Abstract
Objectives:
The objective of this pilot study was to evaluate the effects of three different antiseptic materials on healing processes of direct pulp therapies with Ca(OH)2 histopathologically.
Methods:
Twenty-eight upper and lower first molar teeth from 7 male Wistar rats were used in this study. Four cavities were prepared in each rat in four quadrants, and each quadrant represented different experimental groups. In Group I: 0.5% sodium hypochlorite (NaOCl); in Group II: 2% chlorhexidine digluconate (CHX); in Group III: 0.1% octenidine dihydrochloride (OCT); and in Group IV 0.9% sterile saline was applied to the exposure site with a sterile cotton pellet for 3 minutes. After hemorrhage control, the pulps were capped with hard setting Ca(OH)2 and, finally, restored with IRM. The animals were euthanized at 21 days post-operatively. After sacrificing, routine histological procedures were performed and evaluated statistically with non-parametric Kruskal-Wallis test among the groups and two-by-two comparisons by using the Mann-Whitney U test for inflammatory response and tissue organization scores at the confidence interval of 95%.
Results:
There were significant differences in inflammatory response and tissue organization scores between the groups (P<.05). Statistical evaluation of inflammatory response showed that Group IV was significantly different from Groups I, II and III separately with a higher inflammatory cell response (P<.05) whereas no significant differences were detected between the other groups in two-by-two comparisons (P>.05). Healthy coronal and radicular pulp tissue organization scores indicated that the Group I has better pulp tissue organization than Group IV and this was significantly different (P<.05) whereas no significant differences were observed between the other groups separately (P>.05).
Conclusions:
The antiseptic materials used in this study created an environment that, rather than saline solution, may affect clinical and histological success in a positive way.
Keywords: Antiseptics, Pulpal healing, Calcium hydroxide, Endodontic treatment
INTRODUCTION
Direct pulp therapy is a technique used for the treatment of mechanical or traumatic pulp exposures, without any clinical symptoms of inflammation. Removal of irritation, control of infection and biocompatibility of capping material are important factors in treatment outcome.1 Also, controlling contamination of the pulp exposure site during the treatment process is another important factor. Ultimately, the goal of treating the exposed pulp with an appropriate pulp capping material is to promote the dentinogenic potential of pulp cells.2
The subject of vital pulpal therapy remains controversial, especially regarding which type of pulp dressing provides the most predictable healing. Calcium hydroxide (Ca(OH)2) has been the standard, but dentin bridge formation can occur under a number of pulp capping materials.1,3,4 Application of Ca(OH)2 on exposed pulp tissue results in the release of hydroxyl ions with a bactericidal effect, followed by a combination of lytic and coagulation necrosis in the wound surface. The beneficial effect of Ca(OH)2 has been regarded, to this bactericidal effect and chemical injury, limited by the zone of necrosis, which caused slight irritation of the vital tissue and stimulated the pulp to defend and repair. On the other hand, Hörsted-Bindslev and Lövschall5 stated that pulp capping with Ca(OH)2 induces apoptosis, which is a non-inflammatory controlled cell death mechanism in the underlying pulp, so that the balance of apoptosis and pro-inflammatory response induced by necrosis may have great importance to the prognosis. The opponents of Ca(OH)2 for direct pulp capping procedures cite three major causes of failure: a) the porosity of newly produced dentinal bridge; b) poor adherence to dentin; and c) inability to provide a long-term seal against microleakage.6
Saline solution is one of the most traditional agents used for hemorrhage control in pulp therapies, although it has limited effects on pulp healing.7 On the other hand, many researchers concluded that disinfection of the pulp exposure site and removing the blood coagulum before direct pulp capping has a beneficial effect on pulp healing.3,7,8 For these purposes not only antiseptic agents but also hemostatic agents were used.3,9,10 An ISO study conducted by Garcia-Godoy and Murray11 showed that hemostatic treatment had little effect on systemic pulp physiology or healing. They stated local pulp treatment with various hemostatic agents did not alter systemic blood pressure or heart rate during local pulp application. One of the well-known agents that is biocompatible with exposed pulpal tissues is NaOCl.3,7,9 When used in pulp exposures, NaOCl acts as a hemostatic as well as a bacteriostatic and/or bactericidal agent.7
Alternatively, Pameijer and Stanley12 stated 2% CHX as an effective hemostatic agent in pulp exposure site and recommended 2% CHX as a disinfecting agent for pulp capping procedures.13 The study of Horsted-Bindslev et al14 who found only mild inflammatory reactions after application of 0.2% CHX in human pulps, supported the idea. Swift et al15 suggested the use of NaOCl or CHX solution in hemostasis control for a successful vital pulp therapy in their review, which the clinical techniques discussed.
Recently a new bispyridine antimicrobial compound – 0.1% octenidine dihydrochloride (OCT) – has been developed as a potential antimicrobial/antiplaque agent for use in mouthwash formulations. 16–19 It has been shown to be a mucous membrane antiseptic and is also used in severe burns and for wound healing. OCT has also been suggested as an endodontic irrigant based on its antimicrobial effects and lower cytotoxicity.20–22
The research hypothesis of this study was that antiseptic materials not only impair the healing process of dental pulp capped with Ca(OH)2 but also increase the success of the treatment due to their disinfectant and hemostatic properties. In the present study our aim was to evaluate the histopathological effects of a new antiseptic agent besides well-known ones on the repair process of pulp tissue under Ca(OH)2 comparatively to saline solution.
MATERIALS AND METHODS
Experimental design and direct pulp-capping procedures
Experimental protocols of animals were reviewed by the Gazi University Institutional Ethical Committee. Twenty-eight upper and lower first molar teeth from 7 male Wistar rats were used in this study. All procedures were performed under anesthesia using intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). Teeth were randomly assigned to treatment groups using a statistical randomized teeth table.
After disinfection of the operation field with 3% iodine, class I cavities were prepared using a sterile high-speed ½ round dental bur. To ensure standardization, a pinpoint pulp exposure was performed with a dental explorer. Four cavities were prepared in each rat in four quadrants (four cavities per rat), and each quadrant represented different experimental groups. In Group I, 0.5% sodium hypochlorite (NaOCl)(Gazi University, Faculty of Pharmacy, Ankara, Turkey); in Group II, 2% chlorhexidine digluconate (CHX) (Klorhex, Drogsan ilaçları san ve Tic. A.Ş. Ankara, Turkey); in Group III, 0.1% octenidine dihydrochloride (OCT) (Octenisept, Schülke & Mary GmBH, Wien, Austria); and, as a control in Group IV, 0.9% sterile saline solution was used. All test materials were applied to the respective exposure site with a saturated sterile cotton pellet for 3 minutes. In most cases, all hemorrhage had stopped without the presence of an underlying blood clot. If hemorrhage persisted, another sterile cotton pellet saturated with testing material was placed on the exposure site again for 3 minutes. After hemorrhaging was controlled, all exposures were capped with hard setting Ca(OH)2 (Dycal, Dentsply, Konstanz, Germany), and final restorations were finished with Intermediate Restorative Material (IRM) (DENTSPLY Caulk, Ontario, Canada). The animals were sacrificed twenty-one days post-operatively under general anesthesia with an intraperitoneal injection of sodium pentobarbital (50mg/kg).
Histopathological examination
The specimens were fixed in 10% neutral buffered formalin and decalcified in buffered 10% formic acid. After decalcification, the specimens were rinsed under running water for 4 hours followed by dehydration with ascending concentrations of alcohol and then embedded in paraffin blocks. Five-μm sections were prepared for histological analysis. Each section was stained with hematoxylin and eosin (H&E). Maisson’s Trichrome staining protocol was performed to evaluate pulp tissue organization, while Brown & Brenn staining was used for determining bacterial presence in all specimens.
Sections were examined under the light microscope (Eclipse e-600, Nikon, Tokyo, Japan) x20, x40, x100, x200, and x400 magnifications. Evaluation criteria for inflammatory cell response are given in Table 1 and for tissue disorganization in Table 2. Statistical data of the scores were given in Table 3.
Table 1.
Evaluation criteria for inflammatory cell response.
| Code | Criteria |
|---|---|
| 1 | None or few scattered inflammatory cells present in the pulp at the exposure site beneath the new dentinal bridge |
| 2a | Acute inflammatory cell lesion dominated by polymorphonuclear leukocytes |
| 2b | Chronic inflammatory cell lesion dominated by mononuclear lymphocytes |
| 3 | Severe inflammatory cell lesion appearing as an abscess or dense infiltrate of polymorphonuclear leukocytes involving one third or more of the coronal pulp |
| 4 | Necrotic pulp |
Table 2.
Evaluation criterias for tissue disorganization.
| Code | Criteria |
|---|---|
| 1 | Normal tissue |
| 2 | Odontoblastic layer disorganized but central pulp normal |
| 3 | Total disorganization of the pulp tissue morphology |
| 4 | Pulp necrosis |
Table 3.
Statistical data of the scores.
| Groups | Inflammation scores | Soft Tissue Organization Scores | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean Rank | Chi-square/df | P value | N | Mean Rank | Chi-square/df | P value | |
| I-Sodium hypochloride | 7 | 10.64 | 9.274/3 | <.05 | 7 | 10.5 | 8.467/3 | <.05 |
| II-Chlorhexidinegluconate | 7 | 13.21 | 7 | 12.29 | ||||
| III-Octenidinedihydrochloride | 7 | 11.93 | 7 | 13.36 | ||||
| IV-Saline | 7 | 22.21 | 7 | 21.86 | ||||
Statistical analysis
The criteria for each specimen were determined and the results were submitted to statistical analysis, using the software Statistical Packages for Social Sciences for Windows 15.0 (SPSS Inc., Chicago, IL, USA). The confidence level was set at 95%. The inflammatory cell response and tissue organization scores were subjected to non-parametric Kruskal-Wallis test to detect the significant differences among the groups and the Mann Whitney U test was used for two-by-two comparisons.
RESULTS
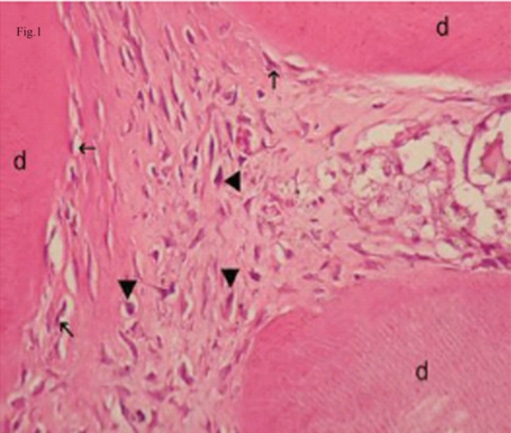
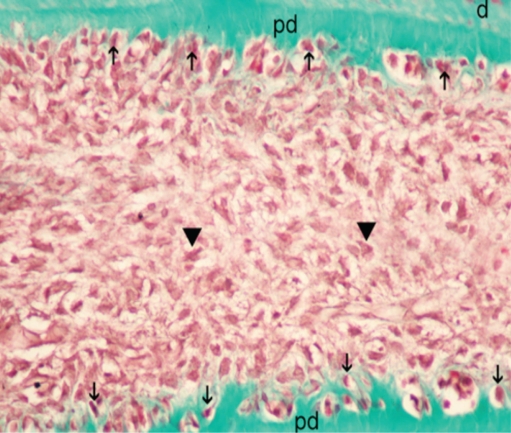
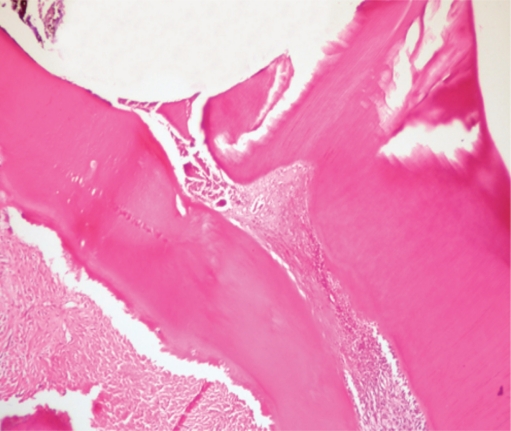
The limited area adjacent to the capping material showed inflammatory infiltrate consisting mostly of mononuclear cells. Pulp tissue containing this infiltrate consisted of collagen fibers, an irregular odontoblastic cell layer, and plump mesenchymal cells. Mild inflammatory cell infiltration beneath the capping material was seen in 6 of 7 samples in Groups I, II, and III, while 4 of 7 samples in Group IV showed the same picture (Figure 1). The pulp tissue with loosely arranged thin collagen fibers, prominent odontoblastic cell layer, dilated capillaries, and mesenchymal cells with angular nuclei was suggested as normal histologic appearance and was observed in 4 of 7 samples in Groups II and III and in 5 of 7 samples in Group I (Figure 2). Pulp tissue morphology was totally disorganized in 6 of 7 samples in Group IV (Figure 3).
Figure 1.
Mild inflammatory cell infiltration beneath of NaOCl. Arrow: odontoblasts, arrowhead: plumped mesenchymal cells, d: dentin. (H&E x100).
Figure 2.
Soft tissue organization almost in normal appearance for Octenidine. d: dentine, pd: predentine, arrowhead: plumped mesenchymal cells. (Masson’s Tricrome x 400).
Figure 3.
A sample of disorganized pulp tissue in saline group. (H&E x40).
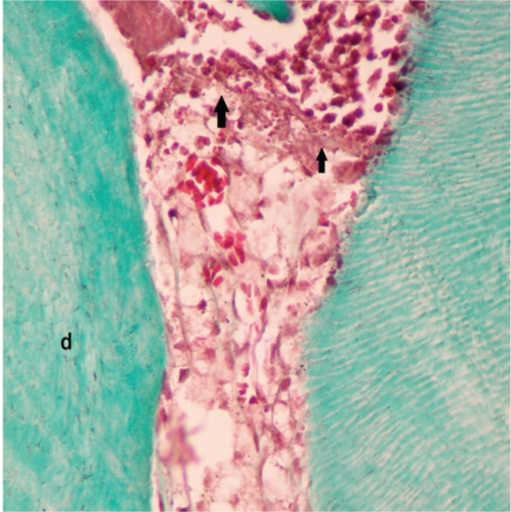
None of the samples showed dentine bridge formation. However, one sample in Group II presented a band-like structure, void of tubule formation, separating the inflammatory infiltrate adjacent to the material from the pulp tissue. This band-like structure was considered to be a precursoring formation of the dentinal bridge (Figure 4). There was no bacterial invasion of the pulp in the Brown & Brenn histochemical staining.
Figure 4.
Band like structure separating the inflammatory infiltrate adjacent to the material from healthy pulp tissue in Octenidine group. d:dentin, arrow: band like structure (Masson’s Tricrome x 400).
Statistical analysis of inflammatory response and tissue organization scores revealed significant differences among the groups tested (P<.05). Statistical evaluation of inflammatory response showed that Group IV was significantly different from Groups I, II and III separately with a higher inflammatory cell response (P<.05) whereas no significant differences were detected between the other groups in two-by-two comparisons (P>.05). Healthy coronal and radicular pulp tissue organization scores indicated that the Group I has better pulp tissue organization than Group IV and this was significantly different (P<.05) whereas no significant differences were observed between the other groups separately (P>.05).
DISCUSSION
It is known that control of pulpal hemorrhage in direct pulp therapies with Ca(OH)2 is a very important step affecting pulpal healing. A light pressure on the exposure with a sterile dry cotton pellet for 3–5 minutes is a traditional clinical practice for hemostatic control in direct pulp therapies. In time this practice has been changed to wet cotton pellet control with sterile saline solution. Today alternatives to wet cotton pellets such as the idea of using antiseptic agents with well-known haemostatic roles and pulp tissue reactions have been discussed to increase the success of vital therapies with Ca(OH)2.4,23,24
Rat teeth’s histological and physiological aspects as well as form and function are very similar to human teeth, so to test new materials and clinical practice they have found wide application areas.25,26 Additionally, rat molar teeth was introduced as a realistic model for pulp and dentine usage test of dental materials.27
Although saline solution has limited effects on pulp healing, it is one of the most traditional and widely used agents for hemorrhage control in pulp therapies.9,13,28–31 Therefore, saline solution is used as control group in this study. However, the statistical evaluation of inflammatory response besides healthy coronal and radicular pulp tissue organization for 0.9% sterile saline (Group IV) showed a significant difference, indicating an acceptable but relatively inferior success on pulpal response.
Sodium hypochlorite is recommended as an alternative irrigation solution in several studies because of its well-known bactericidal action.3,7,9 The disinfecting efficiency of NaOCl depends upon the concentration of undissociated hypochlorus acid (HClO) in solution. HClO exerts its germicidal effect through an oxidative action on sulphydryl groups of bactericidal enzymes. As essential enzymes are inhibited, important metabolic reactions are disrupted, resulting in the death of the bacterial cells.29–31 The major disadvantages of NaOCl are its cytotoxic effects on the periapical tissues and pulp tissue. Although various ISO studies on non-human primate pulps have demonstrated that use of 2% to 5% NaOCl presents no in vivo toxicity to primary odontoblasts or to subjacent pulp cells or capillaries, other studies recommend its use at the lower concentration of 0.5% in order to obtain acceptable cytotoxic and bactericidal levels. 3,13,29–31 In this study, 3 minutes’ application time was preferred for hemostatic control so that 0.5% concentration of NaOCl was selected.
The histopathological evaluation results of this study showed normal histologic appearance in most of the samples of Group I as well as all groups where antiseptic materials were used (P>.05). However, there was a significant difference between 0,5% NaOCl (Group I) and saline solution (Group IV) (P<.05) contrary to the literature review of Schuurs et al,33 who stated that both NaOCl and saline seem suitable for hemostasis and cleaning of the pulp wound, whereas the effectiveness of a 2% CHX solution is questionable.
Chlorhexidine is a cationic bisguanide that seems to act by adsorbing onto the cell wall of the microorganism and causing leakage of intracellular components. At low CHX concentrations, small molecular weight substances will leak out, especially potassium and phosphorus, resulting in a bacteriostatic effect. At higher concentrations, CHX has a bactericidal effect due to precipitation and/or coagulation of the cytoplasm, probably caused by protein cross-linking.30 CHX has been used in endodontics as an irrigating solution and as an antiseptic and/or hemostatic agent in pulp capping procedures during several studies.7,12,13,17 2% CHX was studied for its antimicrobial effect. 7,12,13,29,30 2% CHX was selected as test material for this study, according to the encouraging results of Pamejier and Stanley,12 who found that 2% CHX applied immediately after exposure was an effective hemostatic agent. In another study, Pameijer13 compared 2% CHX and various concentrations of NaOCl during pulp capping with Ca(OH)2, and recommended 2% CHX for disinfecting pulp exposure sites. Also, Ayhan et al29 compared 2% CHX and 0.5% NaOCl as an endodontic irrigant on selected microorganisms and found no statistically significant difference between two groups. Silva et al7 investigated the influence of 0.9% saline solution, 5.25% NaOCl, and 2% CHX on the healing of healthy human pulp tissue capped with Ca(OH)2 and found that three hemostatic agents did not impair the healing process following pulp exposure and capping with Ca(OH)2 at different time intervals investigated. According to the histopathological results of this study the antibacterial agents may affect clinical and histological success in a positive way.
Octenidine dihydrochloride has been used in medicine for many years as a soft tissue antiseptic material. In dental practice, the main usage of OCT is as a mouth rinse material and the antimicrobial/antiplaque effect thereof has been demonstrated in several studies.16–22 It is reported that OCT inhibits dental plaque and caries in rats, dental plaque in primates, and in humans.16–19 Pitten and Kramer20 showed that OCT has antimicrobial efficiency in oral cavities. On the other hand, Shern et al17 compared OCT and CHX as a mouth rinse solution in rats and found no statistical difference between the effect of OCT and CHX in dental plaque and dental carries formation. In a recent study, Dogan et al34 reported the results of antibacterial efficacy of common antiseptic mouth rinses and octenidine dihydrochloride against the Streptococcus mutans and Lactobacillius species. They concluded OCT compared favorably with CHX and Povidone Iodine in its antibacterial effects, both in vitro and in vivo.
Tirali et al35 investigated the antibacterial effects of 100% OCT, 50% OCT and 5.25% NaOCl and 2.5% NaOCl solutions on S. aureus, E. faecalis, and C. albicans over a range of time intervals and found the antimicrobial effect of the most effective concentrations of the tested irrigants were ranked from strongest to weakest as follows: 100% Octenisept, 50% Octenisept, 5.25% NaOCl, and 2.5% NaOCl. No data was found in the literature about the usage of OCT solutions in direct pulp-capping procedures. Moreover, in the view of these studies this antiseptic agent was tested for disinfecting pulp exposure site in this study and found as an acceptable agent for future therapeutic approaches in pulp studies.
Evaluation results for inflammatory cell response and for tissue disorganization showed no difference between NaOCl (Group I), CHX (Group II) and OCT (Group III) besides indicating superior pulpal response at 21 days compared to saline (Group IV).
Despite the short-term results of this study, none of the samples showed dentine bridge formation except for one sample from the OCT group that was considered to be precursoring formation of a dentinal bridge.
Additionally, the routine aseptic clinical protocol followed for treatment and finally a hermetic seal with a hard-setting zinc oxide eugenol (IRM) resulted with no bacterial invasion to the pulp in all groups. In the literature, particularly for long term, adverse effects were reported about the idea of using IRM as a restorative material. In these cases, it was found that the sealing ability of ZnO-eugenol cement might be based rather on its bactericidal properties, than prevention of microleakage. 36 It was also stated that there is a possibility that the eugenol leaching from the cement diffuses through the Ca(OH)2 suspension and liners,33 or the potential effects of reaches the pulp which may result in inflammation and necrosis of the pulp.37 However, Guelmann et al38 investigated the success of pulpotomies performed on an emergency basis and restored with a temporary restorative material. According to the results of that study, the early failures, may be attributed to the inflammatory status of the pulp. In the long term, failures may be associated with the temporary filling material. In this study only the short term results evaluated so the failures could not be related to temporary restorative material.
Total pulp necrosis occurred in one specimen in each of the four groups. This result may be due to the malpractice of the clinician upon the same rat. As we have a small number of samples due to ethical considerations, we could not ignore the pulp necrosis samples for the statistical analysis.
CONCLUSIONS
This study showed a mild inflammatory cell infiltration besides healthy coronal and radicular pulp tissue organization with no statistical importance among Group I, Group II, and Group III, thus indicating affirmative effects in short-term tissue healing. These results signify that OCT can be used alternatively to NaOCl and CHX in direct pulp capping with Ca(OH)2 without any adverse effects. However, the statistical evaluation of inflammatory response noted that traditional saline application (Group IV) was significantly different from the other groups (P<.05) with inferior success on pulpal response and pulp tissue morphology.
As a result, although there was a short time interval (21 days) and a small amount of sample in this pilot study; it can be suggested that the antiseptic materials used in this study, rather than saline solution, created an environment that may affect clinical and histological success in a positive way.
REFERENCES
- 1.Andelin WE, Shabahang S, Wright K, Torabinejad M. Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. J Endod. 2003;10:646–650. doi: 10.1097/00004770-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder U. Effects of calcium-hydroxide-containing pulp-capping agents on pulp cell migration, proliferation and differentiation. J Dent Res. 1985;64:541–548. doi: 10.1177/002203458506400407. [DOI] [PubMed] [Google Scholar]
- 3.Hafez AA, Cox CF, Tarim B, Otsuki M, Akimoto N. An in vivo evaluation of hemorrhage control using sodium hypochlorite and direct capping with a one- or two-component adhesive system in exposed nonhuman primate pulps. Quintessence Int. 2002;33:261–272. [PubMed] [Google Scholar]
- 4.Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulpcapping agents in human teeeth: a priliminary report. Int Endod J. 2002;36:225–231. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 5.Horsted-Bindslev P, Lovschall H. Treatment outcome of vital pulp treatment. Endod Topics. 2002;2:24–34. [Google Scholar]
- 6.Holland R, de Souza V, de Mello W, et al. Permeability of har tissue bridge formed after pulpotomy with calcium hydroxide. A histological study. J Am Dent Assoc. 1979;99:472–475. doi: 10.14219/jada.archive.1979.0317. [DOI] [PubMed] [Google Scholar]
- 7.Silva AF, Tarquinio SBC, Demarco FF, Piva E, Rivero ERC. The influence of haemostatic agents on healing of healthy human dental pulp tissue capped with calcium hydroxide. Int Endod J. 2006;39:309–316. doi: 10.1111/j.1365-2591.2006.01101.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox CF, Tarim B, Kopel H, Gürel G, Hafez A. Tecnique sensivity: Biological factors contrubuting to clinical success with various restorative matreials. Adv Dent Res. 2001;15:85–90. doi: 10.1177/08959374010150012301. [DOI] [PubMed] [Google Scholar]
- 9.Accorinte MLR, Loguercio AD, Reis A, Muench A, Arajujo VC. Response of human pulp capped with a bonding agent after bleeding control with hemostatic agents. Oper Dent. 2005;30:147–155. [PubMed] [Google Scholar]
- 10.Cengiz BS, Batirbaygil Y, Onur MA, Atilla P, Asan E, Altay N, Cehreli ZC. Histological comparision of alendronate, calcium hydroxide and formocresol in amputadet rat molar. Dent Traumatology. 2004;21:1–8. doi: 10.1111/j.1600-9657.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Godoy F, Murray EP. Systemic evaluation of various haemostatic agents following local application prior to direct pulp capping. Braz J Oral Sci. 2005;4:791–797. [Google Scholar]
- 12.Pameijer CH, Stanley HR. The disastrous effects of ‘total etch’ technique in vital pulp capping in primates. Am J Dent. 1998;11:148. [PubMed] [Google Scholar]
- 13.Pameijer CH. Pulp capping with an experimental hemostatic agent and calcium hydroxide. 2002. p. 1811. IADR/AADR/CADR 80th general session.
- 14.Horsted-Bindslev P, Vilkinis V, Sidlauskas A. Direct capping of human pulps with a dentin bonding system or wiyh calcium hydroxide cement. Oral Surg Oral Med Oral Pathol Oral Radiol and Endod. 2003;96:591–600. doi: 10.1016/s1079-2104(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 15.Swift EJ, Trope M, Ritter AV. Vital pulp therapy for the mature tooth-can it work? Endod Topics. 2003;5:49–56. [Google Scholar]
- 16.Slee MA, Cimijotti E, Rothstein S. The effect of daily treatments with an octenidine dentifrice formulation on gingival health in cynomolgus monkeys. J Periodontal Res. 1985;20:542–549. doi: 10.1111/j.1600-0765.1985.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 17.Shern RJ, Monell-Torrens E, Kingman A. Effect of two recently developed antiseptics on dental plaque and carries in rats. Caries Res. 1985;19:458–465. doi: 10.1159/000260882. [DOI] [PubMed] [Google Scholar]
- 18.Beiswanger BB, Mallatt ME, Jackson RD, Hennon DK. The clinical effects of a mouthrinse containing 0.1% octenidine. J Dent Res. 1990;69:454–457. doi: 10.1177/00220345900690020701. [DOI] [PubMed] [Google Scholar]
- 19.Kocak MM, Ozcan S, Kocak S, Topuz O, Erten H. Comparison of the efficacy of three different mouthrinse solutions in decreasing the level of streptococcus mutans in saliva. Eur J Dent. 2009;3:57–61. [PMC free article] [PubMed] [Google Scholar]
- 20.Pitten FA, Kramer A. Antimicrobial efficacy of antiseptic mouthrinse solutions. Eur J Clin Pharmacol. 1999;55:95–100. doi: 10.1007/s002280050601. [DOI] [PubMed] [Google Scholar]
- 21.Ghannoum MA, Elteen KA, Stretton RJ, Whittaker PA. Effects of octenidine and pirtenidine on adhesion of candida species to human buccal epithelial cells in vitro. Arch Oral Biol. 1990;35:249–253. doi: 10.1016/0003-9969(90)90039-d. [DOI] [PubMed] [Google Scholar]
- 22.Patters MR, Nalbandian J, Nichols FC, Niekrash CE, Kennedy JE, Kiel RA, Trummel CL. Effects of octenidine mouthrinse on plaque formation and gingivitis in humans. J Periodontal Res. 1986;21:154–162. doi: 10.1111/j.1600-0765.1986.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshiba K, Yoshiba N, Nakamura H, Iwaku M, Ozawa H. Immunolocalization of fibronectin during reperative dentinogenesis in human teeth after pulp capping with calcium hydroxide. J Dent Res. 1996;75:1590–1597. doi: 10.1177/00220345960750081101. [DOI] [PubMed] [Google Scholar]
- 24.Kirk EEJ, Lim KC, Khan MOG. A comparison of dentinogenesis on pulp capping with calcium hydroxide in paste and cement form. Oral Surg Oral Med Oral Pathol. 1989;68:210–219. doi: 10.1016/0030-4220(89)90195-3. [DOI] [PubMed] [Google Scholar]
- 25.Six N, Lasfargeus JJ, Goldberg B. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J Dent. 2000;28:413–422. doi: 10.1016/s0300-5712(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 26.Murray PE, Matthews JB, Sloan AJ, Smith AJ. Analysis of incisor pulp cell populations in Wistar rats of different ages. Arch Oral Biol. 2002;47:709–715. doi: 10.1016/s0003-9969(02)00055-9. [DOI] [PubMed] [Google Scholar]
- 27.Dammaschke T. Rat molar teeth as a study model for direct pulp cappingresearch in dentistry. Lab Anim. 2010;44:1–6. doi: 10.1258/la.2009.008120. [DOI] [PubMed] [Google Scholar]
- 28.Accorinte Mde L, Loguercio AD, Reis A, Holland R. Effects of hemostatic agents on the histomorphologic response of human dental pulp capped with calcium hydroxide. Quintessence Int. 2007;38:843–852. [PubMed] [Google Scholar]
- 29.Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int Endodon J. 1999;32:99–102. doi: 10.1046/j.1365-2591.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 30.Gomes BPFA, Ferraz CCR, Vianna ME, Berber VB, Teixeria FB, Souza-Filh FJ. In vitro antibacterial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of enterococcus faecalis. Int Endod J. 2001;34:424–428. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 31.Ostravik D. Intracanal medication in: Pitt Ford TR Endodontics in clinical practice. Harty’s 5th edition. Spain: Elsevier; 2004. pp. 95–108. [Google Scholar]
- 32.Dychdala GR. Chlorine and chlorine compounds in: Block SS Disinfection, Sterilization, and Preservation. Philedelphia: Lea&Febiger; 1991. pp. 133–135. [Google Scholar]
- 33.Schuurs AH, Gruythuysen RJ, Wesselink PR. Pulp capping with adhesive resin-based composite vs. calcium hydroxide: a review. Endod Dent Traumatol. 2000;16:240–250. doi: 10.1034/j.1600-9657.2000.016006240.x. [DOI] [PubMed] [Google Scholar]
- 34.Dogan AA, Adiloglu AK, Onal S, Cetin ES, Polat E, Uskun E, Koksal F. Short-term relative antibacterial effect of octenidine dihydrochloride on the oral microflora in orthodontically treated patients. Int J Infect Dis. 2008;12:e19–25. doi: 10.1016/j.ijid.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Tirali RE, Turan Y, Akal N, Karahan ZC. In vitro antimicrobial activity of several concentrations of NaOCl and Octenisept in elimination of endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e117–20. doi: 10.1016/j.tripleo.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Pameijer CH, Wendt SL., Jr Microleakage of “surface-sealing” materials. Am J Dent. 1995;8:43–46. [PubMed] [Google Scholar]
- 37.Watts A, Paterson RC. Pulpal response to a zinc oxide-eugenol cement. Int Endod J. 1987;20:82–86. doi: 10.1111/j.1365-2591.1987.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 38.Guelmann M, Fair J, Turner C, Courts FJ. The success of emergency pulpotomies in primary molars. Pediatr Dent. 2002;24:217–220. [PubMed] [Google Scholar]