Abstract
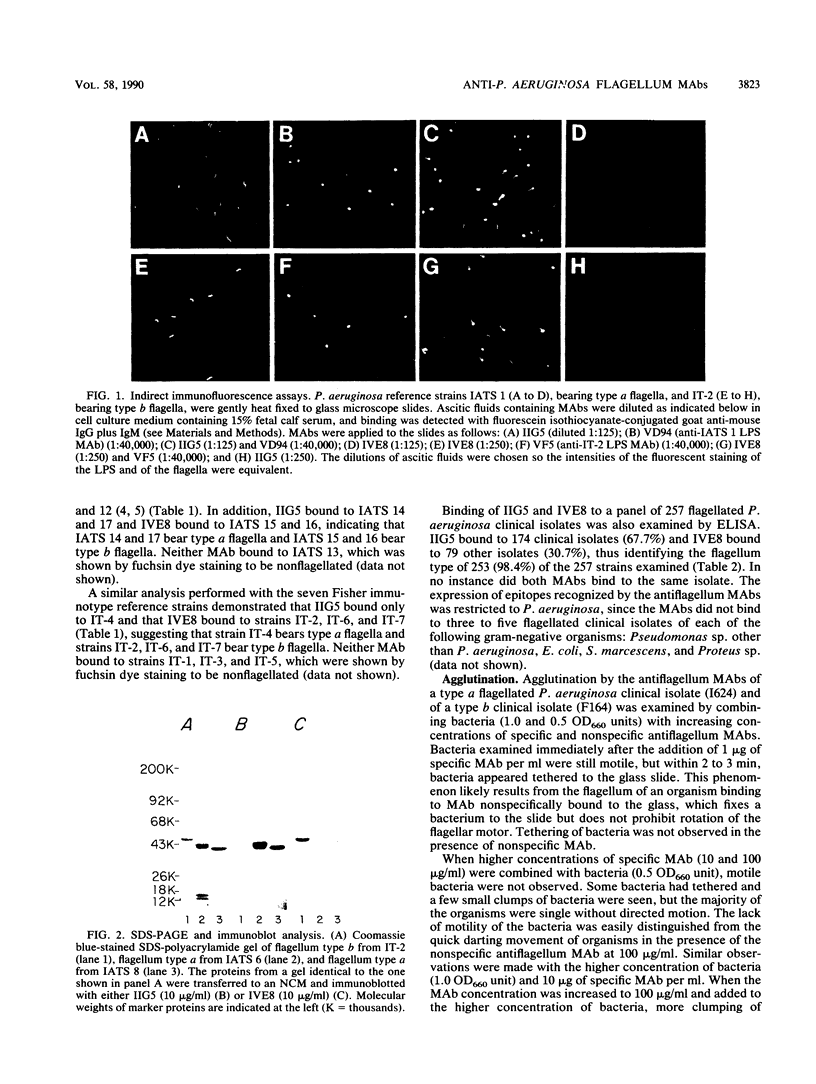
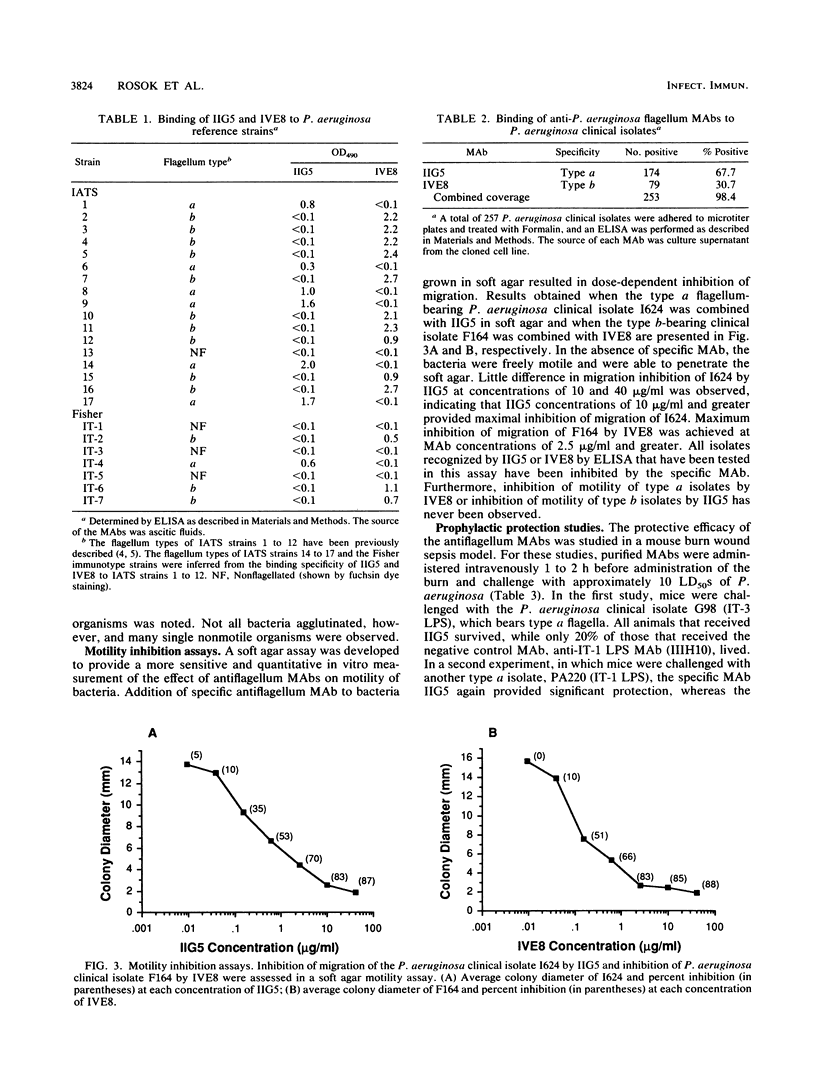
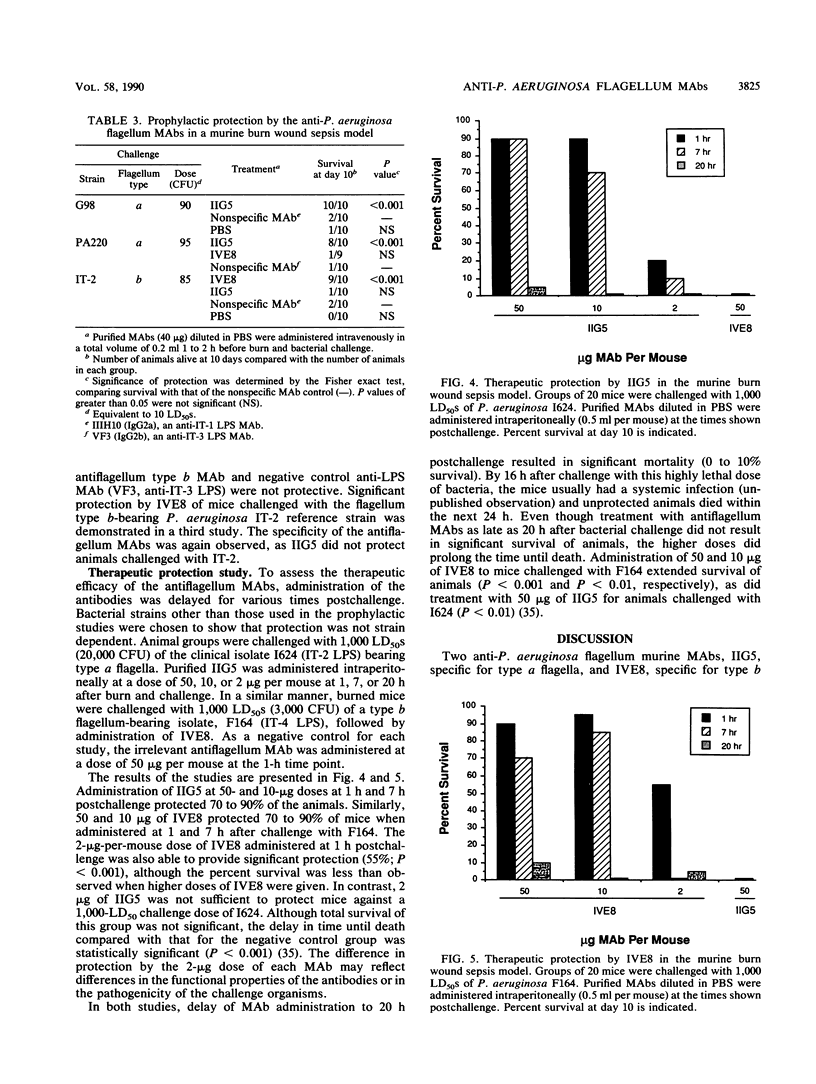
Two murine monoclonal antibodies, IIG5 (IgG3) and IVE8 (IgG2a), that bind to Pseudomonas aeruginosa type a flagella and type b flagella, respectively, were prepared by conventional hybridoma methodology. Specificity of each monoclonal antibody for type a or type b flagella was demonstrated by enzyme-linked immunosorbent assay, indirect immunofluorescence, and immunoblotting. The percentage of P. aeruginosa isolates recognized by each monoclonal antibody was analyzed by enzyme-linked immunosorbent assay. Among a panel of 257 flagellated P. aeruginosa clinical isolates, IIG5 bound to 67.7% of the isolates and IVE8 bound to another 30.7%, for a combined coverage of 98.4%. Inhibition of motility of P. aeruginosa by the monoclonal antibodies was observed in vitro in a soft agar assay and was dose dependent. The protective efficacy of IIG5 and IVE8 was examined in a mouse burn wound sepsis model. The antiflagellum monoclonal antibodies provided specific and significant prophylactic and therapeutic protection against lethal challenge with P. aeruginosa strains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. S., Dawson M., Drake D., Montie T. C. Electrophoretic separation and molecular weight characterization of Pseudomonas aeruginosa H-antigen flagellins. Infect Immun. 1985 Sep;49(3):770–774. doi: 10.1128/iai.49.3.770-774.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. R., Montie T. C. Flagellar antibody stimulated opsonophagocytosis of Pseudomonas aeruginosa associated with response to either a- or b-type flagellar antigen. Can J Microbiol. 1989 Sep;35(9):890–894. doi: 10.1139/m89-148. [DOI] [PubMed] [Google Scholar]
- Anderson T. R., Montie T. C. Opsonophagocytosis of Pseudomonas aeruginosa treated with antiflagellar serum. Infect Immun. 1987 Dec;55(12):3204–3206. doi: 10.1128/iai.55.12.3204-3206.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorg R. A., Knoche M. E., Spies A. F., Kraus C. J. Differentiation of the major flagellar antigens of Pseudomonas aeruginosa by the slide coagglutination technique. J Clin Microbiol. 1984 Jul;20(1):84–88. doi: 10.1128/jcm.20.1.84-88.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorg R. Flagellaspezifisches H-Antigenschema von Pseudomonas aeruginosa. Zentralbl Bakteriol Orig A. 1978 Nov;242(2):228–238. [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Collins M. S., Roby R. E. Anti-Pseudomonas aeruginosa activity of an intravenous human IgG preparation in burned mice. J Trauma. 1983 Jun;23(6):530–534. doi: 10.1097/00005373-198306000-00015. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect Immun. 1984 Mar;43(3):795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Simple model for the study of Pseudomonas aeruginosa infections in leukopenic mice. Infect Immun. 1983 Mar;39(3):1067–1071. doi: 10.1128/iai.39.3.1067-1071.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake D., Montie T. C. Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J Gen Microbiol. 1988 Jan;134(1):43–52. doi: 10.1099/00221287-134-1-43. [DOI] [PubMed] [Google Scholar]
- Drake D., Montie T. C. Protection against Pseudomonas aeruginosa infection by passive transfer of anti-flagellar serum. Can J Microbiol. 1987 Sep;33(9):755–763. doi: 10.1139/m87-130. [DOI] [PubMed] [Google Scholar]
- Engvall E. Quantitative enzyme immunoassay (ELISA) in microbiology. Med Biol. 1977 Aug;55(4):193–200. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick M. R., Cluff L. E. Pseudomonas bacteremia. Review of 108 cases. Am J Med. 1976 Apr;60(4):501–508. doi: 10.1016/0002-9343(76)90716-6. [DOI] [PubMed] [Google Scholar]
- Forbes L. Rapid flagella stain. J Clin Microbiol. 1981 Apr;13(4):807–809. doi: 10.1128/jcm.13.4.807-809.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freireich E. J., Gehan E. A., Rall D. P., Schmidt L. H., Skipper H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966 May;50(4):219–244. [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Naglich J. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: immunization using divalent flagella preparations. J Trauma. 1986 Feb;26(2):118–122. doi: 10.1097/00005373-198602000-00003. [DOI] [PubMed] [Google Scholar]
- Holder I. A. The pathogenesis of infections owing to Pseudomonas aeruginosa using the burned mouse model: experimental studies from the Shriners Burns Institute, Cincinnati. Can J Microbiol. 1985 Apr;31(4):393–402. doi: 10.1139/m85-075. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lányi B. Serological properties of Pseudomonas aeruginosa. II. Type-specific thermolabile (flagellar) antigens. Acta Microbiol Acad Sci Hung. 1970;17(1):35–48. [PubMed] [Google Scholar]
- Montie T. C., Craven R. C., Holder I. A. Flagellar preparations from Pseudomonas aeruginosa: isolation and characterization. Infect Immun. 1982 Jan;35(1):281–288. doi: 10.1128/iai.35.1.281-288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie T. C., Doyle-Huntzinger D., Craven R. C., Holder I. A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect Immun. 1982 Dec;38(3):1296–1298. doi: 10.1128/iai.38.3.1296-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Edman D. C., Leppla S. H., Wretlind B., Lewis L. R., Martin K. E. Protection against experimental Pseudomonas aeruginosa infection in mice by active immunization with exotoxin A toxoids. Infect Immun. 1981 May;32(2):681–689. doi: 10.1128/iai.32.2.681-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Reynolds H. Y., Carbone P. P. Pseudomonas pneumonia. A retrospective study of 36 cases. Am J Med. 1973 Aug;55(2):155–160. doi: 10.1016/0002-9343(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Small G. J., Lostrom M. E., Pier G. B. Polyclonal and monoclonal antibody therapy for experimental Pseudomonas aeruginosa pneumonia. Infect Immun. 1986 Oct;54(1):239–244. doi: 10.1128/iai.54.1.239-244.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt T. L., Bradley D. E. The antibody response to the flagella of Pseudomonas aeruginosa. J Med Microbiol. 1975 Feb;8(1):97–106. doi: 10.1099/00222615-8-1-97. [DOI] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- Sastry P. A., Pearlstone J. R., Smillie L. B., Paranchych W. Studies on the primary structure and antigenic determinants of pilin isolated from Pseudomonas aeruginosa K. Can J Biochem Cell Biol. 1985 Apr;63(4):284–291. doi: 10.1139/o85-042. [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki M., Kawamura T., Fujinaga S., Masuho Y., Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J Infect Dis. 1984 Oct;150(4):570–576. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- Seppälä I., Sarvas H., Péterfy F., Mäkelä O. The four subclasses of IgG can be isolated from mouse serum by using Protein A-Sepharose. Scand J Immunol. 1981 Oct;14(4):335–342. doi: 10.1111/j.1365-3083.1981.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Stoll B. J., Pollack M., Young L. S., Koles N., Gascon R., Pier G. B. Functionally active monoclonal antibody that recognizes an epitope on the O side chain of Pseudomonas aeruginosa immunotype-1 lipopolysaccharide. Infect Immun. 1986 Sep;53(3):656–662. doi: 10.1128/iai.53.3.656-662.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972 Sep;126(3):277–287. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]





