Abstract
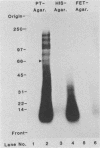
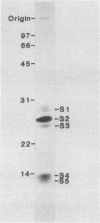
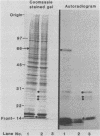
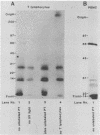
We have investigated human T-lymphocyte receptors for pertussis toxin by affinity isolation and photoaffinity labeling procedures. T lymphocytes were obtained from peripheral human blood, surface iodinated, and solubilized in Triton X-100. The iodinated mixture was then passed through pertussis toxin-agarose, and the fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Autoradiography of the fixed, dried gels revealed several bands in the pertussis toxin-bound fraction that were not observed in fractions obtained from histone or fetuin-agarose. Further investigations employed a photoaffinity labeling reagent, sulfosuccinimidyl 2-(p-azido-salicylamido)-1,3'-dithiopropionate, to identify pertussis toxin receptors in freshly isolated peripheral blood monocytic cells, T lymphocytes, and Jurkat cells. In all three cell systems, the pertussis toxin affinity probe specifically labeled a single protein species with an apparent molecular weight of 70,000 that was not observed when the procedure was performed in the presence of excess unmodified pertussis toxin. A protein comparable in molecular weight to the one detected by the photoaffinity labeling technique was also observed among the species that bound to pertussis toxin-agarose. The results suggest that pertussis toxin may bind to a 70,000-Da receptor in human T lymphocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. D., Hollenberg M. D., Bhaumick B., Bala R. M. Comparative studies on human placental insulin and basic somatomedin receptors. J Cell Biochem. 1982;20(3):283–292. doi: 10.1002/jcb.240200308. [DOI] [PubMed] [Google Scholar]
- Armstrong G. D., Howard L. A., Peppler M. S. Use of glycosyltransferases to restore pertussis toxin receptor activity to asialoagalactofetuin. J Biol Chem. 1988 Jun 25;263(18):8677–8684. [PubMed] [Google Scholar]
- Armstrong G. D., Peppler M. S. Maintenance of biological activity of pertussis toxin radioiodinated while bound to fetuin-agarose. Infect Immun. 1987 May;55(5):1294–1299. doi: 10.1128/iai.55.5.1294-1299.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chong P., Klein M. Single-step purification of pertussis toxin and its subunits by heat-treated fetuin-sepharose affinity chromatography. Biochem Cell Biol. 1989 Jul;67(7):387–391. doi: 10.1139/o89-061. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. II. Membrane localization and binding characteristics. J Immunol. 1988 Aug 1;141(3):1006–1011. [PubMed] [Google Scholar]
- Macintyre E. A., Tatham P. E., Abdul-Gaffar R., Linch D. C. The effects of pertussis toxin on human T lymphocytes. Immunology. 1988 Jul;64(3):427–432. [PMC free article] [PubMed] [Google Scholar]
- Munoz J. J., Peacock M. G. Role of pertussigen (pertussis toxin) on the mouse protective activity of vaccines made from Bordetella species. Microbiol Immunol. 1989;33(4):341–355. doi: 10.1111/j.1348-0421.1989.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Walker R., Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987 Oct 1;139(7):2419–2423. [PubMed] [Google Scholar]
- Shephard E. G., de Beer F. C., von Holt C., Hapgood J. P. The use of sulfosuccinimidyl-2-(p-azidosalicylamido)-1,3'-dithiopropionate as a crosslinking reagent to identify cell surface receptors. Anal Biochem. 1988 Feb 1;168(2):306–313. doi: 10.1016/0003-2697(88)90323-5. [DOI] [PubMed] [Google Scholar]
- Strnad C. F., Carchman R. A. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 1987 Dec 10;225(1-2):16–20. doi: 10.1016/0014-5793(87)81123-7. [DOI] [PubMed] [Google Scholar]
- Strnad C. F., Lin W. Q., Carchman R. A. Interactive effects of pertussis toxin and the phorbol ester tumour promotor, phorbol dibutyrate, on T-lymphocyte mitogenesis and the expression of phenotypic determinants. Immunology. 1989 Apr;66(4):539–545. [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Thom R. E., Casnellie J. E. Pertussis toxin activates protein kinase C and a tyrosine protein kinase in the human T cell line Jurkat. FEBS Lett. 1989 Feb 13;244(1):181–184. doi: 10.1016/0014-5793(89)81188-3. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell G. J., Peppler M. S., Bonnah R. A., Clark C. G., Chong P., Armstrong G. D. Lectinlike properties of pertussis toxin. Infect Immun. 1989 Jun;57(6):1854–1857. doi: 10.1128/iai.57.6.1854-1857.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvliet M. H., Burns D. L., Brennan M. J., Poolman J. T., Manclark C. R. Binding of pertussis toxin to eucaryotic cells and glycoproteins. Infect Immun. 1989 Nov;57(11):3324–3330. doi: 10.1128/iai.57.11.3324-3330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]