Abstract
Understanding the evolutionary forces that produced the human brain is a central problem in neuroscience and human biology. Comparisons across primate species show that both brain volume and gyrification (the degree of folding in the cerebral cortex) have progressively increased during primate evolution and there is a strong positive correlation between these two traits across primate species. The human brain is exceptional among primates in both total volume and gyrification, and therefore understanding the genetic mechanisms influencing variation in these traits will improve our understanding of a landmark feature of our species. Here we show that individual variation in gyrification is significantly heritable in both humans and an Old World monkey (baboons, Papio hamadryas). Furthermore, contrary to expectations based on the positive phenotypic correlation across species, the genetic correlation between cerebral volume and gyrification within both humans and baboons is estimated as negative. These results suggest that the positive relationship between cerebral volume and cortical folding across species cannot be explained by one set of selective pressures or genetic changes. Our data suggest that one set of selective pressures favored the progressive increase in brain volume documented in the primate fossil record, and that a second independent selective process, possibly related to parturition and neonatal brain size, may have favored brains with progressively greater cortical folding. Without a second separate selective pressure, natural selection favoring increased brain volume would be expected to produce less folded, more lissencephalic brains. These results provide initial evidence for the heritability of gyrification, and possibly a new perspective on the evolutionary mechanisms underlying long-term changes in the nonhuman primate and human brain.
Introduction
The genetic basis of human brain evolution is a central question in both neuroscience and human biology. In order to better understand both the neurobiology of modern humans and the evolutionary processes that produced the modern human brain, researchers from various fields have labored to identify and describe the shared genetic mechanisms that govern the neurodevelopment of all mammals, as well as the distinct developmental genetic differences that distinguish various species. The modern human brain is unique among all living primates in a number of ways, but the overall size of the cerebral hemispheres and the remarkable folding (gyrification) of the cerebral cortex are certainly among the most fundamental of human neuroanatomical features. A better understanding of the genetic mechanisms that govern overall brain size and the degree of cortical gyrification would have fundamental implications for both neuroscience and human evolutionary studies.
The history of primate brain evolution over the past 50 million years consists of progressive increase in both brain volume and gyrification. Early primates prior to 40 million years ago exhibited small brains with little or no cortical folding (Martin, 1990; Preuss, 2007a). In more advanced primates such as the New World monkeys (e.g. marmosets or capuchins) and Old World monkeys (e.g. macaques and baboons), brain size is larger in absolute terms and especially as a fraction of body size (brain:body size ratio). This trend toward larger cerebral volume and greater brain:body size ratios (Figure One) was continued with the later appearance of the great apes (chimpanzees, gorillas and orangutans) (Kaas, 2008; Martin, 1990; Preuss, 2007a). In parallel with the increase in brain volume, there has also been an increase in the degree of cortical folding (gyrification). In general, Old World monkeys have cerebral hemispheres with more significantly folded cerebral cortex than do New World monkeys, and the great apes show still more gyrification (Figure One). Thus, across the broad diversity of living primate species, there is a strong positive correlation between absolute brain volume and degree of gyrification. A similar relationship between brain size and gyrification is also observed in other orders of mammals, particularly cetaceans (Pillay and Manger, 2007). Since the human brain stands as the most extreme case of the parallel increase in these two traits among primates, constructing a more detailed understanding of the relevant genetic, developmental and evolutionary processes that produced this pattern of between-species differences will provide new insights into the processes that shaped human brain structure.
Figure 1.
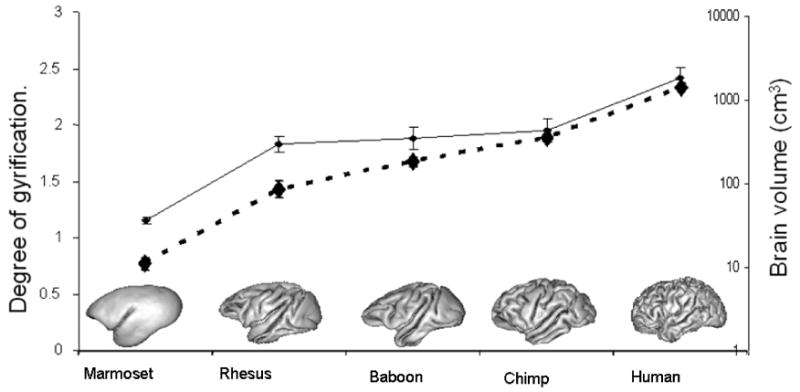
Measurements of brain volume and cerebral gyrification index (GI) were calculated for one species of New World monkey: marmoset (Callithrix (Callithrix) jacchus); three species of Old World Monkeys: Rhesus (Macaca mulatta), Baboon (Papio hamadryas) and Chimpanzee (Pan troglodytes); and humans. Across primate species brain volume and GI were highly correlated (r=0.99).
Recent research has shown than individual variation in brain volume within primate species is under strong genetic control. Various studies in humans and other primates have investigated the heritability (h2) of cerebral volume (i.e. the proportion of total phenotypic variation that can be accounted for by genetic differences among individuals), and reported estimates in the range of h2 = 0.5-0.8 or higher (Cheverud et al., 1990; Posthuma et al., 2002; Rogers et al., 2007; Thompson et al., 2001; Toga and Thompson, 2005). This indicates that 50%-80% or more of individual variation in brain volume is caused by genetic differences among subjects. Despite this evidence for genetic control of individual variation in brain volume, and the widely recognized correlation across species between brain volume and cortical folding, little is known about the influence of genetic differences on individual (within-species) variation in gyrification. The tight correlation between brain volume and cortical folding across primates as well as other mammals implies a degree of genetic control over gyrification that should not be difficult to detect. However, that genetic effect has not been clearly established either in humans or any other primate (Bartley et al., 1997; Lohmann et al., 1999; White et al., 2002).
We undertook this study to test two predictions. First, we predicted that like brain volume, individual variation within species in gyrification will exhibit additive genetic variance, i.e. heritability. Second, we anticipated that the same genes that influence individual variation in brain volume will also influence gyrification. We predicted that the genetic correlation (ρG) between these traits will be positive and substantial, possibly approaching unity (ρG = 1). We conducted our study in parallel in humans and baboons (Papio hamadryas). We used the same magnetic resonance imaging methods to quantify variation in cerebral gyrification among 97 baboons and 242 people. This two-species approach allowed us to simultaneously test our predictions in different species, and determine whether the genetic architecture of these traits is different in humans than in other primates. While brain size has increased throughout the last 45-50 million years of primate evolution, the human brain has undergone extremely rapid expansion during the last three million years (Barton, 2006; Kaas and Preuss, 2003; Schoenemann, 2006a). This unique acceleration in our evolutionary lineage justifies independent assessments in humans and nonhuman primates because the genetic architecture of these traits may have been altered during that process of recent human brain expansion.
Materials and Methods
Study Subjects
Structural MR images were collected for 97 pedigreed adult baboons (Papio hamadryas) housed at the Southwest National Primate Research Center (San Antonio, TX). Animal handling protocols were described previously (Rogers et al., 2007). Study animals consisted of 46 males and 51 females, average age 15.2 ± 4.0 (s.d.) years (range: 7.3-27.3 years). Genealogical relationships included 276 parent-offspring pairs, 25 full sib pairs, 362 half-sib pairs, 172 grandparent-grandoffspring pairs and a large number of more distant relationships. Structural MR images were collected from 242 healthy adult human volunteers (96 males, 146 females), part of the Genetics of Brain Structure and Function (GOBSF) project. The average age of human subjects was 47.1± 13.7 years (range: 19 to 85 years old). Human subjects were screened for neurological and psychiatric disorders and underwent neurological and neuropsychological examinations prior to imaging. Genealogical relationships consisted of 82 parent-offspring pairs, 73 full sib pairs, 29 half-sib pairs, and a large number of more distant relationships.
Inter-species analysis of brain volume and gyrification index (GI) in several primate species (humans, three species of Old World Monkey and one species of New World monkey), using the methods summarized above, replicated the previously reported evolutionary trends in brain volume and GI (Figure One). Across species, brain volume and gyrification index were highly correlated, with about 97% of variability in GI being accounted for by increases in brain volume.
MR imaging
We quantified the gyrification index (GI) by adapting a classical 2-D method developed for histological analysis to 3-D MR images, as proposed by Zilles et al. (Zilles et al., 1989; Zilles et al., 1988). Substantial effort was made to ensure that data were collected and analyzed using imaging and genetic methods that minimized methodological differences between species. In particular, image collection in both groups was performed with allometrically similar spatial resolutions.
Animal subjects and human volunteers underwent structural MR imaging at the Research Imaging Center (Univ. of Texas Health Science Center, San Antonio) using a 3T Siemens Trio MRI scanner (Siemens, Erlangen, Germany). Both baboons and humans were imaged using the same 3-D, motion-corrected protocol optimized for high gray matter/white matter contrast. The average volume of a baboon brain is about one-eighth the average volume of the human brain. To accommodate the smaller brain size, the spatial resolution and scan time for baboons were adjusted to achieve a comparable spatial sampling and signal-to-noise ratio. Baboons were imaged with the isotropic spatial resolution of 500 μm during 58-min imaging session using an 8-channel primate head coil. Human volunteers were imaged with an isotropic spatial resolution of 800 μm during a 26 minute scan session using an 8-channel head coil. Datasets for both species had similar average GM/WM contrast (25 ± 2% for baboons, 24 ± 3% for humans) and signal-to-noise ratio (24 ± 3% for baboons, 25 ± 3% for humans). Animal handling and imaging procedures were reviewed and approved by the IACUC of the SFBR. Human imaging was performed under a signed informed consent and following review and approval by the IRB at the UTHSCSA.
Image processing and analysis
MR images for both baboon and human subjects were processed using the image analysis steps described by Rogers et al. (Rogers et al., 2007). These steps were assembled into a image processing pipeline and consisted of: removal of non-brain tissue, correction for radiofrequency bias field inhomogeneity, and global spatial normalization (Rogers et al., 2007) (Figure 2). Finally, the external (GM/CSF) and gray matter/white matter interfaces were extracted from tissue segmented images using the BrainVisa package (Mangin JF et al., 1995; Mangin JF et al., 2004). GI is defined as the ratio between the area of a gyrated surface and the area of its convex hull while excluding the surface area occupied by the lateral ventricles (Figure 2). Consequently, the values for the GI were calculated as the ratio between each individual's respective surface area and the area of their respective convex hulls. The value of GI was calculated for each hemisphere and then averaged to obtain interhemispheric average GI values for a given subject. The method for GI calculation described here is available as a plug-in for BrainVisa at (http://ric.uthscsa.edu/personalpages/petr/).
Figure 2.
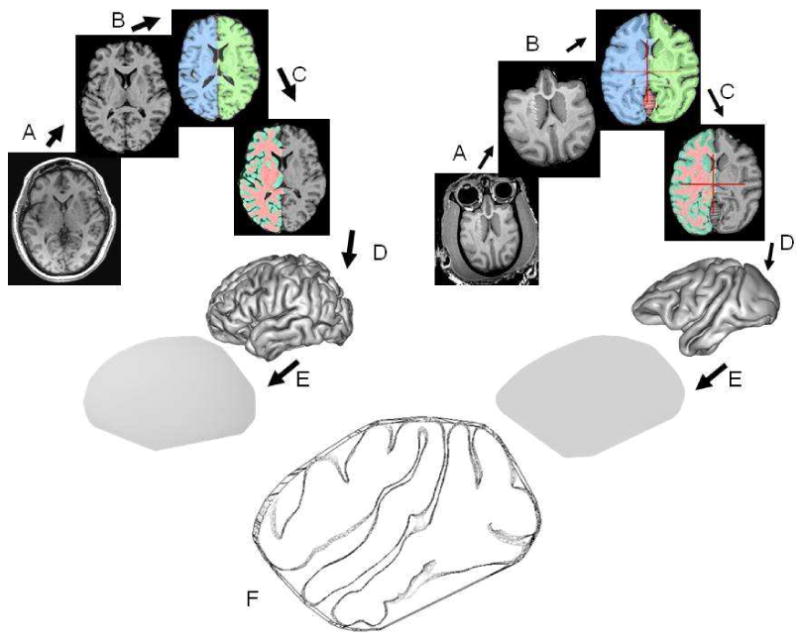
The degree of cerebral gyrification, gyrification index, was calculated as the ratio the surface area of outer cerebral cortex (Scortex) and surface area of the convex hull of the cerebral cortex (Sconvex).
Quantitative genetic analyses
The heritability of and genetic correlations between phenotypes were estimated using maximum likelihood variance decomposition methods implemented in the SOLAR computer package (Almasy and Blangero, 1998, 2009). Univariate genetic analyses of heritability estimated the influence of specific variables (additive genetic variation, covariates including sex, age, and random unidentified environmental effects) on the variance of each trait within each population. This is calculated using maximum likelihood methods to partition the phenotypic variance among individuals into that portion due to genetics (i.e. accounted for by a matrix of pairwise kinship coefficients, which is determined by the known genealogical relationships among study subjects), that due to measured covariates (age and sex) and that attributable to random environmental effects. Genetic correlations between phenotypes were investigated using a multivariate extension of the basic variance components methods, and estimated by conditioning the co-variance between phenotypes on pairwise kinship among individuals within the pedigrees (Comuzzie et al., 1996; Comuzzie et al., 1994). The overall phenotypic correlation between two phenotypes (Trait 1 and Trait 2) can be expressed as a function of the correlation due to shared environmental effects between pairs of individuals (ρ(E)) and the correlation due to shared genetic effects (ρ(G)). For any two traits measured in a series of genealogically related individuals, the relationship among the phenotypic correlation (ρ(p)) and the genetic and environmental correlations is:
In this formulation, h12 and h22 are the heritabilities (the proportion of total phenotypic variance attributable to additive genetic variance) for Trait 1 and Trait 2 respectively. The heritability of each trait is a function of the degree to which kinship across individuals predicts their phenotypic similarity for that trait. To model and estimate the various bivariate effects (i.e. phenotypic, genetic and environmental correlations between traits), we again use a matrix of pairwise kinship coefficients for all pairs of individuals. The phenotypic variance-covariance matrix, as well as the associated genetic and random environmental effects, is obtained by conditioning the covariance among relatives on the kinship matrix. Likelihood methods are used to determine the statistical significance of the estimate of each parameter (i.e. trait heritabilities, and genetic and environmental correlations) by comparing a model in which that parameter is constrained to zero against the general model in which all parameters are estimated simultaneously. Further details regarding the population genetic theory and the specific analytical methods used can be found in (Almasy and Blangero, 2009; Comuzzie et al., 1996; Comuzzie et al., 1997)
Results
We find that the mean gyrification index (GI) for the external gray matter surface was GI = 1.89±0.15 for baboons and GI = 2.29±0.08 for humans. GI values calculated for different primate species appropriately matched the evolutionary trends in gyrification previously reported (Figure One). Neither age nor sex was significantly related to GI in baboons, while both age (p=3.8 × 10-10) and sex (p=2.4 × 10-4) were significantly related to GI in humans. Quantitative genetic analyses showed that brain volume, brain surface area and cortical gyrification are all significantly heritable in both species. The heritability (± s.e.) of brain (cerebral) volume in this sample was h2=0.52±0.18 (p= 0.00005) for humans and h2=0.85 ± 0.29 (p= 0.0012) for baboons. The estimated heritability for GI in humans was h2=0.30 ± 0.16 (p= 0.018), and the heritability of GI in baboons was h2=0.71 ± 0.29 (p= 0.0021). The heritability of cerebral surface area is h2=0.76±0.27 (p= 0.001) for baboons and h2=0.42±0.16 (p= 0.001) for humans. Thus, we can confirm Prediction One that additive genetic differences among individuals influence variation in degree of gyrification in both humans and baboons, with 30% of phenotypic variance in GI among humans attributed to genetic variation, and 71% among baboons.
To test our second prediction, we performed genetic correlation analysis. This analysis uses the known pairwise kinship between members of a pedigree and the individual values for two phenotypic traits to estimate the proportion of genetic variance shared between the two traits. In essence, all pairwise comparisons among related individuals are employed to determine whether the measurement of one phenotype in one individual is correlated with measurement of the second phenotype in closely related individuals that share genes in common due to shared ancestry. If the genetic correlation is statistically significant, this shows that a gene or genes shared among those individuals influence both phenotypes, i.e. there is pleiotropy between traits. A positive correlation indicates that genetic differences that increase the measured values for one phenotype also increase the values for the second, while a negative correlation suggests that genes with positive effects on the first trait have negative effects on the second. The genetic correlations between cerebral volume and GI were estimated as ρG= -0.73 in humans and ρG= -0.77 in baboons. Both genetic correlations are significantly less than 0 (p= 0.045 in humans and p= 0.045 in baboons), and therefore both are also significantly less than +1.0, our predicted result. Figure Three illustrates the phenotypic correlations between these traits in the two species. The genetic correlation between cerebral surface area and GI was estimated as negative, but was not statistically significant in either species (baboons: ρG= -0.56, p = 0.07; humans: ρG= -0.60, p = 0.14).
Figure Three.
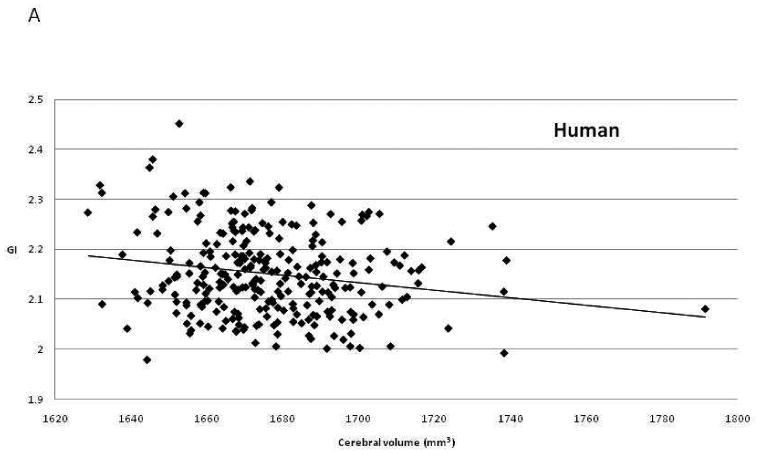
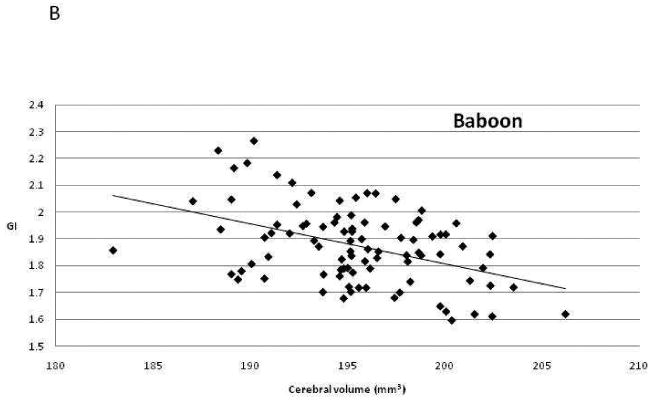
Scatter plots of the relationship between brain volume and gyrification index in (a) humans and (b) baboons.
This study demonstrates significant genetic heritability for individual variation in cortical gyrification in both species, and furthermore suggests that there are shared genetic effects common to both cerebral volume and gyrification. However, contrary to expectation, the calculated genetic correlations between cerebral volume and cortical folding are estimated to be significantly negative in both baboons and humans. Hence, while we can confirm Prediction One, that gyrification is heritable, we must reject Prediction Two, that the positive phenotypic correlation across primate species between gyrification and brain volume results from a common set of genetic changes affecting both traits in the same direction. The negative genetic correlations observed for both humans and baboons strongly suggest that there may be overlapping sets of genes that influence both brain volume and gyrification. But rather than producing parallel increases in both traits, our data suggest that the same genetic changes that increase brain volume within these species seem to exert a negative effect on degree of gyrification.
Discussion
Early fossil primates older than 40 million years have small body sizes and small brain sizes, which reflect their recent divergence from other mammalian orders (Martin, 1990). One hallmark of primate evolutionary history is the origin of new species and new evolutionary groups that exhibit both increased body size and increased brain size relative to body size. As a result, the primate species living today exhibit a broad range of brain sizes and brain:body size ratios (Martin, 1990; Preuss, 2007b). In parallel with increasing brain size, the cerebral cortex of more recently appearing primate groups such as apes and humans shows much more extensive folding or gyrification. Little is known about the specific genetic mechanisms that underlie this suite of parallel evolutionary changes in brain structure, but this pattern is fundamental to the history of primate brain evolution.
Our results indicate that genetic variation now segregating in both baboon and human populations does influence individual differences in gyrification. The specific genes or genetic mechanisms that produce the cortical folding characteristic of Old World monkeys, apes and humans have not yet been identified (but see ref. (Piao et al., 2004)). The embryonic development of cortical convolutions (sulci and gyri) in the human and nonhuman primate brain has been attributed to tension-based developmental mechanisms involving axonal connections between functional regions within the developing cortex (Van Essen, 1997). Under this model, sulci and gyri result from the differential strength of connections in the developing brain, with gyri forming as a result of strong connections between the two cortical regions that grow into close proximity, while sulci form between regions that lack strong connections. This mechanical tension-based model, which is supported by recent studies in nonhuman primates (Hilgetag and Barbas, 2006), connects increases in folding to changes in cortical volume, but does not address the extent of genetic control over gyrification (heritability), or predict whether the same genetic variation that influences cerebral volume also influences degree of cortical folding.
Evidence from congenital malformations in humans does not provide support for either a positive or a negative genetic correlation between these phenotypes (Stevenson, 2006a, b). Known genetic mutations can cause profound congenital malformations of cerebral gyrification such as lissencephaly and poly-microgyria (Stevenson, 2006a), but these conditions are often observed along with apparently normal brain volume. Microcephaly and megalencephaly are caused by processes that interfere with normal brain growth (Stevenson, 2006b) and alter brain volume, but the pattern of gyrification is often well-preserved. These clinical syndromes show that severe pathological mutations can affect one without affecting the other. However, this lack of correlation will not necessarily hold for normal (non-pathological) genetic variation. Overall, the clinical data do not argue for either a positive or negative intra-species genetic correlation between brain volume and gyrification.
We believe that our results have important implications concerning the evolutionary forces that have acted on primate brain structure. It is reasonable to presume that the differences in brain size between hominoids (e.g. living apes and humans) as compared to less encephalized primates (e.g. lemurs and lorises), resulted from natural selection favoring increased cerebral volume, neuron number and overall functional complexity (Schoenemann, 2006b; Vallender et al., 2008). The evolution of larger brain size has been attributed to its positive effects on cognitive and behavioral capability, especially in complex social groups (Dunbar, 2003; Dunbar and Shultz, 2007). Note that the increase in brain size was not a global increase affecting all structures equally, but involved specific additions at specific evolutionary stages (Preuss, 2007b).
Our studies indicate that genetic variation segregating among humans and presumably independent but functionally similar genetic variation segregating among baboons appears to have opposite effects on brain volume and gyrification. The results suggest that in either humans or baboons, selection favoring increased brain volume would produce a correlated reduction in GI. The separate observation of this pattern of genetic architecture in two primate species strongly implies that this genetic architecture may be characteristic of many primate species, and that it was also true for human ancestors. Consequently, assuming that natural selection acted predominantly on brain size (Schoenemann, 2006b), evolutionary genetic changes that resulted in increased brain size during primate evolution would have simultaneously produced less folded, more lissencephalic brains. However comparative primate neuroanatomy and the history of primate brain evolution shows the opposite pattern, with a strong positive correlation between volume and gyrification across species and clades. Therefore we infer that there was a second independent selective force that did not negate the progressive evolutionary increases in brain size, but did generate additional developmental changes resulting in brains with greater degrees of cortical folding. One possibility for this second selective force is based in parturition and reproductive constraints (Rosenberg and Trevathan, 2002). The pelvic inlet and outlet of many anthropoid primates and especially humans are restricted in size by biomechanical constraints related to locomotion. Consequently, the size of the birth canal cannot increase freely to accommodate ever larger neonatal brains and skulls. Indeed, humans are not the only primate for which the size of a newborn's skull can create problems during birth (Leutenegger, 1974).
As adult brain volume increased during various stages of primate evolution, there may have been an advantage within specific primate lineages to maintain neonatal brain volume below certain limits. This natural selection for retaining small neonatal brain size may have resulted in the second set of genetic changes that increased cortical folding in humans, great apes and to a lesser extent in Old World monkeys. This progressive increase in cortical folding could have been achieved through tension-based mechanisms (Van Essen, 1997). Regardless of the specific selective forces or developmental mechanisms at work, our results constitute initial evidence that one set of selective pressures and genetic changes can explain progressively increasing brain size, and that an independent second set of pressures and genetic changes may be needed to explain greater cortical folding. One prediction of the tension-based model of cortical folding (Van Essen, 1997) is that at least some of the genes that influence the degree of cortical folding act through effects on axon tension, either by altering the strength of cortical-cortical connections or affecting the ability of axons to resist elongation as cortical segments grow apart during embryogenesis. Our data predict that detailed analysis of the genetic bases of brain volume and gyrification will identify two sets of evolutionary genetic changes distinguishing humans from smaller brained primates, one set that produced increased cerebral volume and another that leads to greater gyrification.
Table One.
Brain Phenotypes and Genetic Parameters for Baboons and Humans
| Baboons | Humans | |
|---|---|---|
| Mean Cerebral Volume (cc, after spatial normalization) | 195.4 | 1676.1 |
| Mean Gyrification Index (GI) | 1.89 | 2.29 |
| Mean Surface Area (after spatial normalization) | 195.3 | 1880.0 |
| Heritability (h2) of Cerebral Volume | 0.85 (p=0.0012) | 0.52 (p=0.00005) |
| Heritability (h2) of Gyrification Index | 0.71 (p=0.0021) | 0.30 (p=0.018) |
| Heritability (h2) of Surface Area | 0.76 (p=0.001) | 0.42 (p=0.001) |
| Genetic Correlation: Volume vs. GI | -0.77 (p=0.045) | -0.73 (p=0.045) |
| Genetic Correlation: Surface Area vs. GI | -0.56 (p=0.07) | -0.60 (p=0.14) |
Acknowledgments
This work was supported in part by grants from the US National Institute of Mental Health (MH078111, MH059490, and MH078143), the National Institute of Biomedical Imaging and Bioengineering (K01 EB006395) and the National Center for Research Resources base grant to the Southwest National Primate Research Center (P51-RR013986). We are grateful to the participants in the Genetics of Brain Structure Study. The supercomputing facilities used for this work at the AT&T Genetics Computing Center were supported in part by a gift from the AT&T Foundation. The work was carried out in facilities that were constructed with support from Research Facilities Improvement grants C06-RR013556, C06-RR015456 and C06-RR014578 from the National Center for Research Resources, NIH. We also wish to thank two anonymous reviewers for their helpful recommendations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Variance components methods for analysis of complex phenotypes. In: Almasy L, Al-Chalabi A, editors. Genetics of Complex Human Diseases. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. pp. 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120(Pt 2):257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Barton RA. Primate brain evolution: Integrating comparative, neurophysiological and ethological data. Evolutionary Anthropol. 2006;15:224–236. [Google Scholar]
- Cheverud JM, Falk D, Vannier M, Konigsberg L, Helmkamp RC, Hildebolt C. Heritability of brain size and surface features in rhesus macaques (Macaca mulatta) J Hered. 1990;81:51–57. doi: 10.1093/oxfordjournals.jhered.a110924. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Blangero J, Mahaney MC, Haffner SM, Mitchell BD, Stern MP, MacCluer JW. Genetic and environmental correlations among hormone levels and measures of body fat accumulation and topography. J Clin Endocrinol Metab. 1996;81:597–600. doi: 10.1210/jcem.81.2.8636274. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Blangero J, Mahaney MC, Mitchell BD, Stern MP, MacCluer JW. Genetic and environmental correlations among skinfold measures. Int J Obes Relat Metab Disord. 1994;18:413–418. [PubMed] [Google Scholar]
- Comuzzie AG, Rainwater DL, Blangero J, Mahaney MC, VandeBerg JL, MacCluer JW. Shared and unique genetic effects among seven HDL phenotypes. Arterioscler Thromb Vasc Biol. 1997;17:859–864. doi: 10.1161/01.atv.17.5.859. [DOI] [PubMed] [Google Scholar]
- Dunbar R. Psychology. Evolution of the social brain. Science. 2003;302:1160–1161. doi: 10.1126/science.1092116. [DOI] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Preuss TM. Human brain evolution. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. Academic Press; New York: 2003. pp. 1147–1166. [Google Scholar]
- Kaas JH, P TM. Human brain evolution. Elsevier Academic Press; Boston: 2008. [Google Scholar]
- Leutenegger W. Functional aspects of pelvic morphology in simian primates. J Hum Evol. 1974;3:207–222. [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cereb Cortex. 1999;9:754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Mangin JF, Frouin V, Bloch I, Régis J, López-Krahe J. From 3D Magnetic Resonance Images to Structural Representations of the Cortex Topography Using Topology Preserving Deformations. Journal of Mathematical Imaging and Vision. 1995;5:297–318. [Google Scholar]
- Mangin JF, Rivière D, Coulon O, Poupon C, Cachia A, Cointepas Y, Poline JB, Le Bihan D, Regis J, Papadopoulos-Orfanos D. Coordinate-based versus structural approaches to brain image analysis. Artif Intell Med. 2004;30:77–97. doi: 10.1016/S0933-3657(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Martin RD. Primate origins and evolution. Princeton University Press; Princeton, NJ: 1990. [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, Voit T, Ross ME, Michaud JL, Descarie JC, Barkovich AJ, Walsh CA. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- Pillay P, Manger PR. Order-specific quantitative patterns of cortical gyrification. Eur J Neurosci. 2007;25:2705–2712. doi: 10.1111/j.1460-9568.2007.05524.x. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, P TM, editors. Evolution of Nervous Systems: Volume 4 Primates. Elsevier Academic Press; Boston: 2007a. pp. 1–34. [Google Scholar]
- Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, P TM, editors. Evolution of Nervous Systems: A Comprehensive Reference. Elsevier Academic Press; Boston: 2007b. pp. 1–34. [Google Scholar]
- Rogers J, Kochunov P, Lancaster J, Shelledy W, Glahn D, Blangero J, Fox P. Heritability of brain volume, surface area and shape: an MRI study in an extended pedigree of baboons. Hum Brain Mapp. 2007;28:576–583. doi: 10.1002/hbm.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg K, Trevathan W. Birth, obstetrics and human evolution. Bjog. 2002;109:1199–1206. doi: 10.1046/j.1471-0528.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Schoenemann PT. Evolution of the size and functional areas of the human brain. Annual Rev Anthropol. 2006a;35:379–406. [Google Scholar]
- Schoenemann PT. Evolution of the size and functional areas of the human brain. Annual Review of Anthropology. 2006b;35:379–406. [Google Scholar]
- Stevenson RE. Human malformations and related anomalies. Oxford monographs on medical genetics ; no. 52. Oxford University Press, Oxford; New York: 2006a. pp. 546–580. [Google Scholar]
- Stevenson RE. Human malformations and related anomalies. Oxford monographs on medical genetics ; no. 52. Oxford University Press, Oxford; New York: 2006b. pp. 470–525. [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annu Rev Neurosci. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Vallender EJ, Mekel-Bobrov N, Lahn BT. Genetic basis of human brain evolution. Trends Neurosci. 2008;31:637–644. doi: 10.1016/j.tins.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cereb Cortex. 2002;12:486–493. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain Behav Evol. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl) 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]


