: 10.1038/nrd818. [DOI] [PubMed] [Google Scholar]
- The corifollitropin alfa Ensure study group. Corifollitropin alfa for ovarian stimulation in IVF: a randomized trial in lower-body-weight women. Reprod Biomed Online. 2010;21:66–76. doi: 10.1016/j.rbmo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Hum Reprod. 2003;18:1000–1004. doi: 10.1093/humrep/deg133. [DOI] [PubMed] [Google Scholar]
- Wadhwa M, Bird C, Dilger P, Gaines-Das R, Thorpe R. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. J Immunol Methods. 2003;278:1–17. doi: 10.1016/s0022-1759(03)00206-0. [DOI] [PubMed] [Google Scholar]
- WHO Scientific Group. Agents stimulating gonadal function in the human. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1973;514:1–30. [PubMed] [Google Scholar]