Abstract
BACKGROUND
Chromatin configuration of the germinal vesicle (GV) and quality of the cytoplasm are critical factors in achieving oocyte meiotic and developmental capacity during folliculogenesis. Besides gaining new insights into the timing and cellular mechanisms associated with the acquisition and regulation of GV oocyte competence, the domestic cat model was used to examine (i) the relation between GV chromatin configuration and oocyte functionality during folliculogenesis and (ii) the role of the cytoplasmic environment on the GV competence and stability.
METHODS
Structural and functional properties of GV oocytes were characterized after isolation from different follicle stages of non-stimulated cat ovaries. GV transfers, artificial chromatin compaction and oocyte vitrification were used to demonstrate the respective roles of GV and cytoplasm on the oocyte functionality.
RESULTS
GVs acquired the intrinsic capability to resume meiosis during the pre-antral follicle stage, whereas the capacity to support embryo development occurred while the antrum started to form. Chromatin configuration of the GV did not undergo extensive modification during the acquisition of competence or during the arrest of transcriptional activity at the large antral follicle stage. However, the quality and quantity of the cytoplasm regulated and enhanced GV functionality. This finding also held for GVs transferred from incompetent or subpar oocytes into the cytoplasm of good quality oocytes or when chromatin was artificially modified or vitrified.
CONCLUSIONS
The cat model provides a new insight into GV oocyte structure and function during folliculogenesis while challenging current concepts about oocyte quality criteria based on the GV morphology. This suggests alternative evaluative approaches for oocytes from other species too, including humans. Cat GVs also appear competent at an early follicle stage and are resilient to perturbations which designate this organelle as an attractive target for developing novel fertility preservation tactics.
Keywords: germinal vesicle, chromatin, oocyte quality, animal model, assisted reproduction
Introduction
Intrafollicular oocytes in the mammalian ovary are arrested at the first meiotic prophase and contain a nucleus called the germinal vesicle (GV). Progression of the follicle from the primordial to the antral stage is a serial process that empowers the oocyte to resume meiosis after the LH surge and then support embryo development after ovulation and fertilization (Tan et al., 2009; Tingen et al., 2009). In terms of timing, oocyte capacity for meiotic resumption occurs at the early antral follicular stage, whereas developmental competence is acquired during the final growth of the antral follicle in several species including the mouse (Eppig and Schroeder 1989; Zuccotti et al., 2002; Pesty et al., 2008), the pig (Motlik et al., 1986; Wu et al., 2006) and the cow (Pavlok et al., 1992; Lodde et al., 2007). In general, oocyte competence in domestic and laboratory animals appears influenced primarily by epigenetic factors that control overall gene expression while significantly modifying the GV chromatin configuration (De La Fuente, 2006; Tan et al., 2009). Specifically, GV oocytes from antral follicles must end a ‘non-surrounded nucleolus with diffuse chromatin’ (NSN) configuration before gaining full meiotic competence. Subsequently, these oocytes must take on a ‘surrounded nucleolus by more condensed chromatin’ (SN) configuration and stop gene transcription before having the capacity to sustain blastocyst formation (Tan et al., 2009). However, the factors underlying the biological differences in meiotic and developmental competence between NSN and SN oocytes are not fully characterized (Inoue et al., 2008). Furthermore, there appears to be species exceptions; e.g. there is no transition from an NSN to an SN pattern in the goat oocyte (Sui et al., 2005). Such observations support the need for exploring other model systems and the interrelationships among GV chromatin configuration changes, epigenetic modifications, transcriptional activity and eventual oocyte competence (Tan et al., 2009).
Along with the maintenance of GV arrest at the first meiotic prophase, the quality of the cytoplasmic environment is fundamental to sustaining GV integrity and competence until the oocyte is selected for ovulation. In the mouse (Bao et al., 2002; Takeuchi et al., 2005; Inoue et al., 2008) and the cow (Bao et al., 2003), the transfer of nuclei from early antral stage oocytes into the cytoplasm of fully grown stages has demonstrated that the GV competence in oocytes from antral follicles tends to be equal, whereas subsequent developmental failure originates from cytoplasmic ineffectiveness or defects. The nucleo-cytoplasmic volume ratio also appears critical for normal meiotic chromosome segregation, spindle formation, chromosome alignment on the metaphase spindle and embryo development, at least in the mouse oocyte (Kárníková et al., 1998; Cui et al., 2005).
There is now a growing body of evidence of the value of the laboratory cat as a reproductive model for humans, largely due to intriguing morphological and biological similarities between the ovaries and oocytes of the two species, including addressing practical issues such as fertility preservation (Bristol-Gould and Woodruff, 2006; Comizzoli et al., 2010). More specifically, substantial knowledge has emerged on the immature cat oocyte, which our laboratory has asserted is relevant to enhancing our overall understanding of oocyte competence and GV functionality (Comizzoli et al., 2010). First, the morphology of immature cat oocytes has been an accurate metric for predicting in vitro culture success (based on the number of compact cumulus cell layers and cytoplasmic appearance; Wood and Wildt, 1997). Second, best quality cat oocytes (Grade 1) consistently contain GVs with decondensed chromatin and highly acetylated histones (Comizzoli et al., 2008, 2009). This finding suggests that there may be a link among chromatin configuration, epigenetic pattern and oocyte competence in this species, which might be different from others that normally show an SN chromatin pattern in the same class of GV oocytes. Third, cat GV chromatin is remarkably tolerant to osmotic stresses, artificial chromatin compaction and freezing temperatures (Comizzoli et al., 2008, 2009). This observation underpins our contention that the cat is a germane model for exploring the plasticity and resilience of the GV to different external environments. Collectively, this rapidly growing database plus the characteristics of the immature cat oocyte offer opportunities for new insights into what provokes and protects meiotic and developmental capacity in this cell.
The general aim of the present study was to determine the evolution of cat GV structure and function during folliculogenesis. Our main purpose was to better understand what factors and processes are involved in major events, especially the capacity to resume meiosis and support normal embryo development. Our objectives also were to examine (i) the relation between GV chromatin configuration and oocyte functionality during folliculogenesis and (ii) the role of the cytoplasmic environment on the GV competence and stability.
Materials and Methods
Collection and classification of oocytes from diverse ovarian follicles
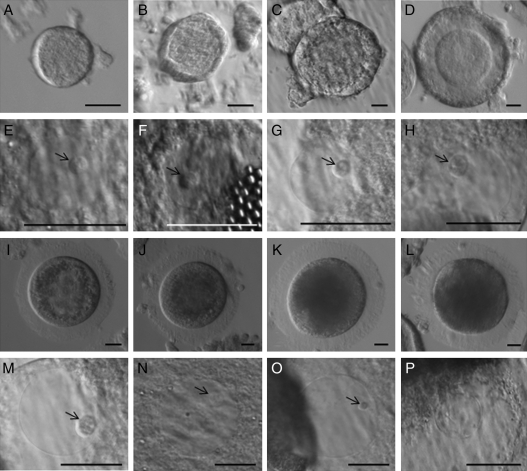
Ovaries from adult domestic cats were recovered after routine ovariohysterectomy at local veterinary clinics and transported in phosphate-buffered saline (PBS) at 4°C to the laboratory within 6–12 h of excision. Cumulus–oocyte complexes (COCs) were collected from large antral follicles (>1 to 3.5 mm in diameter, the latter size being the pre-ovulatory stage; Saint-Dizier et al., 2007) by slicing the large antral follicles visible at the surface of the ovaries with a scalpel blade in Hepes-buffered minimum essential medium (H-MEM; Gibco Laboratories, Grand Island, NY, USA) supplemented with 1 mM pyruvate, 2 mM l-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin and 4 mg/ml bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA). Each COC was classified according to the standard quality criteria (Wood and Wildt, 1997) as Grade 1 (uniformly dark cytoplasm, ≥5 compact layers of cumulus cells), Grade 2 (same as Grade 1, but with <5 cell layers) or Grade 3 (transparent or fragmented cytoplasm, partial loss of cell layers). Denuded oocytes (Grade 4) as well as degenerated oocytes (fragmented cytoplasm) were discarded. Remaining antral follicles (smaller and deeper in the ovarian cortex) were dissected from the ovarian tissue with needles and forceps and then classified according to diameters (≤0.5 mm, early antral follicle; 0.6–1 mm, small antral follicle). Oocytes from early and small antral follicles then were released and cultured separately (see below). Further tissue dissection with needles also enabled isolating early follicular stages designated as primordial, primary, secondary and pre-antral follicles on the basis of the diameter, numbers of surrounding granulosa cells and presence of basal lamina, as described by Jewgenow (1998) (Fig. 1A–D).
Figure 1.
Morphology of intraovarian cat oocytes (A–D and I–K) and respective GVs delimited by a nuclear envelope (E–H and M–O) at different stages of follicular development. (A and E) Primordial. (B and F) Primary. (C and G) Secondary. (D and H) Pre-antral. (I and M) Early antral. (J and N) Small antral. (K and O) Large antral. (L and P) After artificial chromatin compaction. Black arrows indicate the presence of the nucleolar-like body. Bar = 20 µm.
Morphological assessments of oocytes and GVs
The oocyte from each antral follicle was denuded (0.2 % hyaluronidase for 5 min) and centrifuged (15 000g for 5 min) to visualize the GV. Due to few surrounding cells and clear cytoplasm, oocytes from primordial, primary, secondary and pre-antral follicles did not require hyaluronidase treatment or centrifugation. The diameter of every oocyte and GV (average of two perpendicular diameters) was measured under phase-contrast microscopy (Olympus BX41, Olympus Corporation, Melville, NY, USA) using SPOT software 3.5.9 (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). Oocytes then were fixed by air-drying on a microscope slide followed by exposure to 95% ethanol for at least 6 h at room temperature. GV chromatin was stained with a PBS solution containing 5 µg/ml ethidium homodimer (Invitrogen, Eugene, OR, USA) plus 10 µg/ml Hoechst 33342 (Sigma) in mounting medium (Vectashield; Vector Laboratories, Burlingame, CA, USA) and examined by epifluorescence microscopy (Olympus BX 41).
Assessment of transcriptional activity in GVs from diverse-stage oocytes
Transcription was assayed by immunofluorescent detection of Bromo-UTP (BrUTP, Sigma) incorporated into nascent RNAs, according to the method of Bouniol et al. (1995) with some modifications. Oocytes from each follicular stage were incubated separately in PBS containing 0.025% Triton X-100 (Sigma) plus 50 mM BrUTP for 30 min. After being rinsed three times in PBS, oocytes were cultured in MEM (Sigma; supplemented as above) for 1.5 h at 38.5°C in air with 5% CO2. Oocytes then were fixed in 5% paraformaldehyde for 30 min at 38.5°C. After washing in PBS and saturating non-specific antigenic sites [PBS with 0.5% Triton X-100 plus 20% fetal calf serum (FCS; Irvine Scientific, Santa Ana, CA, USA)] for 30 min at 38.5°C, oocytes were incubated overnight at 4°C with anti-5-bromo-29-desoxyuridine (anti-BrdU) monoclonal antibody (Sigma) diluted 1:200 in PBS containing 0.5% Triton X-100 plus 2% FCS. After three washings (15 min each) in PBS, oocytes were incubated with an FITC-labeled anti-mouse IgG (Sigma) diluted 1:150 for 1 h at 38.5°C. GV chromatin then was stained with ethidium homodimer plus Hoechst and mounted as described previously before making observations with epifluorescence (Olympus BX 41). A set of negative controls (exposing the oocytes to 10 µg/ml α-amanitine—a transcription inhibitor—during BrUTP incubation or omitting the first antibody) was included in each series and used as a reference for the absence of transcriptional activity.
Oocyte micromanipulations
Donor and recipient oocytes were denuded of cumulus cells (with 0.2% hyaluronidase and gentle pipetting) to visualize the GV and then exposed to 7.5 µg/ml cytochalasin B at 38.5°C and centrifuged (15 000g; 5 min) in H-MEM to polarize the cytoplasmic lipid droplets. Micromanipulations were conducted in microdrops covered with mineral oil and maintained on the heated stage (38.5°C) of an inverted microscope (Olympus IX 70) equipped with micromanipulators (Narishige, Sterling, VA, USA). After partial zona dissection with a microlance (Humagen, Charlottesville, VA, USA), donor or recipient oocytes were enucleated by extrusion of a small GV karyoplast that was aspirated in a biopsy micropipette (40 µm inner diameter; Humagen). Grade 1 recipient cytoplasts then were reconstructed with a donor GV karyoplast by membrane fusion induced with Sendai virus (HVJ, 2700 hemagglutinating activity U/ml; Cosmo Bio Co., Ltd, Tokyo, Japan). Reconstructed oocytes were cultured in MEM at 38.5°C in air with 5% CO2 for 25–35 min until the fusion occurred and then were extensively washed.
To modify the nucleo-cytoplasmic volume ratio of Grade 1 oocytes, diverse volumes of cytoplasmic extrusions (without the GV) were removed after partial zona dissection. The final oocyte diameter was used as the reference to classify the extrusion volume as 25, 50 or 75% of the original volume based on the volume formula V = 4π[(diameter/2)3]/3. GV karyoplasts enclosed in the zona pellucida were obtained by removing the maximum amount of cytoplasm.
In vitro compaction of GVs and vitrification of oocytes
GV chromatin compaction was achieved by exposing denuded oocytes to 1 mM resveratrol (Sigma) for 1 h at 38.5°C and oocyte vitrification was performed as described previously (Comizzoli et al., 2009). Briefly for the latter, denuded oocytes were exposed to 10% (v/v) ethylene glycol (EG) plus 10% (v/v) dimethylsulfoxide (DMSO) for 30 s followed by exposure to the vitrification solution (VS) consisting of 20% EG plus 20% DMSO plus 1.0 M sucrose for 20 s at room temperature. Oocytes then were deposited in a minimum volume (<0.5 µl) of VS on a plastic gutter (2 oocytes/gutter) made at the tip of a 0.25 ml plastic straw and then directly plunged into liquid nitrogen. After at least 7 days of storage, vitrified oocytes were warmed by plunging the plastic gutter into 2.5 M sucrose at 38.5°C and then extensively washed stepwise in 1.0, 0.5 and 0 M sucrose solutions (5 min each).
In vitro culture and assessments of oocytes and embryos
In vitro maturation (IVM) medium consisted of MEM (Sigma) supplemented as above plus 1 µg/ml FSH (NIDDK-ovine FSH-18; National Hormone and Pituitary Program, Rockville, MD, USA), 1 µg/ml LH (NIDDK-oLH-25; National Hormone and Pituitary Program) and 1 µg/ml estradiol (Sigma). Oocytes denuded for micromanipulation or vitrification were co-cultured with intact oocytes (ratio 1:1; Godard et al., 2009) in 50 µl microdrops under mineral oil (28 h; at 38.5°C in air with 5% CO2). After fixation in 2.5% paraformaldehyde and immunostaining of microtubules and chromatin staining (Comizzoli et al., 2004), incidence of nuclear maturation was defined as the number of oocytes at the metaphase II (MII) stage relative to the total number of oocytes cultured in vitro. The proportion of normal MII spindles was relative to the total number of MII oocytes. IVF then was performed using a standard protocol routinely used in our laboratory (Comizzoli et al., 2008). Briefly, frozen/thawed, motile spermatozoa from a single sperm donor (three males were alternatively used) were selected by swim-up processing in Ham's F10 medium (Irvine Scientific) supplemented with 25 mM Hepes, 1 mM pyruvate, 2 mM glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin and 5% FCS (Irvine Scientific; complete Ham's with Hepes). Co-cultured groups of intact and denuded oocytes (ratio 1:1; Godard et al., 2009) were inseminated with 1 × 106 motile spermatozoa/ml in 50 µl microdrops of complete Ham's without Hepes under equilibrated mineral oil at 38.5°C in air with 5% CO2. Approximately 10% of total oocytes in each treatment were incubated without spermatozoa to assess the incidence of spontaneous activation (parthenogenetic control). At 18 h post-insemination (hpi), oocytes were cleaned by gentle pipetting. Presumptive zygotes were cultured in vitro for 7 days in complete Ham's F10 (38.5°C, in air with 5% CO2) before fixation in ethanol and Hoechst staining (as described above). Embryo stages were determined by the number of blastomeric nuclei. A cat embryo with 25–45 blastomeres without a blastocoele was defined as a morula (Comizzoli et al., 2008). An embryo with a visible blastocoele before fixation and comprising more than 50 blastomeres was classified as a blastocyst. Blastocyst quality was evaluated by the total number of blastomeres (with more cells indicative of higher quality; Comizzoli et al., 2008).
Experimental design and statistical analysis
Oocytes from each ovarian follicle phenotype or grade were selected from a pool of different ovaries collected on a given day (one replicate).
Experiment 1 evaluated the morphology, transcriptional activity and meiotic competence of intraovarian oocytes from different stages of growing follicles. In each replicate and for each follicular stage (primordial, primary, secondary, pre-antral, early antral, small antral and large antral), some oocytes (n = 808; five replicates) were measured and then fixed to assess GV chromatin configuration or transcriptional activity. The remainder (n = 695; five replicates) was fixed after IVM to evaluate nuclear stage as well as the proportion of intact MII spindles.
Experiment 2 examined the role of the cytoplasmic environment on GV capacity to resume meiosis and subsequently sustain normal embryo development. Experiment 2a compared the meiotic and developmental competence of GV oocytes from different growing stages (pre-antral, early antral, small antral and large antral follicles) to oocytes reconstructed with the GV from the same growing stages and a Grade 1 cytoplast. In each replicate, half of the intact or reconstructed oocytes (n = 1101; six replicates) was fixed after IVM to determine the percentage of fusion, nuclear stage and MII spindle integrity. The other half (n = 1069; six replicates) was inseminated (except for 115 additional parthenogenetic controls), cultured in vitro for 7 days and fixed to examine the proportion of cleaved embryos and the number of blastomeres per embryo. In Experiment 2b, the role of the cytoplasmic volume on GV functionality in Grade 1 oocytes was explored. In each replicate and after each extrusion (0, 25, 50 and 75% of the total volume or GV karyoplast), half of the extruded oocytes (n = 366; six replicates) was fixed after IVM to assess the percentage of fusion, nuclear stage and MII spindle integrity. The other half (n = 369; six replicates) was inseminated (except for 48 additional parthenogenetic controls), cultured in vitro for 7 days and fixed to assess the proportion of cleaved embryos and the number of blastomeres per embryo.
Experiment 3 characterized the impact of non-physiological conditions (artificial chromatin compaction and vitrification) on meiotic and developmental competences of GVs from Grade 1 oocytes and the effect of transferring a ‘treated’ GV into a fresh, untreated Grade 1 cytoplast. Treatment was defined as GV compaction, vitrification, GV compaction plus vitrification or no manipulation (control, intact oocytes only). In this experiment, oocytes were directly cultured in vitro or subjected to GV transfer before in vitro culture. For each treatment, oocytes either were fixed after IVM (n = 739; 6 replicates) to determine the percentage of fusion, nuclear stage and MII spindle integrity or were inseminated (n = 729 including; 6 replicates; except for 95 additional parthenogenetic controls), cultured in vitro for 7 days and assessed for cleavage and blastomere number as described in Experiment 2.
Percentage data were transformed using arcsine transformation before statistical analysis. Comparisons between treatments and among replicates were evaluated by analysis of variance (ANOVA), Tukey's multiple test for mean comparison and a Bartlett test for the homogeneity of the variances. Data not normally distributed were analyzed by the Kruskal–Wallis ANOVA on ranks and the Dunn method for all pairwise comparisons. Differences were considered significant at P < 0.05 (SAS Institute Inc., Cary, NC, USA).
Results
Experiment 1: morphology, transcriptional activity and meiotic competence of intraovarian oocytes from diverse follicle stages
Oocyte diameter (excluding the zona pellucida) increased progressively (P < 0.05) from the primordial follicle through the large antral stage, where size was maximal and uninfluenced (P ≥ 0.05) by quality grade (Table I, and Fig. 1A–D and I–K). Oocytes retained a clear cytoplasm (Fig. 1A–D and I) until the early antral stage, a transitional interval of increasing lipidization of the cytoplasm (Fig. 1I). Oocytes of small and large antral follicles were composed of dark cytoplasmic lipid droplets (Fig. 1J and K). Regardless of the follicular stage, oocytes always had a GV clearly delimited by a continuous nuclear envelope (Fig. 1E–H and M–O). Despite increasing follicle size, GV diameter did not change (P≥ 0.05) in oocytes contained in primordial to secondary follicles (Table I and Fig. 1E–G). However, GV diameter increased (P< 0.05) thereafter and through the large antral stage (Table I, and Fig. 1H and M–O). GV size was maximal and comparable (P≥ 0.05) in oocytes from large antral follicles while being unrelated to quality grade (Table I).
Table I.
Morphology, transcriptional activity and meiotic competence of intraovarian cat oocytes from diverse follicle stages.
| Oocyte origin | n | Oocyte diameter1 (µm) | GV diameter (µm) | Chromatin configuration and activity |
n | Nuclear status after in vitro maturation (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NLB | F | R | TA | GV | GVBD | MI | MII | Normal MII spindle | |||||
| Primordial follicle | 86 | 33.6 ± 1.3a | 15.2 ± 1.8a | Large | + | − | − | 80 | 100a | 0a | 0a | 0a | |
| Primary follicle | 92 | 47.5 ± 3.2b | 16.9 ± 0.9a | Large | + | − | + | 78 | 100a | 0a | 0a | 0a | |
| Secondary follicle | 96 | 63.2 ± 2.7c | 17.0 ± 1.1a | Large | + | − | + | 80 | 100a | 0a | 0a | 0a | |
| Pre-antral follicle | 93 | 73.6 ± 4.2d | 24.8 ± 2.5b | Large | ± | ± | + | 72 | 100a | 0a | 0a | 0a | |
| Antral follicles | |||||||||||||
| Early (≤0.5 mm) | 82 | 93.9 ± 3.5e | 31.0 ± 2.2c | Large | − | + | + | 74 | 46.3 ± 4.8b | 12.5 ± 6.5b,c | 26.2 ± 4.8b,d | 15.0 ± 4.1b | 81.8 ± 23.6ª |
| Small (0.6–1 mm) | 91 | 104.0 ± 3.6f | 36.5 ± 3.8d | Large | − | + | + | 79 | 12.3 ± 2.5c | 16.5 ± 2.4b | 25.2 ± 4.8b,d | 46.0 ± 6.3c | 83.3 ± 10.2ª |
| Large (>1 mm) | |||||||||||||
| Grade 1 | 95 | 115.7 ± 4.2g | 40.2 ± 3.5e | Small/absent | − | + | − | 80 | 0d | 0a | 18.8 ± 3.0c | 81.2 ± 3.0d | 90.1 ± 7.8ª |
| Grade 2 | 84 | 116.2 ± 2.8g | 39.8 ± 3.7d,e | Small/absent | − | + | − | 78 | 0d | 9.2 ± 3.0c | 24.8 ± 5.0b | 66.0 ± 5.7e | 90.4 ± 8.2ª |
| Grade 3 | 89 | 109.2 ± 5.1g | 40.9 ± 1.1e | Small/absent | − | + | − | 74 | 0d | 16.5 ± 3.7b | 34.5 ± 5.4d | 49.0 ± 6.4c | 41.7 ± 11.4b |
Within columns, values (mean ± SD; five replicates) with different superscripts are different (P< 0.05). NLB, nucleolar-like body; F, filamentous chromatin; R, reticular chromatin; TA, transcriptional activity; GV, germinal vesicle; GVBD, germinal vesicle breakdown; MI, metaphase I; MII, metaphase II.
1Measurement without the zona pellucida.
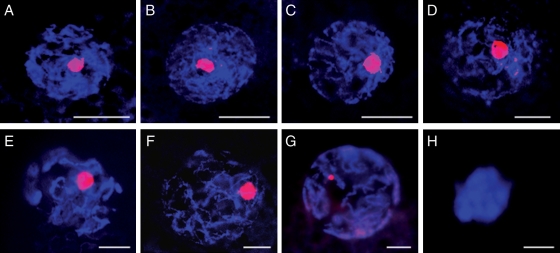
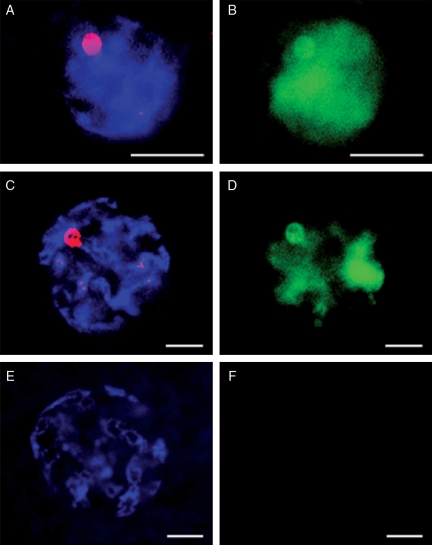
Oocytes from primordial, primary and secondary follicles contained diffuse GV chromatin in the nucleoplasm with a filamentous configuration (Table I; Fig. 2A–C) and a large nucleolar-like body (range, 3.1–6.2 µm diameter) (Table I, and Figs 1E–G and 2A–C). Oocytes of pre-antral follicles also contained a large nucleolar-like body, but the GV chromatin had a transitional, filamentous-reticular configuration (Table I, and Figs 1H and 2D). Oocytes from either an early or small antral follicle had GV chromatin with a fully reticular configuration and a large nucleolar-like body (Table I, and Figs 1M and N and 2E and F). The same reticular conformation was observed in the GV chromatin of oocytes from large antral follicles (regardless of the grade), but the nucleolar-like body was smaller compared with earlier stages (range, 1.4–2.1 µm diameter; P< 0.05; Table I, and Figs 1O and 2G) or absent in >60% of the oocytes. Transcriptional activity was detected in all GVs from the primary follicle through small antral follicle stages with newly transcribed mRNAs detected throughout the nucleoplasm and in the nucleolar-like body (Table I; Fig. 3B and D). Transcription was never measurable in GVs from oocytes contained in large antral follicles, regardless of the quality grade (Table I and Fig. 3F).
Figure 2.
Chromatin configuration of GVs in intraovarian oocytes in different follicular stages. Chromatin was stained with Hoechst (blue), whereas the nucleolar-like body was stained with ethidium homodimer (red). (A) Primordial. (B) Primary. (C) Secondary. (D) Pre-antral. (E) Early antral. (F) Small antral. (G) Large antral. (H) After artificial compaction. Bar = 10 µm.
Figure 3.
Chromatin configuration (A, C and E; Hoechst and ethidium staining) and transcription activity (B, D and F; FITC staining) in GVs of oocytes from diverse stages of follicles. (A and B) Primary. (C and D) Small antral follicle. (E and F) Large antral follicle. Bar = 10 µm.
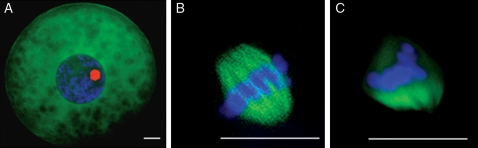
After the IVM interval, the oocytes from primordial through pre-antral follicle stages remained at the GV stage (Table I) with the same chromatin configuration and microtubules dispersed in the cytoplasm (Fig. 4A). However, oocytes from all antral stages had the capacity to resume meiosis, reach MII stage and have (mostly) normal metaphase spindles (Table I and Fig. 4B). The ability to reach MII increased progressively (P < 0.05) in oocytes from early antral to large antral follicles (Table I). Oocyte quality markedly influenced (P< 0.05) the ability to achieve nuclear maturation, with the highest incidence of MII in cells with a Grade 1 rating and lower ability (P< 0.05) in subpar counterparts (Table I). There was evidence of poor chromosomal alignment on the metaphase plate of Grade 3 oocytes (Fig. 4C) associated with a ∼50% reduction in normal MII spindles (compared with Grade of 1 or 2 counterparts).
Figure 4.
Microtubule (FITC staining) and chromatin configuration (Hoechst and ethidium homodimer staining) after IVM. (A) GV stage. (B) Normal MII spindle. (C) Abnormal MII spindle. Bar = 10 µm.
Experiment 2a: impact of a Grade 1 cytoplasmic environment on expression of meiotic and developmental competence of GVs from oocytes of diverse follicle stages
Without GV transfer, the meiotic competence of oocytes (and incidence of normal MII spindles) from pre-antral to large antral follicles (Table II) was consistent with observations made in Experiment 1 (Table I). None of the oocytes from pre-antral follicles reached MII and, therefore, all failed to be fertilized after in vitro insemination (Table II). Despite a modest ability to reach the MII stage (∼16%), none of the oocytes from early antral follicles could be fertilized (Table II). The ability of oocytes from the small antral group to achieve nuclear maturation more than tripled (to ∼47%), although half of these formed 2- to 4-cell embryos post-insemination and with equal-sized blastomeres but none advanced further in vitro (Table II). Only oocytes from large antral follicles (>1 mm) developed into morulae and blastocysts after insemination and culture (Table II). Although there was no influence of quality on the proportion of morulae produced (P ≥ 0.05), successful blastocyst formation was quality-dependent, and no blastocysts formed from Grade 3-inseminated oocytes (Table II).
Table II.
Impact of GV transfer (GVT) into Grade 1 cytoplasts on the meiotic and developmental competence of GVs from diverse follicles stages.
| Donor GV origin | n | GVT | Fused (%) | Nuclear status after in vitro maturation (%) |
n | Developmental competence after in vitro fertilization (%) |
|||
|---|---|---|---|---|---|---|---|---|---|
| MII or MII/fused | Normal MII spindle | Cleaved/MII | Morulae/cleaved | Blastocysts/cleaved | |||||
| Pre-antral follicle | 89 | − | 0a | 72 | 0a | 0a | 0a | ||
| 89 | + | 74.1 ± 2.2a | 20.0 ± 8.9b | 38.5 ± 16.2a | 74 | 0a | 0a | 0a | |
| Early antral (≤0.5 mm) | 91 | − | 16.1 ± 6.0b | 80.0 ± 13.8b | 91 | 0a | 0a | 0a | |
| 92 | + | 70.0 ± 4.3a | 54.8 ± 10.9c | 85.7 ± 10.2b | 90 | 60.9 ± 7.9b | 14.3 ± 7.2b | 7.1 ± 5.9b | |
| Small antral (0.6–1 mm) | 91 | − | 46.7 ± 9.0c | 88.1 ± 9.8b | 93 | 54.0 ± 11.9b | 0a | 0a | |
| 89 | + | 71.2 ± 3.9a | 55.2 ± 9.2c | 85.7 ± 11.4b | 92 | 64.5 ± 5.6b | 29.7 ± 6.6c | 11.3 ± 6.1b | |
| Large antral (>1 mm) | |||||||||
| Grade 1 | 95 | − | 85.5 ± 3.5d | 91.5 ± 6.8b | 95 | 76.6 ± 6.2c | 31.3 ± 3.0c | 34.6 ± 5.4c | |
| 92 | + | 72.4 ± 5.2a | 59.5 ± 4.8c | 87.5 ± 7.2b | 92 | 64.0 ± 9.6b,c | 29.5 ± 8.5c | 12.5 ± 8.4b | |
| Grade 2 | 98 | − | 71.4 ± 3.6e | 85.7 ± 6.5b | 90 | 75.0 ± 8.0c | 30.0 ± 2.4c | 25.7 ± 3.2d | |
| 95 | + | 68.3 ± 5.4a | 56.1 ± 7.3c | 83.3 ± 7.1b | 95 | 63.9 ± 6.7b | 29.4 ± 7.5c | 11.8 ± 8.3b | |
| Grade 3 | 91 | − | 50.9 ± 4.8c | 43.5 ± 8.2a | 93 | 57.1 ± 5.2b | 31.3 ± 3.9c | 0a | |
| 89 | + | 71.2 ± 7.3a | 57.1 ± 6.1c | 83.3 ± 10.1b | 92 | 66.7 ± 7.2b | 18.8 ± 9.4b,c | 11.3 ± 9.1b | |
Within columns, values (mean ± SD; six replicates) with different superscripts are different (P< 0.05). MII, metaphase II.
Following GV transfer into a Grade 1 cytoplast (from large antral follicles) and then culture, fusion success was the same (P≥ 0.05), regardless of origin of the donor GV used for reconstruction (Table II). About 20% of reconstructed oocytes with a GV from a pre-antral follicle reached MII, but with a lower (P < 0.05) incidence of normal MII spindle formation compared with later stage groups and a sustained inability to be fertilized (Table II). Enhanced nuclear maturational ability (P < 0.05; ∼3-fold) also occurred when GVs from early antral follicles were transferred into Grade 1 cytoplasts (Table II). More than 60% of these reconstructed oocytes cleaved and a few advanced to morulae (∼14%) and blastocysts (∼7%) (Table II). There was no advantage for achieving MII after transferring more advanced donor GVs (from oocytes of small or large antral follicles), with all reconstructions expressing comparable (P≥ 0.05) meiotic competence (Table II). Proportions of normal MII spindle formation also were the same (P ≥ 0.05) with the exception of a 2-fold improvement (P < 0.05) for GV from Grade 3 oocytes. Oocytes reconstructed with GVs from early and small antral follicles also exhibited better developmental competence (P < 0.05) than in the absence of GV transfer (Table II). By contrast, there was an ∼12% or more reduction (P < 0.05) in embryo formation and development for GVs from Grade 1 and 2 oocytes after reconstruction compared with similar quality, non-transferred counterparts (Table II). Blastocyst formation was comparable among all reconstructed oocytes (P ≥ 0.05) that included ∼11% success with GVs from Grade 3 cells. Regardless of treatment, no parthenogenetic activation was observed in any group, and blastomere numbers in blastocysts were not different (range, 69–92; P ≥ 0.05).
Experiment 2b: influence of the nuclear–cytoplasmic volume ratio on expression of meiotic and developmental competence of GVs in Grade 1 oocytes
Grade 1 oocytes with as much as 50% of cytoplasmic volume extruded retained the capacity to resume meiosis and in similar proportions (P≥ 0.05) to controls or counterparts with 25% removed (Table III). However, oocytes losing half of their cytoplasm had a decreased (P < 0.05) incidence of normal MII spindles and fewer (P< 0.05) cleaved embryos and morula-blastocyst formations (Table III). With a 50% reduction in cytoplasm, >80% of embryos arrested before the 8–16 cell stage. In contrast, normal spindle formation and embryo development through the blastocyst stage were retained in oocytes with 25% of cytoplasm extruded (Table III). However, blastomere numbers in blastocysts were not different (range, 72–94; P ≥ 0.05) regardless of the treatment group. The ability to reach the MII stage was completely inhibited (P< 0.05) when oocytes were extruded of more than 50% of their original cytoplasmic volume (Table III). When 75% of the original volume was removed, oocytes arrested at the MI stage (Table III) with >50% of spindle abnormalities. Lastly, GV karyoplasts did not progress beyond the GVBD stage after IVM (Table III). No parthenogenetic activation was observed regardless of the treatment.
Table III.
Impact of the nuclear–cytoplasmic volume ratio on the meiotic and developmental competence of Grade 1 oocytes; influence of extruding various proportions of cytoplasm.
| Extrusion of total oocyte volume | n | Nuclear status after in vitro maturation (%) |
n | Developmental competence after in vitro fertilization (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GV | GVBD | MI | MII | Normal MII spindle | Cleaved/MII | Morulae/cleaved | Blastocysts/cleaved | |||
| None | 75 | 0a | 0a | 18.5 ± 4.2a | 81.5 ± 4.2a | 90.1 ± 8.9ª | 73 | 71.7 ± 6.3a | 28.9 ± 3.3a | 34.2 ± 4.4a |
| 25% | 77 | 0a | 0a | 20.9 ± 2.9a | 79.1 ± 2.9a | 88.5 ± 7.8ª | 75 | 69.8 ± 7.3a | 31.8 ± 6.4a | 32.4 ± 3.6a |
| 50% | 71 | 0a | 0a | 23.0 ± 5.7a | 77.0 ± 5.7a | 54.5 ± 9.1b | 73 | 46.8 ± 5.8b | 13.6 ± 4.8b | 4.5 ± 6.4b |
| 75% | 72 | 29.0 ± 5.2b | 33.9 ± 3.1b | 37.1 ± 8.1b | 0b | 74 | 0c | 0c | 0c | |
| GV karyoplast | 71 | 54.1 ± 5.8c | 45.9 ± 5.8c | 0c | 0b | 74 | 0c | 0c | 0c | |
Within columns, values (mean ± SD; six replicates) with different superscripts are different (P< 0.05). GV, germinal vesicle; GVBD, germinal vesicle breakdown; MI, metaphase I; MII, metaphase II.
Experiment 3: impact of a fresh Grade 1 cytoplasmic environment on meiotic and developmental competence of GVs that were artificially compacted and/or vitrified
In vitro compaction led to GV diameters (range, 15–19 µm; Figs 1P and 2H) less than half the size found in oocytes from antral follicles and comparable to those in primordial and primary follicles (Table I). With compaction alone and no GV transfer, oocyte meiotic and developmental competences were similar (P ≥ 0.05) to control treatment, including the ability to produce blastocysts (Table IV). Proportions of oocytes reaching MII stage with normal spindles and success of embryo development were affected adversely (P < 0.05) by vitrification, including reducing the ability to develop into morulae or blastocysts. Pre-vitrification compaction of the chromatin mitigated some of this damage, resulting in normal levels of nuclear maturation, spindle formation, and embryo cleavage. Chromatin compaction also was partially protective for advanced embryo development with equivalent proportions of morulae and less (P < 0.05), but still ∼16% blastocyst production (Table IV).
Table IV.
Impact of GVT into fresh Grade 1 cytoplasts on the meiotic and developmental competence of GVs artificially compacted and/or vitrified.
| Treatment | n | GVT | Fused (%) | Nuclear status after in vitro maturation (%) |
n | Developmental competence after in vitro fertilization (%) |
|||
|---|---|---|---|---|---|---|---|---|---|
| MII or MII/fused | Normal MII spindle | Cleaved/MII | Morulae/cleaved | Blastocysts/cleaved | |||||
| None | 95 | − | 78.2 ± 6.5a,c | 89.2 ± 5.8a | 91 | 77.1 ± 5.4a | 29.4 ± 3.2a | 35.3 ± 6.0a | |
| 92 | + | 75.0 ± 2.9a | 56.4 ± 4.9b | 82.0 ± 12.5a | 92 | 63.6 ± 8.1b | 28.6 ± 9.2a | 14.3 ± 8.3b | |
| GV compaction | 91 | − | 80.4 ± 3.3c | 89.0 ± 6.3a | 90 | 73.2 ± 4.1a,b | 30.0 ± 3.9a | 36.7 ± 2.7a | |
| 91 | + | 69.7 ± 6.1a | 60.6 ± 6.5b | 84.2 ± 9.2a | 93 | 68.3 ± 5.5a,b | 27.8 ± 9.2a | 16.7 ± 8.3b | |
| Vitrification | 95 | − | 52.7 ± 6.3b | 44.0 ± 12.0b | 89 | 31.0 ± 7.9c | 0b | 0c | |
| 92 | + | 73.5 ± 4.4a | 51.5 ± 8.1b | 83.3 ± 7.8a | 91 | 58.8 ± 7.5b | 10.4 ± 6.3c | 14.5 ± 9.2b | |
| GV compaction + vitrification | 91 | − | 70.6 ± 5.7a | 89.1 ± 8.2a | 88 | 69.4 ± 7.6a,b | 21.0 ± 9.0a,c | 16.4 ± 10.2b | |
| 92 | + | 71.5 ± 8.5a | 53.1 ± 5.2b | 82.9 ± 10.8a | 95 | 64.7 ± 7.0b | 27.3 ± 5.6a | 15.1 ± 9.4b | |
Within columns, values (mean ± SD; six replicates) with different superscripts are different (P< 0.05). MII, metaphase II.
After GV transfer, percentages of fusion were not different (P≥ 0.05) in any treatment group (control, compaction, vitrification, compaction plus vitrification). With no other treatment, GV transfer alone decreased the incidence of MII, embryo cleavage, and blastocyst formation, but not normal spindle or morulae formation (Table IV). In general, other treatments combined with GV transfer into a fresh Grade 1 cytoplast reduced (P < 0.05) the proportion of oocytes achieving MII, but without compromising normal spindle formation. In contrast, reconstruction increased (P < 0.05) spindle integrity by nearly 2-fold in the vitrification alone group. After in vitro insemination, the developmental competence of reconstructed oocytes was comparable (P > 0.05) to controls for the compaction and compaction plus vitrification groups (Table IV). However, the transfer of a previously vitrified GV to a fresh cytoplast doubled (P < 0.05) cleavage success while also resulting in advanced embryo production (Table IV). Regardless of the treatment, no parthenogenetic activation was observed, and blastomere numbers in blastocysts were not different (range, 68–95; P ≥ 0.05).
Discussion
This study revealed that the cat oocyte's ability to achieve meiosis and sustain preimplantation embryo development was acquired progressively and through the early stages of follicular growth. The onset of oocyte meiotic competence occurred during the early antral stage. However, the ability to sustain embryo development post-fertilization was acquired in oocytes during the small antral stage. More importantly, it was possible to elucidate that the GV competence was being acquired before the whole oocyte and when this exactly occurred. GV transfers into cytoplasts of Grade 1 oocytes demonstrated that intrinsic meiotic as well as developmental capacity of the GV was acquired during and right after the antrum formation, a period with slight chromatin changes. Subsequently, the only modification of the GV chromatin was the disappearance of the nucleolar-like body which coincided with the arrest in transcriptional activity. The GV transfers and cytoplasmic extrusions also unequivocally demonstrated that the quality and quantity of the cytoplasm were the primary determining factors for the GV ability to express full meiotic and developmental competence. Particularly significant was that the optimal cytoplasmic environment (from Grade 1 oocytes) also enhanced meiotic and developmental competence of GVs ‘rescued’ from oocytes that were rated subpar, recovered from earlier stage follicles or following GV chromatin perturbations (compaction and/or vitrification). The latter observations highlighted the remarkable resilience of the GV to non-physiological conditions.
Follicle and oocyte diameters at the various growing stages were consistent with previous descriptions in the domestic cat that separately have focused on the primordial, pre-antral and large follicle phenotypes (Wood et al., 1997; Jewgenow, 1998; Bristol-Gould and Woodruff, 2006; Reynaud et al., 2009; Carrijo et al., 2010). Here, we observed that intraovarian cat oocytes from fully mature follicles reached a final diameter of ∼110 µm without the zona pellucida, which was near the size reported for human (105 µm; Miyara et al., 2003; Escrich et al., 2010), and larger than in laboratory rodents (80 µm; Tan et al., 2009). However, the cat GV at varying growing stages was consistently larger than in other species, eventually being ∼40 µm in diameter, which was greater than the range of 25–30 µm reported for the mouse (Liu et al., 1999), cow (Lodde et al., 2007), pig (Sun et al., 2004), non-human primates (Schramm et al., 1993), human (Escrich et al., 2010) and even other carnivores (dog, Viaris de Lesegno et al., 2008; ferret, Sun et al., 2009a).
We suspect that the unusual size of the cat GV was perhaps related to the organelle's other traits that were revealed during our investigation. Distinctive were the cat's filamentous and reticular chromatin patterns across follicular stages compared with similar GV stages in other mammals (Tan et al., 2009), including other carnivores (Viaris de Lesegno et al., 2008; Sun et al., 2009a). Cat GV chromatin also maintained a homogeneous configuration within each follicular stage as well as a single nucleolar-like body containing RNA (as determined by ethidium homodimer staining). Interestingly, the diffuse chromatin configurations and the presence of a single nucleolar-like body (known to actively synthesize RNA) were analogous to what occurs in the human (Parfenov et al., 1989; Combelles et al., 2002; Escrich et al., 2010) and cow (Lodde et al., 2008) oocyte. The onset of transcriptional activity in the cat GV coincided with primordial follicle activation (as has been described for the mouse; De La Fuente and Eppig, 2001), then stopped in the largest antral follicles (>1 mm diameter) when the nucleolus decreased in size or disappeared. In the latter context, the cat was similar to laboratory rodents, the cow and human (Kopecný et al., 1995; Miyara et al., 2003; Lodde et al., 2008), with transcriptional arrest occurring in large antral follicles but, in the case of the cat, this was considerably in advance of reaching a pre-ovulatory stage. Transcriptional silencing occurred while cat GV chromatin remained decondensed and was unrelated to a switch from an NSN to an SN pattern, compared with what has been described for the mouse (Debey et al., 1993), monkey (Schramm et al., 1993) and human (Miyara et al., 2003). The only noticeable marker for chromatin modification in the cat was the ‘dismissal’ of the nucleolus which has been shown to reflect arrest of ribosomal RNA transcription in the oocyte (Maddox-Hyttel et al., 2005) and dispersal of proteins that assist in cell-cycle regulations (Andersen et al., 2005). Histone deacetylation was likely not the epigenetic factor involved in gene silencing because histones are highly acetylated in cat GV oocytes recovered from large antral follicles (Comizzoli et al., 2009). Thus, the cat model appeared unique as transcriptional arrest occurred, whereas GV chromatin was sustained in a decondensed state compared with a condensed status in mouse (De La Fuente, 2006) and pig (Endo et al., 2006) GVs. Because studies from our laboratory (Comizzoli et al., 2008) and that of another (Luvoni et al., 2006) have revealed active and open communications between the cat oocyte and its cumulus investment up to the pre-ovulatory stage, it may well be that enveloping cumulus cells play a significant role in regulating GV transcription, even more than indicated in previous studies of the mouse (De La Funente, 2006) and the cow (Lodde et al., 2007). This mechanism probably involves a direct regulation of RNA polymerase phosphorylations/activity like in the mouse and human (Miyara et al., 2003), but not a modification of GV chromatin accessibility to transcriptional factors.
Besides the interesting absence of major GV chromatin modification related to the follicular stage or transcriptional activity, no differences related to atresia were observed. With ∼65% of atretic oocytes in the cat ovary (Wood et al., 1997; Jewgenow, 1998), no differences in GV chromatin configurations was detected, especially within small antral follicles (0.6–1 mm in diameter) that have the highest incidence of oocyte atresia or in lower-grade oocytes collected from large antral follicles (Wood et al., 1997). This clearly demonstrated that atresia was likely not affecting the cat GV structure in contrast with other species studied to date (Tan et al., 2009). Thus, our observations here were evidence for a remarkable homogeneity and stability of the cat GV, a finding that has been confirmed in another (as yet unpublished) study from our laboratory. That investigation is revealing that chromatin configuration remains unperturbed in cat GV oocytes collected from pre-ovulatory follicles after hCG stimulation in vivo or immediately before GV ‘break-down’ (GVBD). Likewise, cat oocytes remaining at the GV stage after IVM retain unaltered chromatin configuration. Collectively, these observations are substantially different from what has been reported in mammal species to date (Tan et al., 2009) where the LH surge and events preceding GVBD are associated with GV chromatin condensation. Interestingly, most domestic cats only experience an LH surge and ovulation after copulation or an exogenous LH-type stimulus (Pelican et al., 2006), and it is unlikely that the oocyte donors in our study had been exposed to mating. Thus, another advantage of the induced-ovulating cat model is the ability to study follicles and oocytes that have been unbiased and non-confounded by LH exposure.
The ability to achieve meiosis was secured in the cat oocyte as the antrum was beginning to form, which is what occurs in the mouse and marmoset (Eppig and Schroeder 1989; Gilchrist et al. 1995), but which is substantially earlier during the folliculogenesis than in other studied species, including the pig (Motlik et al., 1986), goat (de Smedt et al. 1994) and cow (Lodde et al., 2007). We focused our assessment of normal chromosomal segregation during meiosis by examining MII spindle quality because normality in the latter and in the metaphase plates associated with subsequent equal-sized blastomeres in early cleavages are good indicators of euploidy (Comizzoli et al., 2004). Cytoplasmic quality in lower-grade oocytes, cytoplasmic extrusion experiments as well as vitrification of whole oocytes clearly demonstrated that the cytoplasmic environment also played a fundamental role on the proper spindle formation. However, GV transfer experiments revealed that the ability to resume meiosis appeared in GVs from pre-antral follicles but that the intrinsic capacity to properly condense in chromosomes was fully acquired at the early antral stage and therefore depended on the GV too as previously reported in other species (Gao et al., 2002; Mattiske et al., 2009).
Following the ability to resume meiosis, the developmental competence of the oocyte became optimal in large antral follicles, but varied according to the quality grade of the cell, as reported earlier by our research group (Wood and Wildt, 1997; Comizzoli et al., 2008). The present study further determined that oocytes residing in early and small-sized antral follicles apparently failed to develop beyond 8 cells after fertilization which is the stage of the major embryonic genome activation (Hoffert et al., 1997; Waurich et al., 2010). Similarly, a recent report in non-human primates has indicated that high proportions of oocytes from small antral follicles do not resume meiosis or lead to a partial embryo development (Peluffo et al., 2010). In contrast and compared with other species, including the cow (Pavlok et al., 1992), mouse (Zuccotti et al., 2002) and pig (Wu et al., 2006), cat oocytes became competent for embryo development in small antral follicles (1 mm diameter) relative to the final pre-ovulatory diameter (3.5 mm). Furthermore, GV transfers demonstrated that this ability to sustain early embryo growth was fully acquired by the GV at the early antral stage (≤0.5 mm diameter). Thus, both meiotic and developmental competence were secured in the GV before being conferred to the whole oocyte (cytoplasm). Again, this occurred at an earlier follicle stage than observed in other mammal species studied to date (Bao et al., 2002, 2003; De La Fuente, 2006; Pesty et al., 2008; Inoue et al., 2008; Tan et al., 2009), re-emphasizing the uniqueness of the cat model.
Our collective structural and functional results clearly suggested that progression from pre-antral to the antral follicle stage appeared to be the primary transition point for acquiring GV meiotic and developmental competence. The key indicator included a 30% increase in diameter of the oocyte and 20% in GV size, whereas chromatin configuration modestly morphed from a filamentous to a reticular pattern. DNA and histone methylation currently are examined as the markers of acquisition since they may occur independently of the change in GV chromatin configuration in the mouse (Kageyama et al., 2007; Inoue et al., 2008). It also has been suggested that GVs from pre-antral or earlier follicle stages could progressively acquire a nucleoplasmic factor that may be essential for meiotic resumption and subsequent embryo development as suggested in the mouse (Bao et al., 2002; Gao et al., 2002) and pig (Sugiura et al., 2001). At last, the ‘signal’ that is necessary to form an antrum might also impact the acquisition of GV competence to resume meiosis and develop into a normal embryo (Rodgers and Irving-Rodgers, 2010).
The other notable change was from the early antral stage to the large antral stage. The oocyte and GV diameters increased (20% and 30%, respectively) while the nucleolus dismissal was the only notable change in the chromatin configuration when gene expression was silenced. Furthermore, although early and small antral follicles contained proficient GVs, the cytoplasm of these particular stage oocytes was incompatible with allowing expression of full GV competency. Therefore, cytoplasm quality was the regulatory force controlling whole oocyte function in this species. A Grade 1 cytoplast (from large antral follicle) containing homogeneous cytoplasmic lipid droplets was a good environment for any GV regardless of follicle origin. Optimal grade ensured favorable communication by maintaining GV arrest as well as enhancing GV functional properties. Most importantly, the cat oocyte was notably different from the mouse counterpart where meiotic competence largely relies on cytoplasmic factors whereas developmental competence in fully-grown oocytes is GV dependent (Inoue et al., 2008). We suggest that the key factors contributing to the quality of a Grade 1 cytoplast are originating from the surrounding cumulus cells communicating with the oocyte (Luvoni et al., 2006; Comizzoli et al., 2008) or from the synthesis of specific proteins (Vitale et al., 2007). These observations underscore the fascinating and complex interactions routinely occurring between the oocyte nucleus and the surrounding cytoplasm.
The GV compaction and vitrification experiments were useful in further proving the absence of linkage between chromatin configuration and competence while demonstrating the plasticity and resilience of the organelle to significant perturbations. We have previously documented the benefits of chromatin compaction in withstanding the stress of vitrification storage (Comizzoli et al., 2009). The transfer of compacted and/or vitrified GVs followed by successful maturation, fertilization and embryo development demonstrated that ribosomal proteins, nucleoplasmin and remodeling factors, normal constituents of GVs, were also successfully retained. Again, the quality of the cytoplasm from Grade 1 oocytes appeared to strictly control GV competence by helping reanimate the vitrified, compacted organelles and then allow resumption of meiosis as well as fertilization and development. Final affirmation of the significant role of cytoplasm was observed in the extrusion experiments. While removing 25% of cytoplasmic volume from Grade 1 oocytes had no effect, a negative impact was detected when eliminating half the volume, as revealed by more abnormal MII spindles and impaired cleavage and morula/blastocyst formation. Oocyte functional capacity essentially was abolished by extruding 75% of the cytoplasm. Our observations confirm earlier findings in the mouse oocyte that demonstrated the need for a critical quantity of cytoplasmic volume for normal meiotic chromosome segregation, spindle formation, chromosome alignment on the metaphase spindle and developmental competence (Kárníková et al., 1998; Cui et al., 2005). However, the cat oocyte appeared to be more sensitive than mouse counterparts at a comparable level of cytoplasmic extrusion (Cui et al., 2005). Additionally, it was interesting that half of the cat oocytes experiencing high proportions of extrusion (resulting in a GV karyoplast) remained arrested at the GV stage post-IVM. This likely was a natural arrest related to low amounts of cAMP and cGMP (Sun et al. 2009b) and is noteworthy as perhaps a potential means of preserving the GV karyoplast alone to ensure genome stability before reconstruction with a fresh recipient cytoplast.
In conclusion, we have generated new knowledge about the structural and functional properties of a mammalian oocyte and its resident GV as both progress through each follicle stage. Especially important was our discovery that there is a clear asynchrony between early acquisition of nuclear and later cytoplasmic competence during folliculogenesis in the cat model. We believe that our findings also support the contention that chromatin configuration is an unreliable indicator of GV and oocyte functionality. Interestingly, although GV chromatin condensation in human oocytes appears to follow an SN-like pattern just before resumption of the meiosis (Combelles et al., 2002; Miyara et al., 2003), there also is recent evidence that GV morphometry and chromatin configuration are poor predictors of human oocyte competence (Escrich et al., 2010, 2011). One major challenge is that studies of human GV oocytes generally rely on surplus gametes derived from patients who have failed to respond to exogenous hormonal stimulation for in vivo maturation. Therefore, these cells already may be compromised or not true representatives of normal oocytes collected at any time of the cycle (Combelles et al., 2002; Miyara et al., 2003; Vanhoutte et al., 2007; Escrich et al. 2010, 2011). Additionally, chromatin condensations reported in primates and humans also generally appear related to the early event of the meiotic resumption preceding GVBD rather than a real SN pattern (Schramm et al., 1993; Miyara et al., 2003). Thus, the slight chromatin modifications and the major role of the Grade 1 cytoplasm described in the cat oocyte may be useful in considering and then exploring new and alternative quality criteria for human oocytes. For example, this could include novel indicators of oocyte competence based on the cytoplasmic quality, proteomics (Powell et al., 2010) or cumulus cell biomarkers (Assidi et al., 2011). Our findings also are likely to be of broad-scale interest because incomplete meiotic maturation and embryonic development failure are major causes of human infertility (Tan et al., 2009) and reliable predictors of the oocyte quality before IVM are still needed in human reproductive medicine (Combelles and Racowsky, 2005). Furthermore, studying GV-stage oocytes from non-stimulated ovaries is analogous to human situations when oocyte collection must be performed without prior exogenous hormonal induction to prevent hyperstimulation syndromes or before ovariectomy for medical reasons (Woodruff, 2010). Finally, we are encouraged by the discovery that the GV can be competent at an early stage, stable in various cytoplasmic conditions and resilient to cryo-storage. This suggested that the ovary is a reservoir for a large pool of competent GVs that represents an untapped source for female fertility preservation at any time of the ovarian cycle and without the need for follicle culture, at least as long as GV can be transferred into a Grade 1 cytoplast. Such results imply the potential of using the GV alone as a means of storing female fertility long term, which is the subject of ongoing investigations in our laboratory.
Authors' roles
P.C. contributed to the design of the study, acquisition and analysis of data and manuscript preparation; B.S.P contributed to the study design and manuscript preparation; and D.E.W contributed to the design of the study, data interpretation and the manuscript review.
Funding
This study was supported by grant numbers K01 RR020045 and R01 RR026064 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Acknowledgements
We thank Drs Michael Cranfield and Brent Whitaker (Maryland Line Animal Rescue) and Dr Darby Thornburgh (Petworth Animal Hospital) for providing domestic cat ovaries.
References
- Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CEl. Nucleolar proteome dynamics. Nature. 2005;433:77–83. doi: 10.1038/nature03207. [DOI] [PubMed] [Google Scholar]
- Assidi M, Montag M, Van Der Ven K, Sirard MA. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: a preliminary study. J Assist Reprod Genet. 2011;28:173–188. doi: 10.1007/s10815-010-9491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Obata Y, Ono Y, Futatsumata N, Niimura S, Kono T. Nuclear competence for maturation and pronuclear formation in mouse oocytes. Hum Reprod. 2002;17:1311–1316. doi: 10.1093/humrep/17.5.1311. [DOI] [PubMed] [Google Scholar]
- Bao S, Ushijima H, Hirose A, Aono F, Ono Y, Kono T. Development of bovine oocytes reconstructed with a nucleus from growing stage oocytes after fertilization in vitro. Theriogenology. 2003;59:1231–1239. doi: 10.1016/s0093-691x(02)01174-3. [DOI] [PubMed] [Google Scholar]
- Bouniol C, Nguyen E, Debey P. Endogenous transcription occurs at the 1-cell stage in the mouse embryo. Exp Cell Res. 1995;218:57–62. doi: 10.1006/excr.1995.1130. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould S, Woodruff TK. Folliculogenesis in the domestic cat (Felis catus) Theriogenology. 2006;66:5–13. doi: 10.1016/j.theriogenology.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Carrijo OA, Jr, Marinho AP, Campos AA, Amorim CA, Báo SN, Lucci CM. Morphometry, estimation and ultrastructure of ovarian preantral follicle population in queens. Cells Tissues Organs. 2010;191:152–160. doi: 10.1159/000225935. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Racowsky C. Assessment and optimization of oocyte quality during assisted reproductive technology treatment. Semin Reprod Med. 2005;23:277–284. doi: 10.1055/s-2005-872456. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Effect of 1,2-propanediol versus 1,2-ethanediol on subsequent oocyte maturation, spindle integrity, fertilization, and embryo development in vitro in the domestic cat. Biol Reprod. 2004;71:598–604. doi: 10.1095/biolreprod.104.027920. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisosmotic conditions on structural and functional integrity of cumulus–oocyte complexes at the germinal vesicle stage in the domestic cat. Mol Reprod Dev. 2008;75:345–354. doi: 10.1002/mrd.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. In vitro compaction of germinal vesicle chromatin is beneficial to survival of vitrified cat oocytes. Reprod Domest Anim. 2009;44:269–274. doi: 10.1111/j.1439-0531.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Songsasen N, Wildt DE. Protecting and extending fertility options for females of wild and endangered species. Cancer Treat Res. 2010;156:87–100. doi: 10.1007/978-1-4419-6518-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui LB, Huang XY, Sun FZ. Nucleocytoplasmic ratio of fully grown germinal vesicle oocytes is essential for Cancer Treat Res mouse meiotic chromosome segregation and alignment, spindle shape and early embryonic development. Hum Reprod. 2005;20:2946–2953. doi: 10.1093/humrep/dei143. [DOI] [PubMed] [Google Scholar]
- De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;292:1–12. doi: 10.1016/j.ydbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. doi: 10.1006/dbio.2000.9947. [DOI] [PubMed] [Google Scholar]
- de Smedt V, Crozet N, Gall L. Morphological and functional changes accompanying the acquisition of meiotic competence in ovarian goat oocyte. J Exp Zool. 1994;269:128–139. doi: 10.1002/jez.1402690206. [DOI] [PubMed] [Google Scholar]
- Debey P, Szöllösi MS, Szöllösi D, Vautier D, Girousse A, Besombes D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev. 1993;36:59–74. doi: 10.1002/mrd.1080360110. [DOI] [PubMed] [Google Scholar]
- Endo T, Naito K, Kume S, Nishimura Y, Kashima K, Tojo H. Activities of maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) are not required for the global histone deacetylation observed after germinal vesicle breakdown (GVBD) in porcine oocytes. Reproduction. 2006;131:439–447. doi: 10.1530/rep.1.00924. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation and fertilization in vitro. Biol Reprod. 1989;54:268–276. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- Escrich L, Grau N, Meseguer M, Pellicer A, Escribá MJ. Morphologic indicators predict the stage of chromatin condensation of human germinal vesicle oocytes recovered from stimulated cycles. Fertil Steril. 2010;93:2557–2564. doi: 10.1016/j.fertnstert.2009.05.077. [DOI] [PubMed] [Google Scholar]
- Escrich L, Grau N, Mercader A, Rubio C, Pellicer A, Escribá MJ. Spontaneous in vitro maturation and artificial activation of human germinal vesicle oocytes recovered from stimulated cycles. J Assist Reprod Genet. 2011;28:111–117. doi: 10.1007/s10815-010-9493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Gasparrini B, McGarry M, Ferrier T, Fletcher J, Harkness L, De Sousa P, Wilmut I. Germinal vesicle material is essential for nucleus remodeling after nuclear transfer. Biol Reprod. 2002;67:928–934. doi: 10.1095/biolreprod.102.004606. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Nayudu PL, Nowshari MA, Hodges JK. Meiotic competence of marmoset monkey oocytes is related to follicle size and oocyte-somatic cell associations. Biol Reprod. 1995;52:1234–1243. doi: 10.1095/biolreprod52.6.1234. [DOI] [PubMed] [Google Scholar]
- Godard NM, Pukazhenthi BS, Wildt DE, Comizzoli P. Paracrine factors from cumulus-enclosed oocytes ensure the successful maturation and fertilization in vitro of denuded oocytes in the cat model. Fertil Steril. 2009;91:2051–2060. doi: 10.1016/j.fertnstert.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert KA, Anderson GB, Wildt DE, Roth TL. Transition from maternal to embryonic control of development in IVM/IVF domestic cat embryos. Mol Reprod Dev. 1997;48:208–215. doi: 10.1002/(SICI)1098-2795(199710)48:2<208::AID-MRD8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Inoue A, Nakajima R, Nagata M, Aoki F. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Hum Reprod. 2008;23:1377–1384. doi: 10.1093/humrep/den096. [DOI] [PubMed] [Google Scholar]
- Jewgenow K. Role of media, protein and energy supplements on maintenance of morphology and DNA-synthesis of small preantral domestic cat follicles during short-term culture. Theriogenology. 1998;49:1567–1577. doi: 10.1016/s0093-691x(98)00102-2. [DOI] [PubMed] [Google Scholar]
- Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction. 2007;133:85–94. doi: 10.1530/REP-06-0025. [DOI] [PubMed] [Google Scholar]
- Kárníková L, Urban F, Moor R, Fulka J., Jr. Mouse oocyte maturation: the effect of modified nucleocytoplasmic ratio. Reprod Nutr Dev. 1998;38:665–670. doi: 10.1051/rnd:19980608. [DOI] [PubMed] [Google Scholar]
- Kopecný V, Landa V, Pavlok A. Localization of nucleic acids in the nucleoli of oocytes and early embryos of mouse and hamster: an autoradiographic study. Mol Reprod Dev. 1995;41:449–458. doi: 10.1002/mrd.1080410407. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang CW, Grifo JA, Krey LC, Zhang J. Reconstruction of mouse oocytes by germinal vesicle transfer: maturity of host oocyte cytoplasm determines meiosis. Hum Reprod. 1999;14:2357–2361. doi: 10.1093/humrep/14.9.2357. [DOI] [PubMed] [Google Scholar]
- Lodde V, Modina S, Galbusera C, Franciosi F, Luciano AM. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence. Mol Reprod Dev. 2007;74:740–749. doi: 10.1002/mrd.20639. [DOI] [PubMed] [Google Scholar]
- Lodde V, Modina S, Maddox-Hyttel P, Franciosi F, Lauria A, Luciano AM. Oocyte morphology and transcriptional silencing in relation to chromatin remodeling during the final phases of bovine oocyte growth. Mol Reprod Dev. 2008;75:915–924. doi: 10.1002/mrd.20824. [DOI] [PubMed] [Google Scholar]
- Luvoni GC, Chigioni S, Perego L, Lodde V, Modina S, Luciano AM. Effect of gonadotropins during in vitro maturation of feline oocytes on oocyte-cumulus cells functional coupling and intracellular concentration of glutathione. Anim Reprod Sci. 2006;96:66–78. doi: 10.1016/j.anireprosci.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Maddox-Hyttel P, Bjerregaard B, Laurincik J. Meiosis and embryo technology: renaissance of the nucleolus. Reprod Fertil Dev. 2005;17:3–14. doi: 10.1071/rd04108. [DOI] [PubMed] [Google Scholar]
- Mattiske DM, Han L, Mann JR. Meiotic maturation failure induced by DICER1 deficiency is derived from primary oocyte ooplasm. Reproduction. 2009;137:625–632. doi: 10.1530/REP-08-0475. [DOI] [PubMed] [Google Scholar]
- Miyara F, Migne C, Dumont-Hassan M, Le Meur A, Cohen-Bacrie P, Aubriot FX, Glissant A, Nathan C, Douard S, Stanovici A, et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol Reprod Dev. 2003;64:458–470. doi: 10.1002/mrd.10233. [DOI] [PubMed] [Google Scholar]
- Motlik J, Fulka J, Fléchon JE. Changes in intercellular coupling between pig oocytes and cumulus cells during maturation in vivo and in vitro. J Reprod Fertil. 1986;76:31–37. doi: 10.1530/jrf.0.0760031. [DOI] [PubMed] [Google Scholar]
- Parfenov V, Potchukalina G, Dudina L, Kostyuchek D, Gruzova M. Human antral follicles: oocyte nucleus and the karyosphere formation (electron microscopic and autoradiographic data) Gamete Res. 1989;22:219–231. doi: 10.1002/mrd.1120220209. [DOI] [PubMed] [Google Scholar]
- Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev. 1992;31:63–67. doi: 10.1002/mrd.1080310111. [DOI] [PubMed] [Google Scholar]
- Pelican KM, Wildt DE, Pukazhenthi B, Howard J. Ovarian control for assisted reproduction in the domestic cat and wild felids. Theriogenology. 2006;66:37–48. doi: 10.1016/j.theriogenology.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Peluffo MC, Barrett SL, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus–oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro. Biol Reprod. 2010;83:525–532. doi: 10.1095/biolreprod.110.084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesty A, Broca O, Poirot C, Lefèvre B. The role of PLC beta 1 in the control of oocyte meiosis during folliculogenesis. Reprod Sci. 2008;15:661–672. doi: 10.1177/1933719108322434. [DOI] [PubMed] [Google Scholar]
- Powell MD, Manandhar G, Spate L, Sutovsky M, Zimmerman S, Sachdev SC, Hannink M, Prather RS, Sutovsky P. Discovery of putative oocyte quality markers by comparative ExacTag proteomics. Proteomics Clin Appl. 2010;4:337–351. doi: 10.1002/prca.200900024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud K, Gicquel C, Thoumire S, Chebrout M, Ficheux C, Bestandji M, Chastant-Maillard S. Folliculogenesis and morphometry of oocyte and follicle growth in the feline ovary. Reprod Domest Anim. 2009;44:174–179. doi: 10.1111/j.1439-0531.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- Saint-Dizier M, Malandain E, Thoumire S, Remy B, Chastant-Maillard S. Expression of follicle stimulating hormone and luteinizing hormone receptors during follicular growth in the domestic cat ovary. Mol Reprod Dev. 2007;74:989–996. doi: 10.1002/mrd.20676. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Tennier MT, Boatman DE, Bavister BD. Chromatin configurations and meiotic competence of oocytes are related to follicular diameter in nonstimulated rhesus monkeys. Biol Reprod. 1993;48:349–356. doi: 10.1095/biolreprod48.2.349. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Naito K, Iwamori N, Kagii H, Goto S, Ohashi S, Yamanouchi K, Tojo H. Germinal vesicle materials are not required for the activation of MAP kinase in porcine oocyte maturation. Mol Reprod Dev. 2001;59:215–220. doi: 10.1002/mrd.1025. [DOI] [PubMed] [Google Scholar]
- Sui HS, Liu Y, Miao DQ, Yuan JH, Qiao TW, Luo MJ, Tan JH. Configurations of germinal vesicle (GV) chromatin in the goat differ from those of other species. Mol Reprod Dev. 2005;71:227–236. doi: 10.1002/mrd.20251. [DOI] [PubMed] [Google Scholar]
- Sun XS, Liu Y, Yue KZ, Ma SF, Tan JH. Changes in germinal vesicle (GV) chromatin configurations during growth and maturation of porcine oocytes. Mol Reprod Dev. 2004;69:228–234. doi: 10.1002/mrd.20123. [DOI] [PubMed] [Google Scholar]
- Sun X, Li Z, Yi Y, Ding W, Chen J, Engelhardt JF, Leno GH. Chromatin configurations in the ferret germinal vesicle that reflect developmental competence for in vitro maturation. Reprod Domest Anim. 2009a;44:320–325. doi: 10.1111/j.1439-0531.2008.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QY, Miao YL, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle. 2009b;8:2741–2747. doi: 10.4161/cc.8.17.9471. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Neri QV, Katagiri Y, Rosenwaks Z, Palermo GD. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol Reprod. 2005;72:584–592. doi: 10.1095/biolreprod.104.032391. [DOI] [PubMed] [Google Scholar]
- Tan JH, Wang HL, Sun XS, Liu Y, Sui HS, Zhang J. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol Hum Reprod. 2009;15:1–9. doi: 10.1093/molehr/gan069. [DOI] [PubMed] [Google Scholar]
- Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte L, De Sutter P, Nogueira D, Gerris J, Dhont M, Van der Elst J. Nuclear and cytoplasmic maturation of in vitro matured human oocytes after temporary nuclear arrest by phosphodiesterase 3-inhibitor. Hum Reprod. 2007;22:1239–1246. doi: 10.1093/humrep/dem007. [DOI] [PubMed] [Google Scholar]
- Viaris de Lesegno C, Reynaud K, Pechoux C, Chebrout M, Chastant-Maillard S. Ultrastructural evaluation of in vitro-matured canine oocytes. Reprod Fertil Dev. 2008;20:626–639. doi: 10.1071/rd08021. [DOI] [PubMed] [Google Scholar]
- Vitale AM, Calvert ME, Mallavarapu M, Yurttas P, Perlin J, Herr J, Coonrod S. Proteomic profiling of murine oocyte maturation. Mol Reprod Dev. 2007;74:608–616. doi: 10.1002/mrd.20648. [DOI] [PubMed] [Google Scholar]
- Waurich R, Ringleb J, Braun BC, Jewgenow K. Embryonic gene activation in in vitro produced embryos of the domestic cat (Felis catus) Reproduction. 2010;140:531–540. doi: 10.1530/REP-10-0298. [DOI] [PubMed] [Google Scholar]
- Wood TC, Wildt DE. Effect of the quality of the cumulus–oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J Reprod Fertil. 1997;110:355–360. doi: 10.1530/jrf.0.1100355. [DOI] [PubMed] [Google Scholar]
- Wood TC, Montali RJ, Wildt DE. Follicle-oocyte atresia and temporal taphonomy in cold-stored domestic cat ovaries. Mol Reprod Dev. 1997;46:190–200. doi: 10.1002/(SICI)1098-2795(199702)46:2<190::AID-MRD9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Woodruff TK. The Oncofertility Consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–475. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Cheung QC, Wen L, Li J. A growth-maturation system that enhances the meiotic and developmental competence of porcine oocytes isolated from small follicles. Biol Reprod. 2006;75:547–554. doi: 10.1095/biolreprod.106.051300. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Ponce RH, Boiani M, Guizzardi S, Govoni P, Scandroglio R, Garagna S, Redi CA. The analysis of chromatin organisation allows selection of mouse antral oocytes competent for development to blastocyst. Zygote. 2002;10:73–78. doi: 10.1017/s0967199402002101. [DOI] [PubMed] [Google Scholar]