Abstract
The distribution, toxicity, and clearance of 131I-labeled macroaggregated albumin were studied in the coronary circulation of the dog. Twenty-five percent of the injected label traversed the coronary capillary bed within 3 min after injection and the biologic half-clearance time of the remainder was approximately 8 hr. After coronary artery injection of macroaggregate, no deterioration of left ventricular function was apparent from hemodynamic measurements, and no histologic evidence of myocardial damage was found after doses up to ten times the suggested diagnostic dose. The results of computer-controlled external scintiscans in the excised heart were shown as three-dimensional displays of count distribution.
Studies of particulate deposits suggest a potential applicability to regional myocardial blood flow delineation (1–6) because, for large tissue regions, the distribution is most likely to be in direct proportion to the flow pattern (3,7–9). This method has been used in human studies (5,6); the particles were found to be nontoxic and they resided in the organ for a sufficiently long interval to be detected.
Radioactive carbonized microspheres are never cleared from their sites of deposits (10). Depending on their size, they may be too dependent on the local hydrodynamic factors to delineate flows to small tissue regions (3).
Radioactive macroaggregated albumin (MAA) (10,11) or radioactive macroaggregated human albumin microspheres (HAM) (12–14) are removed from sites of deposition in the intact organism, and hence their long-term effect may not be damaging. MAA has the disadvantages of heterogeneity of particle size and of being friable (15,16). Both MAA and HAM have been widely used to detect flows to different organs, to detect shunts, and to detect areas of vascular obstruction (1,5,6,11–13,17–26). Varied biologic response to both of these substances has been found.
Burdine, et al (12,13), Endo, et al (5), Schelbert, et al (26), and Ashburn, et al (6) reported no significant changes in animals or humans with regard to cardiovascular or immunologic abnormalities for up to 48 hr after introducing albumin microspheres. Mishkin and Brashear (14) gave large doses to dogs and found a significant decrease of aortic pressure without alteration of heart rate or pulmonary pressure. Poe (4) studied both albumin and ceramic microspheres and found that the response invariably was a change in flow or contractility in hearts of open-chest dogs even at relatively low doses, but these observations were neither predictable nor reproducible.
The present report describes further observations on the properties of MAA in dog hearts and, in addition, shows the pattern of deposition in some of these hearts when the conditions are most ideal for detection by scintiscanning techniques.
Materials and Methods
Mongrel dogs weighing between 13 and 35 kg (the larger dogs were used in histopathologic studies) were anesthetized with sodium pentobarbital (30 mg/kg) and ventilated through an endotracheal tube with a Harvard pump respirator.
Macroaggregated albumin (kindly supplied by Abbott Laboratories, North Chicago, Ill.) (2 mg/ml, and 1 ml contains about 2 × 106 particles) was labeled with 127I for hemodynamic and pathologic experiments and with 125I or 131I for clearance and scintiscan studies.
The amount of unbound radioactive iodide in the MAA was determined by the Somogyi precipitation method (27) and high-voltage electrophoresis (28). Less than 4% of the total radioactivity of the MAA used was due to unbound iodide.
Fraction of MAA deposited
The coronary effluent was collected sequentially after intra-aortic injection in a retrogradely perfused, nonworking, isolated dog heart (29) and also after left atrial injection in an open-chest preparation with ligation of the aortic arch. The total radioactivity collected at the venous effluent was expressed as a percentage of the injected dose.
Clearance of deposited MAA from coronary bed
In two dogs the heart was exposed and supported in a pericardial cradle. About 150 μCi of 131I-MAA (0.08 mg/kg animal weight) was injected into the left anterior descending coronary (LAD) artery through an indwelling 25-gage needle. The radioactivity was monitored for 2 hr by a gamma probe placed 5 cm from the surface of the beating left ventricle.
Hemodynamic studies
In four dogs the heart was exposed and supported as before. The pressures in the femoral artery, aorta, left atrium, and left ventricle were monitored by Statham p23Dd strain gages. Cardiac output was measured by the conventional indocyanine green method with a cuvette densitometer (XC100A, Waters Instruments, Inc.). Myocardial blood flow was measured by the washout of 133Xe after injection of 100 μCi into the artery (29).
From one to four injections of MAA (127I), ranging from 0.5 to 10 ml (from 0.06 to 1.0 mg/kg animal weight), were made into the LAD coronary artery at timed intervals of 10–100 sec. The time of the measured flows and pressures was divided into three intervals: control, early (within 5 min), and later (10–29 min). Coronary arterial resistance was calculated as the quotient of mean aortic pressure (mmHg) and myocardial blood flow (ml/min per 100 gm).
Histopathologic studies
In four intact dogs, selective injection of MAA (127I) was made through the left coronary circulation by using fluoroscopy for intracoronary catheterization. When it was ensured that the catheter remained in the coronary artery after the injection, the catheter (Sones, 6.5 F) was removed. The injected volumes were from 0.5 to 5 ml (0.07 to 0.33 mg/kg). The dogs were killed by intra-aortic injection of excess sodium pentobarbital at 42, 120, 93, and 24 hr, respectively.
Histologic sections were made from different areas of the left ventricle and stained with hematoxylin and eosin.
Distribution of MAA in hearts
These studies were done in ten dogs with the open-chest preparation. In four, 131I-MAA was injected into the occluded aortic root of animals with normal coronary vessels. In three, MAA was introduced selectively into the upper third of the LAD coronary artery through an indwelling 25-gage needle. In the remaining three, the MAA was introduced into the occluded aorta after acute complete ligation of the LAD coronary artery and its two largest diagonal branches on the anterior surface of the left ventricle. The hearts were excised 2 min after injection of 160–220 μCi of MAA (0.04–0.2 mg/kg) and stored in isotonic saline at 4°C for 12–18 hr before scanning.
The external scanning was performed on the isolated unbeating heart with a modified Picker Magna-scanner with a 31-hole focusing collimator and a 3 × 3 in. NaI(Tl) crystal linked to a CDC 3200 computer (30). It had been shown that less than 10% of the counts from the adjacent sampling locations contributed to the data from the single location (30).
After the scanning procedure, autoradiographs of 0.5-cm-thick coronal slices of each of the hearts were obtained. The slices were wrapped in plastic sheets (Mylar) and placed on “no screen” x-ray film (Eastman Kodak, Rochester, N.Y.) for 16–24 hr.
Results
Fraction of MAA deposited
At coronary venous flows of 110 and 233 ml/min in the isolated heart preparation and of 57 ml/min in the ligated aorta in the open-chest preparation, 24.6, 24.9, and 23.2%, respectively, of the radioactivity was collected in the venous effluent. The venous dilution curves (Fig. 1) showed that the last sample was less than 3% of the peak activity. This suggested that about a quarter of the injected radioactivity was contributed by particles too small to be trapped in the capillaries of the heart.
FIG. 1.

Venous dilution curves. Washout of MAA (131I) from isolated dog heart at two levels of coronary flow. Solid symbols, 110 ml/min; open symbols, 230 ml/min.
Clearance study
Half-clearance time was calculated to be 7½ and 8¼ hr in the two hearts studied based on the 2 hr of study. The disappearance rate could be approximated to a single exponential decay after the first 5 min after the injection. This suggests that the clearance is sufficiently long to allow detection by external scintiscanning techniques.
In Fig. 2 are shown the effects of MAA injection on coronary flow and resistance and cardiac output. Myocardial blood flow increased during the first 5 min after MAA injection (for difference from control, p < 0.001); the magnitude of the response was related to the dose injected. With the low doses (≤0.12 mg/kg), the mean increase was 18% (range, 3.1–20.6%) and with the higher doses (up to 1 mg/kg) it was 29.7% (range, 16.7–43.4%). After 10 min the flow decreased toward the control level (p < 0.1 from control) in eight of the ten injections. The mean differences from control values were 1.9 and 9% for the low and high doses, respectively.
FIG. 2.
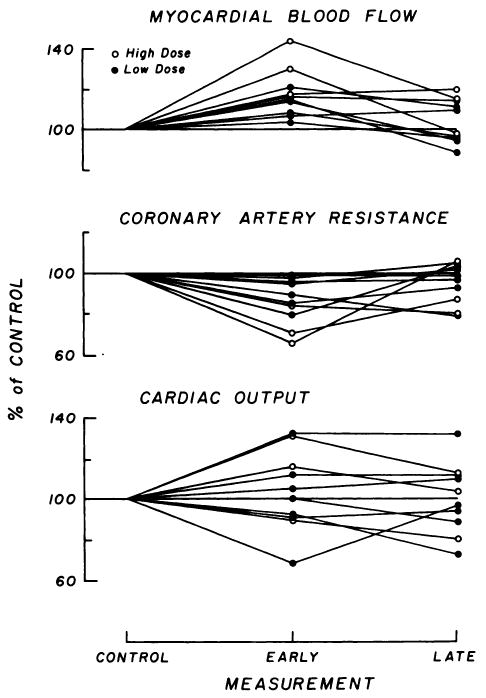
Hemodynamic results after ten injections of labeled macroaggregate into left anterior descending coronary artery in four dogs. Changes from control values are expressed in percent. “Early” curves were obtained at 2–5 min and “late” curves at 10–29 min after MAA injection. Top: Myocardial blood flow (mean control, 103.8 ± 10.9 s.d. ml/100 gm/min). Middle: Coronary arterial resistance (mean control, 1.34 ± 0.20 mmHg/(ml/100 gm/min). Bottom: Cardiac output (mean control, 2.28 ± 0.46 liters/min).
There were no significant changes in systemic blood pressure (control values had means of 100–150 mmHg), which means that there were significant decreases in coronary arterial resistance (p < 0.05) in the initial periods; the mean changes were −8.4% (range, −1.1 to −21%) for the low doses and −26.3% (range, −15.1 to −24.4%) for the high doses.
There were no systematic changes in cardiac output (control values were 0.10–0.18 liters/min/kg) or in the diastolic and systolic components of the left atrial pressure as a function of time or of doses. The cardiac output ranged from −10 to +31.6% of that in the control state.
Histopathologic studies
There were no macroscopic or microscopic abnormalities detected in the tissue studies.
Distribution in hearts
The scintiscan from an intact coronary circulation is shown in Fig. 3 as four three-dimensional plots of counts versus x and y spatial axes surrounding a surface contour plot; the autoradiograph of a midcoronal section from the same heart is shown in Fig. 4 (left). These scans show a single peak maximized over the left ventricle. Atrial activities were detected as minor peaks in the posterior views but in two of the four hearts these formed a small secondary peak distinct from the ventricular mass.
FIG. 3.
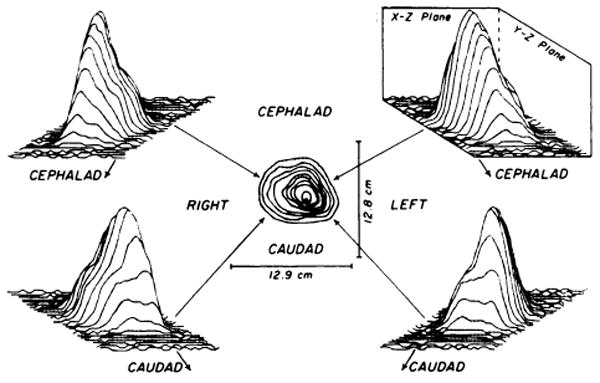
External scintiscans of excised heart with intact coronary circulation obtained by injecting 200 μCi of MAA (131l) into occluded aortic root. Sampling period was 1 sec at each of 31 × 26 (x,y) locations over a surface 12.7 × 12.7 cm. Distance between consecutive data points was 0.41 cm in × direction and 0.51 cm in y direction. Surface contour plot is shown in center with hand-drawn lines joining regions of isocount levels. Three-dimensional count distributions are arranged around surface contour plot in projections from which data are viewed; lines join counts sampled during single horizontal pass. Magnitude of deflection in vertical (z) plane is proportioned to count concentration scaled to maximum of peak counts. In scan, peak counts were 767 counts/sec and background was between 40 and 50 counts/sec.
FIG. 4.
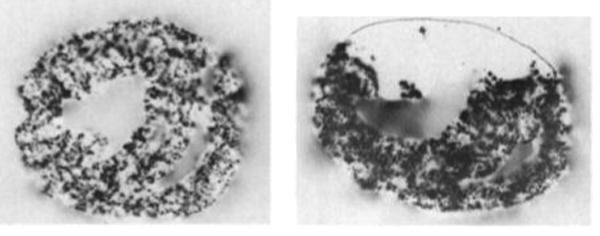
Autoradiographs of two hearts after aortic root injection of MAA (131I). Only coronal slices from mid-portions of ventricles are shown. Left: Heart with intact coronary circulation showing relatively homogeneous distribution of isotope throughout myocardium. Right: Simulated anterior myocardial infarction showing anterior region of unperfused myocardium. Hand-drawn line has been added to show contour of unperfused region.
The distribution of deposited MAA with simulated anterior myocardial infarction is shown in Fig. 4 (right) by an autoradiograph from a midcoronal section and Fig. 5 for the external scintiscan of the whole heart. In the latter, there are two peaks of activity enclosing a valley or “cold” area; this is best illustrated in the second and fourth quadrants (it is obscured by the lateral walls of both ventricles in the first and third quadrants). A lateral scan (not illustrated) further localized the nonperfused region to the anterior surface.
FIG. 5.

External scintiscan of excised heart with ligated left anterior descending coronary artery obtained by injecting 200 μCi of MAA (131I) into occluded aortic root. Sampling period was 1 sec and scan was made over 12.7 × 12.7-cm surface in manner identical to that described for Fig. 3. Peak counts were 331 counts/sec and background was between 20 and 30 counts/sec. In contrast to scan of heart with intact coronary circulation, two count peaks are observed with deep valley or “cold” area between them.
Discussion
Macroaggregated albumin has an advantage over plastic microspheres in that it can be cleared from the capillary bed and metabolized by the reticuloendothelial system (10,11). Uniformity in shapes and sizes can be achieved by the use of macroaggregated microspheres.
Fraction of MAA deposited
In the present study a quarter of the injected activity traversed the capillary bed, a finding not grossly different from previous. reports on the pulmonary and systemic beds (8,10,11). Recirculation of these smaller-size particles and subsequent selective deposition in various organs may lead to errors in the estimates of regional flow. Aside from the heterogeneity of particle sizes, no study has been made pertaining to the heterogeneity of activities in relation to particle sizes.
Clearance
The half-clearance times of almost 8 hr were similar to those reported by Tow, et al (11) and Burdine, et al (12) for the lung and by Endo, et al (5) and Ashburn, et al (6) for the heart. It was much longer than the 2 hr found for the lung by Hawkins, et al (31) using 99mTc-colloid albumin and was much shorter than the 15 hr reported by Johnson, et al (23) for macroaggregated albumin in abdominal viscera and lung. For the liver the disappearance curve was reported (23) to be not mono-exponential. Thus, depending on the isotope used, the clearance from the tissue is probably fast enough for the residual radioactivity not to be a hazard.
Hemodynamic activities
Our maximum dose of MAA was 1 mg/kg while Endo, et al (5) stated that human diagnostic dose was 0.01 mg/kg. The only consistent but transient changes were the initial increases in myocardial flow without pressure alterations. Endo, et al (5), Schelbert, et al (26), and Ashburn, et al (6) did not find significant changes in left ventricular or left atrial pressures, left ventricular dp/dt, and ECG when the amount of MAA injected was as high as 100 times the acceptable dose. Using dog heart and lycopodium spores (20–40 microns in diam) and measuring coronary venous flow directly, West, et al (2) found an initial decrease in venous flow followed by a reactive increase lasting up to 10 min. These changes were related to the dose of spheres but not to autonomic blocking agents or to surgical denervation of the heart, suggesting a local mechanism for the effect on flow. Stone, et al (32) used large plastic spheres (285 microns in diam) and observed severe depression of left ventricular function.
In contrast to our study, Poe (4) did not find predictable or reproducible alterations but reported a reactive increase in flow after an initial decrease. Mishkin and Brashear (14) used doses as high as 500 times those acceptable for human infusions and detected a significant decrease in aortic pressure without changes in pulmonary pressure or heart rate. The flows were not recorded in these cases.
Histology
Histologic abnormalities were not detected in the organs studied. This agrees with findings by Kennady and Taplin (33) in the brain of monkeys, by Burdine, et al (13) using albumin microspheres in different organs, and by Schelbert, et al (26) in dog hearts. In all these reports including the present one the studies were done on essentially normal vascular trees. Clinical toxicity in human patients with regard to gross parameters such as blood pressure, cardiac output, BUN, SGOT, EEG, and urinalysis, is reported to be minimal (12,16, 20–22). Two patients with severe pulmonary vascular disease died after injection of the macroaggregate (34,35). In one patient who recovered from a severe reaction (17) the toxicity was most likely a hypersensitivity to the prophylactic use of Lugol's iodine solution rather than to the macroaggregates. In 6 of their 42 patients who were investigated with brain scan Rosenthall, et al (20) found no histologic evidence of brain changes related to the macroaggregate at autopsy.
Scanning data
This report was not intended to show the superiority of the present scanning system but rather to show the result of scanning in a nearly idealized condition [nonbeating hearts removed from thorax, and maximized counting efficiency and geometry (30)]. Even with very large areas of infarction, not all the views of the scan depicted the nonperfused area clearly. Cannon, et al (36), using 133Xe washout, and Endo, et al (5) and Schelbert, et al (26), using MAA and beating hearts of dogs, were able to detect induced nonperfused regions, but again these were presumably large areas of infarction. Quinn, et al (1), using macroaggregated albumin in closed-chest dogs but with nonbeating hearts, and Love, et al (37), using 42K or 86Rb clearance in beating hearts in dogs, were able to show differences in pattern for presumably smaller areas of induced infarcts. In addition Love, et al (37) showed differences in patterns between human patients with clinically normal hearts and those with pericardial effusion or with left ventricular hypertrophy.
The report by Ashburn, et al (6) on heart imaging in 29 patients gives encouragement to (A) clearance and nontoxicity (acute and long-term) of MAA and (B) possible spatial resolution of 1.0- to 1.5-cm-diameter areas of perfusion images.
Acknowledgments
David R. Richmond was a Fellow of the Minnesota Heart Association; Craig M. Coulam was a Postdoctoral Fellow of the National Institutes of Health; Tada Yipintsoi was a Special Fellow of the National Institutes of Health; and James B. Bassingthwaighte is the recipient of the NIH Career Development Award.
This investigation was supported in part by Research Grants HL43128, K3HL22649, HL-9719, and FR-7 from the National Institutes of Health and by grants-in-aid from the Minnesota Heart Association and the American Heart Association.
The authors appreciate the help of Jane Irving in the preparation of the manuscript and of James F. Greenleaf in the computer-controlled scintiscanning.
References
- 1.Quinn JL, Serratto M, Kezdi P. Coronary artery bed photoscanning using radioiodine albumin macroaggregates (RAMA) J Nucl Med. 1966;7:107–113. [Google Scholar]
- 2.West JW, Kobayashi T, Anderson FS. Effects of selective coronary embolization on coronary blood flow and coronary sinus venous blood oxygen saturation in dogs: with special reference to coronary reflexes. Circ Res. 1962;10:722–738. doi: 10.1161/01.res.10.5.722. [DOI] [PubMed] [Google Scholar]
- 3.Domenech RJ, Hoffman JIE, Noble MIM, et al. Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res. 1969;25:581–596. doi: 10.1161/01.res.25.5.581. [DOI] [PubMed] [Google Scholar]
- 4.Poe N. Cardiodynamic effects of intracoronary artery injection of albumin macroaggregates. J Nucl Med. 1970;11:350–351. [Google Scholar]
- 5.Endo M, Yamazaki T, Konno S, et al. The direct diagnosis of human myocardial ischemia using 131I-MAA via the selective coronary catheter: preliminary report. Amer Heart J. 1970;80:498–506. doi: 10.1016/0002-8703(70)90198-5. [DOI] [PubMed] [Google Scholar]
- 6.Ashburn WL, Braunwald E, Simon AL, et al. Myocardial perfusion imaging with radioactive-labeled particles injected directly into the coronary circulation of patients with coronary artery disease. Circulation. 1971;44:851–865. doi: 10.1161/01.cir.44.5.851. [DOI] [PubMed] [Google Scholar]
- 7.Randolph AM, Heymann MA. The circulation of the fetus in utero: methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 1967;21:163–184. doi: 10.1161/01.res.21.2.163. [DOI] [PubMed] [Google Scholar]
- 8.Kaihara S, Van Heerden PD, Migita T, et al. Measurement of distribution of cardiac output. J Appl Physiol. 1968;25:696–700. doi: 10.1152/jappl.1968.25.6.696. [DOI] [PubMed] [Google Scholar]
- 9.Katz MA, Blantz RC, Rector FC, et al. Measurement of intrarenal blood flow. I. Analysis of microsphere method. Amer J Physiol. 1971;220:1903–1913. doi: 10.1152/ajplegacy.1971.220.6.1903. [DOI] [PubMed] [Google Scholar]
- 10.Taplin GV, Johnson DE, Dore EK, et al. Medical Radioisotope Scanning. Vol. 2. Vienna: IAEA; 1964. Organ visualization by photoscanning using micro- and macroaggregates of radioalbumin; pp. 3–31. [Google Scholar]
- 11.Tow DE, Wagner HN, Lopez-Majano V, et al. Validity of measuring regional pulmonary arterial blood flow with macroaggregates of human serum albumin. Amer J Roentgen. 1966;96:664–676. doi: 10.2214/ajr.96.3.664. [DOI] [PubMed] [Google Scholar]
- 12.Burdine JA, Sonnemaker RE, Ryder LA, et al. Perfusion studies with technetium-99m human albumin microspheres (HAM) Radiology. 1970;95:101–107. doi: 10.1148/95.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Burdine JA, Ryder LA, Sonnemaker RE, et al. 99mTc-human albumin microspheres (HAM) for lung imaging. J Nucl Med. 1971;12:127–130. [PubMed] [Google Scholar]
- 14.Mishkin FS, Brashear RE. Pulmonary and systemic blood pressure responses to large doses of albumin microspheres. J Nucl Med. 1971;12:251–252. [PubMed] [Google Scholar]
- 15.Richmond DR, Tauxe WN, Bassingthwaighte JB. Albumin macroaggregates and measurements of regional blood flow: validity and application of particle sizing by Coulter counter. J Lab Clin Med. 1970;75:336–346. [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner HN, Sabiston DC, McAfee JG, et al. Diagnosis of massive pulmonary embolism in man by radioisotope scanning. N Eng J Med. 1964;271:377–384. doi: 10.1056/NEJM196408202710801. [DOI] [PubMed] [Google Scholar]
- 17.Bliek AJ, Bachynski JE. Two severe reactions following a pulmonary scan in a patient with idiopathic pulmonary haemosiderosis. J Nucl Med. 1971;12:90–92. [PubMed] [Google Scholar]
- 18.Tauxe WN, Burchell HB, Chaapel DW, et al. Quantitating the effect of gravity on lung scans of macroaggregates of albumin-I-131. J Appl Physiol. 1966;21:1381–1386. doi: 10.1152/jappl.1966.21.4.1381. [DOI] [PubMed] [Google Scholar]
- 19.Friedman WF, Braunwald E. Alterations in regional pulmonary blood flow in mitral valve disease studied by radioisotope scanning: a simple nontraumatic technique for estimation of left atrial pressure. Circulation. 1966;34:363–376. doi: 10.1161/01.cir.34.3.363. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthall L, Aguayo A, Stratford J. A clinical assessment of carotid and vertebral artery injection of macroaggregates of radioiodinated albumin (MARIA) for brain scanning. Radiology. 1966;86:499–505. doi: 10.1148/86.3.499. [DOI] [PubMed] [Google Scholar]
- 21.Nagai T, Jimbo M, Sano K. Cerebro-pulmonary scan using macroaggregated albumin as a quantitation of intracerebral arterio-venous shunting. J Nucl Med. 1967;8:709–722. [PubMed] [Google Scholar]
- 22.Friedman WF, Braunwald E, Morrow AG. Alterations in regional pulmonary blood flow in patients with congenital heart disease studied by radioisotope scanning. Circulation. 1968;37:747–758. doi: 10.1161/01.cir.37.5.747. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PM, Kantor IE, Schwartz AJ, et al. Scanning with macroaggregates of human serum albumin in visceral arteriography. Can Med Assoc J. 1969;101:97–101. [PMC free article] [PubMed] [Google Scholar]
- 24.Shida H, Ohara I. Study on peripheral circulation using 131I-macroaggregated serum albumin. Tohoku J Exp Med. 1970;101:311–316. doi: 10.1620/tjem.101.311. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthall L. Combined inferior vena cavography, iliac venography, and lung imaging with 99mTc albumin macroaggregates. Radiology. 1971;98:623–626. doi: 10.1148/98.3.623. [DOI] [PubMed] [Google Scholar]
- 26.Schelbert HR, Ashburn WL, Covell JW, et al. Feasibility and hazards of the intracoronary injection of radioactive serum albumin macroaggregates for external myocardial perfusion imaging. Invest Radiol. 1971;6:379–387. doi: 10.1097/00004424-197111000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Somogyi M. A method for the preparation of blood filtrates for the determination of sugar. J Biol Chem. 1930;86:655–663. [Google Scholar]
- 28.Yipintsoi T, Gustafson DC, Bassingthwaighte JB. Separation of unbound iodide in 125I-labeled antipyrine. J Nucl Med. 1971;12:149–152. [PMC free article] [PubMed] [Google Scholar]
- 29.Bassingthwaighte JB, Strandell T, Donald DE. Estimation of coronary blood flow by washout of diffusible indicators. Circ Res. 1968;23:259–278. doi: 10.1161/01.res.23.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulam CM, Dunnette WH, Wood EH. A computer-controlled scintiscanning system and associated computer graphic techniques for study of regional distribution of blood flow. Comput Biomed Res. 1970;3:249–273. doi: 10.1016/0010-4809(70)90059-5. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins LA, Highet T, McAlister J. A new radiopharmaceutical for lung scanning (abstract) Brit J Radiol. 1970;43:830. [PubMed] [Google Scholar]
- 32.Stone HL, Bishop VS, Guyton AC. Cardiac function after embolization of coronaries with microspheres. Amer J Physiol. 1962;204:16–20. doi: 10.1152/ajplegacy.1963.204.1.16. [DOI] [PubMed] [Google Scholar]
- 33.Kennady JC, Taplin GV. Regional cortical blood flow studies with radioalbumin macroaggregates. In: Bain WH, Harpers AM, editors. Blood Flow Through Organs and Tissues. Baltimore: Williams & Wilkins; 1967. pp. 239–249. [Google Scholar]
- 34.Dworkin HJ, Smith JR, Bull FE. Reaction after administration of macroaggregated albumin for a lung scan. N Eng J Med. 1966;275:376. doi: 10.1056/NEJM196608182750709. [DOI] [PubMed] [Google Scholar]
- 35.Vincent WR, Goldberg SJ, Desilets D. Fatality immediately following rapid infusion of macroaggregates of 99mTc albumin (MAA) for lung scan. Radiology. 1968;91:1181–1184. [PubMed] [Google Scholar]
- 36.Cannon PJ, Haft JI, Johnson PM. Visual assessment of regional myocardial perfusion utilizing radioactive xenon and scintillation photography. Circulation. 1969;40:277–288. doi: 10.1161/01.cir.40.3.277. [DOI] [PubMed] [Google Scholar]
- 37.Love WD, Smith RO, Pulley PE. Mapping myocardial mass and regional coronary blood flow by external monitoring of 48K or 86Rb clearance. J Nucl Med. 1969;10:702–707. [PubMed] [Google Scholar]


