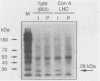
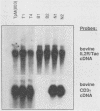
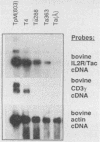
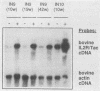
Abstract
The Tac antigen component of the bovine interleukin-2 receptor was expressed as a Cro-beta-galactosidase fusion protein in Escherichia coli and used to raise antibodies in rabbits. These antibodies were used for flow cytofluorimetric analysis to investigate the expression of Tac antigen in a variety of Theileria parva-infected cell lines and also in three Theileria annulata-infected cell lines. Cells expressing Tac antigen on their surface were found in all T. parva-infected cell lines tested whether these were of T- or B-cell origin. T cells expressing Tac antigen could be CD4- CD8-, CD4+ CD8-, CD4- CD8+, or CD4+ CD8+. Tac antigen expression was observed both in cultures which had been maintained in the laboratory for several years and in transformed cell lines which had recently been established by infection of lymphocytes in vitro with T. parva. Northern (RNA) blot analysis demonstrated Tac antigen transcripts in RNA isolated from all T. parva-infected cell lines. Three T. annulata-infected cell lines which were not of T-cell origin were also tested. Two of them expressed Tac antigen on their surface. Abundant Tac antigen mRNA was detected in these T. annulata-infected cell lines, but only trace amounts were demonstrated in the third cell line, which contained very few Tac antigen-expressing cells. In all cell lines tested, whether cloned or uncloned, a proportion of the cells did not express detectable levels of Tac antigen on their surface. This was also the case for a number of other leukocyte surface markers. In addition, we showed that the interleukin-2 receptors were biologically functional, because addition of recombinant interleukin-2 to cultures stimulated cell proliferation. Recombinant interleukin-2 treatment also resulted in increased amounts of steady-state Tac antigen mRNA. The relevance of interleukin-2 receptor expression on Theileria-infected cells is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed J. S., Rehbein G., Schein E. Characterization of Theileria annulata infected lymphoblastoid cells. Z Parasitenkd. 1984;70(6):819–821. doi: 10.1007/BF00927134. [DOI] [PubMed] [Google Scholar]
- Ahmed J. S., Sanft S., Lendner G., Schein E. Effect of chemotherapy on the biological functions of lymphoblastoid cells infected with Theileria annulata. Zentralbl Veterinarmed B. 1987 Aug;34(6):465–470. doi: 10.1111/j.1439-0450.1987.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Baccarini M., Schwinzer R., Lohmann-Matthes M. L. Effect of human recombinant IL-2 on murine macrophage precursors. Involvement of a receptor distinct from the p55 (Tac) protein. J Immunol. 1989 Jan 1;142(1):118–125. [PubMed] [Google Scholar]
- Baldwin C. L., Black S. J., Brown W. C., Conrad P. A., Goddeeris B. M., Kinuthia S. W., Lalor P. A., MacHugh N. D., Morrison W. I., Morzaria S. P. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect Immun. 1988 Feb;56(2):462–467. doi: 10.1128/iai.56.2.462-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. L., Machugh N. D., Ellis J. A., Naessens J., Newson J., Morrison W. I. Monoclonal antibodies which react with bovine T-lymphocyte antigens and induce blastogenesis: tissue distribution and functional characteristics of the target antigens. Immunology. 1988 Mar;63(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. L., Teale A. J., Naessens J. G., Goddeeris B. M., MacHugh N. D., Morrison W. I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986 Jun 15;136(12):4385–4391. [PubMed] [Google Scholar]
- Bismuth G., Moreau J. L., Sommé G., Duphot M., Dautry-Varsat A., Robb R. J., Théze J. Regulation of interleukin 2 (IL2) receptor expression: IL2 as an inducing signal for the expression of its own receptor on a murine T helper cell line. Eur J Immunol. 1985 Jul;15(7):723–727. doi: 10.1002/eji.1830150716. [DOI] [PubMed] [Google Scholar]
- Brown C. G., Stagg D. A., Purnell R. E., Kanhai G. K., Payne R. C. Letter: Infection and transformation of bovine lymphoid cells in vitro by infective particles of Theileria parva. Nature. 1973 Sep 14;245(5420):101–103. doi: 10.1038/245101a0. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Logan K. S. Bovine T-cell clones infected with Theileria parva produce a factor with IL 2-like activity. Parasite Immunol. 1986 Mar;8(2):189–192. doi: 10.1111/j.1365-3024.1986.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Shaw M. K., Conrad P. A., Dolan T. T. Theileria parva: reappearance of schizonts in infected lymphoblastoid cells treated with parvaquone is dependent on interleukin 2-like growth factors. Exp Parasitol. 1989 Apr;68(3):308–325. doi: 10.1016/0014-4894(89)90113-6. [DOI] [PubMed] [Google Scholar]
- Büscher G., Morrison W. I., Nelson R. T. Titration in cattle of infectivity and immunogenicity of autologous cell lines infected with Theileria parva. Vet Parasitol. 1984 Jul;15(1):29–38. doi: 10.1016/0304-4017(84)90107-9. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Clevers H., MacHugh N. D., Bensaid A., Dunlap S., Baldwin C. L., Kaushal A., Iams K., Howard C. J., Morrison W. I. Identification of a bovine surface antigen uniquely expressed on CD4-CD8- T cell receptor gamma/delta+ T lymphocytes. Eur J Immunol. 1990 Apr;20(4):809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- Coquerelle T. M., Eichhorn M., Magnuson N. S., Reeves R., Williams R. O., Dobbelaere D. A. Expression and characterization of the interleukin 2 receptor in Theileria parva-infected bovine lymphocytes. Eur J Immunol. 1989 Apr;19(4):655–659. doi: 10.1002/eji.1830190413. [DOI] [PubMed] [Google Scholar]
- Dobbelaere D. A., Coquerelle T. M., Roditi I. J., Eichhorn M., Williams R. O. Theileria parva infection induces autocrine growth of bovine lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4730–4734. doi: 10.1073/pnas.85.13.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere D. A., Spooner P. R., Barry W. C., Irvin A. D. Monoclonal antibody neutralizes the sporozoite stage of different Theileria parva stocks. Parasite Immunol. 1984 Jul;6(4):361–370. doi: 10.1111/j.1365-3024.1984.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Eichhorn M., Magnuson N. S., Reeves R., Williams R. O., Dobbelaere D. A. IL-2 can enhance the cyclosporin A-mediated inhibition of Theileria parva-infected T cell proliferation. J Immunol. 1990 Jan 15;144(2):691–698. [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Emery D. L. Kinetics of infection with Theileria parva (East Coast fever) in the central lymph of cattle. Vet Parasitol. 1981 Oct;9(1):1–16. doi: 10.1016/0304-4017(81)90002-9. [DOI] [PubMed] [Google Scholar]
- Emery D. L., Morrison W. I. Generation of autologous mixed leucocyte reactions during the course of infection with Theileria parva (East Coast Fever) in cattle. Immunology. 1980 Jun;40(2):229–237. [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., Doxsey S., Stagg D. A., Young A. S. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Electron microscopic observations. Eur J Cell Biol. 1982 Apr;27(1):10–21. [PubMed] [Google Scholar]
- Goddeeris B. M., Morrison W. I. The bovine autologous Theileria mixed leucocyte reaction: influence of monocytes and phenotype of the parasitized stimulator cell on proliferation and parasite specificity. Immunology. 1987 Jan;60(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J. The human interleukin-2 receptor. Annu Rev Immunol. 1986;4:69–95. doi: 10.1146/annurev.iy.04.040186.000441. [DOI] [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Levine H., Griffin J. D. Expression of interleukin 2 receptors and binding of interleukin 2 by gamma interferon-induced human leukemic and normal monocytic cells. J Exp Med. 1985 Sep 1;162(3):1111–1116. doi: 10.1084/jem.162.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Lindemann A., Blohm D., Rambaldi A., Mertelsmann R. H., Griffin J. D. Functional consequences of monocyte IL-2 receptor expression. Induction of IL-1 beta secretion by IFN gamma and IL-2. J Immunol. 1989 Jan 1;142(1):139–143. [PubMed] [Google Scholar]
- Herrmann T., Ahmed J. S., Diamantstein T. The intermediate-affinity interleukin (IL)2 receptor expressed on Theileria annulata-infected cells comprises a single IL 2-binding protein. Partial characterization of bovine IL2 receptors. Eur J Immunol. 1989 Jul;19(7):1339–1342. doi: 10.1002/eji.1830190729. [DOI] [PubMed] [Google Scholar]
- Holter W., Grunow R., Stockinger H., Knapp W. Recombinant interferon-gamma induces interleukin 2 receptors on human peripheral blood monocytes. J Immunol. 1986 Mar 15;136(6):2171–2175. [PubMed] [Google Scholar]
- Howard C. J., Parsons K. R., Jones B. V., Sopp P., Pocock D. H. Two monoclonal antibodies (CC17, CC29) recognizing an antigen (Bo5) on bovine T lymphocytes, analogous to human CD5. Vet Immunol Immunopathol. 1988 Sep;19(2):127–139. doi: 10.1016/0165-2427(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Ivanov V., Stein B., Baumann I., Dobbelaere D. A., Herrlich P., Williams R. O. Infection with the intracellular protozoan parasite Theileria parva induces constitutively high levels of NF-kappa B in bovine T lymphocytes. Mol Cell Biol. 1989 Nov;9(11):4677–4686. doi: 10.1128/mcb.9.11.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor P. A., Morrison W. I., Goddeeris B. M., Jack R. M., Black S. J. Monoclonal antibodies identify phenotypically and functionally distinct cell types in the bovine lymphoid system. Vet Immunol Immunopathol. 1986 Sep;13(1-2):121–140. doi: 10.1016/0165-2427(86)90054-1. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Zubler R. H., Nabholz M., MacDonald H. R. Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature. 1985 Jun 20;315(6021):669–672. doi: 10.1038/315669a0. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Molineaux S. M., Maddon D. E., Zimmerman K. A., Godfrey M., Alt F. W., Chess L., Axel R. Structure and expression of the human and mouse T4 genes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9155–9159. doi: 10.1073/pnas.84.24.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Pepin K. G. Suppression of lectin-stimulated DNA synthesis in bovine lymphocytes by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Sep;40(9):3307–3312. [PubMed] [Google Scholar]
- Mingari M. C., Gerosa F., Carra G., Accolla R. S., Moretta A., Zubler R. H., Waldmann T. A., Moretta L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature. 1984 Dec 13;312(5995):641–643. doi: 10.1038/312641a0. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Baldwin C. L., MacHugh N. D., Teale A. J., Goddeeris B. M., Ellis J. Phenotypic and functional characterisation of bovine lymphocytes. Prog Vet Microbiol Immunol. 1988;4:134–164. [PubMed] [Google Scholar]
- Morrison W. I., Buscher G., Murray M., Emery D. L., Masake R. A., Cook R. H., Wells P. W. Theileria parva: kinetics of infection in the lymphoid system of cattle. Exp Parasitol. 1981 Oct;52(2):248–260. doi: 10.1016/0014-4894(81)90080-1. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Lundin L. B., Dolan T. T., Stagg D. A. Cell-mediated immunity to Theileria-transformed cell lines. Nature. 1979 Oct 25;281(5733):678–680. doi: 10.1038/281678a0. [DOI] [PubMed] [Google Scholar]
- Pinder M., Kar S., Withey K. S., Lundin L. B., Roelants G. E. Proliferation and lymphocyte stimulatory capacity of Theileria-infected lymphoblastoid cells before and after the elimination of intracellular parasites. Immunology. 1981 Sep;44(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Pinder M., Musoke A. J., Morrison W. I., Roelants G. E. The bovine lymphoid system. III. A monoclonal antibody specific for bovine cell surface and serum IgM. Immunology. 1980 Jul;40(3):359–365. [PMC free article] [PubMed] [Google Scholar]
- Pinder M., Withey K. S., Roelants G. E. Theileria parva parasites transform a subpopulation of T lymphocytes. J Immunol. 1981 Jul;127(1):389–390. [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H., Urdal D. L., Kilian P. L., Farrar J. J. Induction and upregulation by interleukin 2 of high-affinity interleukin 2 receptors on thymocytes and T cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8663–8666. doi: 10.1073/pnas.82.24.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintelen M., Schein E., Ahmed J. S. Buparvaquone but not cyclosporin A prevents Theileria annulata-infected bovine lymphoblastoid cells from stimulating uninfected lymphocytes. Trop Med Parasitol. 1990 Jun;41(2):203–207. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roditi I., Dobbelaere D., Williams R. O., Masterson W., Beecroft R. P., Richardson J. P., Pearson T. W. Expression of Trypanosoma brucei procyclin as a fusion protein in Escherichia coli. Mol Biochem Parasitol. 1989 Apr;34(1):35–43. doi: 10.1016/0166-6851(89)90017-0. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner R. L., Innes E. A., Glass E. J., Brown C. G. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology. 1989 Feb;66(2):284–288. [PMC free article] [PubMed] [Google Scholar]
- Spooner R. L., Innes E. A., Glass E. J., Millar P., Brown C. G. Bovine mononuclear cell lines transformed by Theileria parva or Theileria annulata express different subpopulation markers. Parasite Immunol. 1988 Nov;10(6):619–629. doi: 10.1111/j.1365-3024.1988.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Uchino H. Expression of Tac antigen on activated normal human B cells. J Exp Med. 1984 Aug 1;160(2):612–617. doi: 10.1084/jem.160.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A. D., Shaw J., Paetkau V., Bleackley R. C., Magnuson N. S., Reeves R., Magnuson J. A. Cloning of cDNA for the bovine IL-2 receptor (bovine Tac antigen). Immunology. 1988 Apr;63(4):603–610. [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lowenthal J. W., Erard F., Hashimoto N., Devos R., MacDonald H. R. Activated B cells express receptors for, and proliferate in response to, pure interleukin 2. J Exp Med. 1984 Oct 1;160(4):1170–1183. doi: 10.1084/jem.160.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]