Abstract
OBJECTIVE
Our objective was to assess the relationship between di(2-ethylhexyl)phthalate (DEHP) exposure during pregnancy and gestational age at delivery among 311 African American or Dominican women from New York City.
METHODS
Forty-eight-hour personal air and/or spot urine samples were collected during the third trimester. DEHP levels were measured in air samples and 4 DEHP metabolite levels were measured in urine. Specific gravity was used to adjust for urinary dilution. Gestational age was abstracted from newborn medical records (n = 289) or calculated from the expected date of delivery (n = 42). Multivariate linear regression models controlled for potential confounders.
RESULTS
DEHP was detected in 100% of personal air samples (geometric mean: 0.20 μg/m3 [95% confidence interval [CI]: 0.18–0.21 μg/m3]); natural logarithms of air concentrations were inversely but not significantly associated with gestational age. Two or more of the DEHP metabolites were detected in 100% of urine samples (geometric mean: 4.8–38.9 ng/mL [95% CI: 4.1–44.3 ng/mL]). Controlling for potential confounders, gestational age was shorter by 1.1 days (95% CI: 0.2–1.8 days) for each 1-logarithmic unit increase in specific gravity-adjusted mono(2-ethylhexyl)phthalate concentrations (P = .01) and averaged 5.0 days (95% CI: 2.1–8.0 days) less among subjects with the highest versus lowest quartile concentrations (P = .001). Results were similar and statistically significant for the other DEHP metabolites.
CONCLUSIONS
Prenatal DEHP exposure was associated with shorter gestation but, given inconsistencies with previous findings for other study populations, results should be interpreted with caution, and additional research is warranted.
Keywords: di(2-ethylhexyl)phthalate, gestational age, pregnancy, inner city
Di(2-ethylhexyl)phthalate (DEHP) is a plasticizer that is used to impart flexibility to polyvinyl chloride and is used widely in consumer products.1–4 Because DEHP is not bound in the polymer, leaching is common and has resulted in ubiquitous societal exposures.3,5 Once it is absorbed into the human body, DEHP is hydrolyzed rapidly to its monoester metabolite, mono(2-ethylhexyl)phthalate (MEHP), by enzymes present in the lung, blood, and gut.6 MEHP can cross the placenta and enter fetal circulation.7–11 The monoester can be further metabolized to more-hydrophilic oxidative metabolites, including mono(2-ethyl-5-hydroxy hexyl)phthalate (MEHHP), mono(2-ethyl-5-oxohexyl)phthalate(MEOHP),andmono (2-ethyl-5-carboxypentyl)phthalate (MECPP).12 Urinary excretion is the major route of elimination, with most metabolites being excreted within 24 hours.13 Previous epidemiological studies generally measured metabolites in urine as internal dosimeters indicating exposure, because urinary enzymatic activity is negligible and most of the monoesters present arise from elimination of endogenous DEHP. Our previous research on phthalate metabolite levels in repeat urine samples collected biweekly from 23 women in the last 6 to 8 weeks of pregnancy showed reasonable reproducibility for most metabolites.11 The sum of MEHP and the 3 oxidative metabolites measured in urine in the current study is estimated to account for ~65% to 70% of the external DEHP dose.14
Preliminary epidemiological results suggested that prenatal DEHP exposure may modulate the timing of labor. However, findings have been inconsistent, with both positive and negative associations between prenatal DEHP exposure and gestational age being observed.9,15,16 In addition, inconsistent mechanisms for the relationship between prenatal DEHP exposure and parturition have been hypothesized. MEHP is an agonist for peroxisome proliferator-activated receptor γ (PPAR-γ) in placenta and other tissues.17–21 Because PPAR-γ ligands inhibit secretion of cytokines and suppress inflamma-tory responses in gestational tissues, it has been hypothesized that exposure to PPAR-γ ligands during pregnancy may reduce the risk of preterm labor.16,22 However, DEHP exposure in vitro has been shown to cause an inflammatory response in other tissues, and MEHP and other PPAR agonists have been shown to induce cyclooxygenase 2 expression.23–25 This has led to an alternative hypothesis that prenatal DEHP exposure may induce an intrauterine inflammatory response through cyclooxygenase 2 expression and/or related prostaglandin release, resulting in shortened gestation.26,27
The current study was undertaken to assess the relationship between DEHP exposure during pregnancy and gestational age at delivery among a cohort of inner-city pregnant women from New York City. Our previous research demonstrated widespread prenatal exposure to phthalates, including DEHP, in this cohort.11 Levels of several phthalates were higher than those seen among women of reproductive age sampled through the National Health and Nutrition Examination Survey.11
METHODS
Study Group
The study included 331 African American or Dominican women selected from the longitudinal birth cohort being studied by the Columbia Center for Children's Environmental Health (CCCEH). Enrollment criteria for the CCCEH cohort were described elsewhere.28,29 The study was restricted to women 18 to 35 years of age who self-identified as either African American or Dominican and who had resided in northern Manhattan or the South Bronx for ≥1 year before pregnancy. Women were excluded at enrollment if they reported that they smoked cigarettes or used other tobacco products during pregnancy, used illicit drugs, had diabetes mellitus, hypertension, or known HIV infection, or had their first prenatal visit after the 20th week of gestation. Signing of an institutional review board-approved consent form was performed at enrollment. Selection criteria for inclusion in the current study were based on the availability in the parent study of maternal personal air sample extracts (311 of 331 women) and/or urine samples (298 of 331 women), for DEHP and metabolite measurements, respectively. The 331 women did not differ from the remaining women in the CCCEH cohort in terms of marital status, income, proportion receiving Medicaid, education, or gestational age of the newborn at delivery. However, a greater proportion of women were Dominican or other Hispanic (72% vs 59%; P < .001, χ2 test) and the women were somewhat older (average age: 25.5 vs 24.7 years; P = .03, group t test).
Questionnaire and Medical Record Data
A 45-minute questionnaire, administered to each woman in her home by a trained bilingual interviewer during the third trimester of pregnancy, collected information on demographic features, home characteristics, history of active and passive smoking, maternal marital status, education, income level, and alcohol and drug use during pregnancy. Information also was abstracted from the mothers' and infants' medical records. Gestational age (rounded to weeks) was calculated on the basis of the expected date of delivery; the expected date of delivery was based on the date of the last menstrual period in 24% of cases and on sonographic findings (with or without last menstrual period data) in 76%. Gestational age also was extracted from the newborn medical record. Both estimates were available for 262 births; the estimates were correlated (Pearson's correlation coefficient r = 0.79; P < .001) and were within 1 week of each other in 95% of cases. We used clinical estimates of gestational age when they were available (n = 289), because they took into account ultra-sound data and newborn examination findings in cases in which there were inconsistencies between last menstrual period dating and clinical presentation. We used the estimate based on the expected date of delivery when the gestational age from the birth medical record was not available (n = 42).
Collection and Analysis of Personal Air Samples
Women in the cohort were asked to wear a small backpack holding a personal ambient air monitor during the daytime hours for 2 consecutive days during the third trimester and to place the monitor near the bed at night.28,29 The personal air-sampling pumps operated continuously at 4 L/minute during this period, collecting particles of ≤2.5μm in diameter on a precleaned, quartz, microfiber filter and semivolatile vapors and aerosols on a back-up, polyurethane foam cartridge. The monitoring took place between January 2000 and July 2006, with the majority (83%) after 2001. Extracts were kept at −20°C and were analyzed at the Southwest Research Institute when the women were identified for the study. Analysis of the extracts took place between October 2000 and November 2007. The Southwest Research Institute is currently conducting a study of the storage stability of DEHP and other phthalates, and no loss has occurred through 573 days of extract storage. A number of steps were incorporated into the study in 2002 to reduce the risk of contamination of the air extract during processing and analysis. Air levels were significantly higher among subjects monitored before 2002 but did not differ according to year in 2002–2006. Therefore, we conducted statistical analyses for the full cohort and for women from whom the personal air samples were collected in 2002–2006. Results were similar, and results for the full cohort are presented here. Laboratory matrix blanks were extracted and analyzed with each batch of samples, to assess laboratory-introduced phthalate contamination. The amount of DEHP in the laboratory blank averaged 245 ± 279 ng per extract (n=47), which was considerably lower than the average extract amount in the personal air samples (2890 ± 5420 ng per extract).
Collection and Analysis of Urine Samples
A urine sample was collected from the participating women at the end of the personal air monitoring. The samples were collected during 1999 –2006, with the majority (83%) collected after 2001. The urinary DEHP metabolite concentrations were measured at the Centers for Disease Control and Prevention (CDC).30,31 Each analytical run included calibration standards, reagent blanks, and quality control materials of high and low concentrations, for monitoring of accuracy and precision. The urine samples were analyzed between 2004 and 2007. Specific gravity was measured at the CDC by using a handheld refractometer (Atago PAL 10-S-P14643C0 urine specific gravity refractometer [Atago, Bellevue, WA]). Urinary phthalate metabolite concentrations were corrected for dilution through specific gravity adjustment, by using a modification of the formula described by Hauser et al.32 There was no significant difference in metabolite concentrations according to the year of sample collection (1999 –2006).
Statistical Analyses
Descriptive analyses preceded formal hypothesis testing. Concentrations of DEHP in personal air and metabolites in urine were skewed rightward and were transformed by using natural logarithms. For concentrations below the limit of detection (LOD), we assigned a value equal to one half the LOD. Gestational age values were normally distributed and were not transformed. We conducted both univariate and multivariate linear regression analyses to measure the contribution of prenatal DEHP exposure (the independent variable) to gestational age (the dependent variable). Because results were nearly identical, only the latter are presented. Covariates were selected from variables known to be or suspected of being associated with gestational age and/or phthalate levels. All covariates that were associated (P < .15) with phthalate levels (in air or urine) or gestational age were entered into the multivariate models. Because levels of the 4 urinary metabolites were highly correlated (r ≥ 0.8), urinary MEHP levels and personal air DEHP levels were the independent variables used in the model building. Variables with P values of >.50 were subsequently removed if removal did not alter the model fit or change parameter estimates (defined as ≥10% change in the R2 or slope of the independent variable). As a final check, all addition variables identified initially as potential confounders were entered individually into the final models and were retained if they improved the model fit or altered parameter estimates; none did. The final multivariate models controlled for maternal race/ethnicity, age, prepregnancy weight and height, planned cesarean section, premature rupture of membranes, and maternal prenatal asthma, hypertension, and diabetes mellitus. In addition, although the study excluded current smokers at enrollment, 11 women subsequently reported active smoking during pregnancy, either on questionnaires or in the medical record; active smoking was associated with gestational age and was controlled for. Environmental tobacco smoke was not controlled for, because it was not associated with gestational age or phthalate levels and did not improve model fit. None of the phthalate urinary metabolites was associated with maternal prepregnancy weight, BMI, or weight gain during pregnancy. However, prepregnancy weight and height were included in the final models because height was associated with gestational age and prepregnancy weight was weakly correlated with personal air DEHP concentrations. Inclusion of BMI or weight gain during pregnancy did not improve the model fit or alter parameter estimates. Analyses also were conducted by removing subjects with a planned cesarean section (n = 46); results were not appreciably changed from those presented here. When models indicated a significant association between gestational age and concentrations of one of the phthalates, measured as a logarithmically transformed continuous variable, then the phthalate concentrations were categorized into quartiles, and dummy variables were used in the regression model to compare gestational ages for subjects in the second, third, and fourth quartiles versus the first quartile. Differences in gestational ages between quartiles of phthalate exposure levels also were assessed through analysis of covariance. In addition, phthalate urinary metabolite levels were converted to nanomoles per liter and summed as an estimate of total internal dose. All analyses were conducted by using SPSS 16 (SPSS Inc, Chicago, IL). Results were considered statistically significant at P < .05 (2-tailed).
RESULTS
Demographic characteristics are presented in Table 1. Gestational ages averaged 39.3 ± 1.3 weeks; 10 infants (3%) were born before 37 weeks (range: 34 –36 weeks) and 4 (1%) were born after 41 weeks. Table 2 shows exposure distributions and Table 3 presents correlations among the exposure variables. DEHP concentrations in the personal air samples were weakly correlated with the urinary metabolite concentrations; correlations among the urinary metabolite concentrations were highly significant (Table 3). There was no significant difference in the concentrations of DEHP in air or metabolites in urine between African American and Dominican subjects.
TABLE 1.
Demographic Features, Gestational Age (N = 331)
| Maternal age, mean ± SD, y | 25.5 ± 4.8 |
| Ethnicity, % | |
| Black | 28 |
| Dominican or other Hispanic | 72 |
| Maternal education, % | |
| Less than high school degree | 39 |
| High school diploma or general equivalency diploma | 35 |
| More than high school | 25 |
| Marital status, % | |
| Never married | 63 |
| Marrieda | 30 |
| Separated, widowed, or divorced | 7 |
| Income, % | |
| Less than $10 000 | 45 |
| $10 000–$30 000 | 42 |
| More than $30 000 | 13 |
| Reported active smoking during pregnancy, % | 3 |
| Reported smoker in home, % | 26 |
| Maternal height, mean ± SD, cm | 159.7 ± 10.4 |
| Maternal prepregnancy weight, mean ± SD, kg | 67.1 ± 17.4 |
| Gestational age, mean ± SD, wk | 39.3 ± 1.3 |
| Female newborn, % | 52 |
Missing data were as follows: maternal age, n = 1; marital status, n = 1; income, n = 30; active smoking, n = 1; passive smoking, n = 2; maternal height, n = 12; maternal weight, n= 10; newborn gender, n = 1.
Includes living with the same partner for >7 years.
TABLE 2.
Distributions of DEHP Levels in Personal Air Samples and DEHP Metabolite Levels in Urine Samples Collected From Mothers During the Third Trimester of Pregnancy
| No. Above LOD/Total (%) | Level, μg/m3 or ng/mL |
||||||
|---|---|---|---|---|---|---|---|
| Geometric Mean (95% CI) | Percentile |
||||||
| 10th | 25th | 50th | 75th | 95th | |||
| DEHP, μg/m3 | 311/311 (100) | 0.20 (0.18–0.21) | 0.09 | 0.14 | 0.20 | 0.29 | 0.51 |
| MEHP, ng/mL | 248/298 (83) | 4.8 (4.1–5.7) | Below LOD | 1.8 | 4.8 | 12.8 | 58.2 |
| MEHHP, ng/mL | 298/298 (100) | 21.3 (18.4–24.6) | 4.6 | 10.3 | 20.3 | 44.4 | 208.8 |
| MEOHP, ng/mL | 297/298 (99.6) | 18.0 (15.6–20.8) | 3.9 | 8.9 | 17.2 | 35.1 | 155.6 |
| MECPP, ng/mL | 298/298 (100) | 38.9 (34.2–44.3) | 10.6 | 18.7 | 35.7 | 76.2 | 300.2 |
LODs were 1.2 ng/mL (MEHP), 0.7 ng/mL (MEHHP and MEOHP), and 0.6 ng/mL (MECPP).
TABLE 3.
Pearson's Correlation Coefficients for DEHP Levels in Personal Air Samples and DEHP Metabolite Levels in Urine Samples Collected From Mothers During the Third Trimester of Pregnancy
| MECPP Levels in Urine |
MEOHP Levels in Urine |
MEHHP Levels in Urine |
MEHP Levels in Urine |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Levels in urine not adjusted for specific gravity | ||||||||
| DEHP levels in personal air | 0.14 | .02 | 0.18 | .003 | 0.16 | .009 | 0.18 | .003 |
| MEHP levels in urine | 0.82 | <.001 | 0.85 | <.001 | 0.86 | <.001 | ||
| MEHHP levels in urine | 0.95 | <.001 | 0.98 | <.001 | ||||
| MEOHP levels in urine | 0.96 | <.001 | ||||||
| Levels in urine adjusted for specific gravity | ||||||||
| DEHP levels in personal air | 0.07 | .28 | 0.12 | .04 | 0.10 | .10 | 0.12 | .04 |
| MEHP levels in urine | 0.76 | <.001 | 0.81 | <.001 | 0.83 | <.001 | ||
| MEHHP levels in urine | 0.93 | <.001 | 0.98 | <.001 | ||||
| MEOHP levels in urine | 0.94 | <.001 | ||||||
For correlations of personal air levels with urinary metabolite levels, N = 278 before adjustment for specific gravity and N = 277 after adjustment; for correlations between urinary metabolite levels, N = 298 before adjustment for specific gravity and N = 297 after adjustment.
Table 4 shows the results of the multivariate linear regression analyses of the association between the DEHP exposure variables and gestational age (in weeks), controlling for potential confounders. For the urinary metabolites, results after adjustment for urinary dilution are presented but were similar to those before adjustment for dilution. DEHP levels in personal air samples were inversely related to gestational age, but the association was not significant. A significant inverse association between gestational age and DEHP urinary metabolite concentrations was observed. Gestational age was shorter by 1.1 days (95% confidence interval [CI]: 0.2–1.8 days) for each 1-logarithmic unit increase in MEHP concentrations (P = .01), with adjustment for dilution and controlling for potential confounders. Results were similar and significant for the other metabolites and for the sum of all metabolites (Table 4). Results also were similar and significant after removal of the 10 subjects with delivery at <37 weeks of gestation (data not shown). With stratification of the analyses according to ethnicity, gestational age was shorter by 1.8 days (n=72; P = .046) among African American subjects and by 0.9 days (n=177; P=.047) among Dominican subjects for each 1-logarithmic unit increase in MEHP metabolite concentrations.
TABLE 4.
Multivariate Linear Regression Analyses of Changes in Gestational Age in Weeks for Each 1-Logarithmic Unit Increase in DEHP Levels in Personal Air Samples (N = 259) and DEHP Metabolite Levels in Urine Samples, With Adjustment for Specific Gravity (N = 249)
| B (95% CI) | P | |
|---|---|---|
| Model 1: personal air DEHP levels | −0.15 (−0.39 to 0.09) | .23 |
| Model 2: urinary MEHP levels | −0.15 (−0.26 to −0.03) | .01 |
| Model 3: urinary MEHHP levels | −0.18 (−0.31 to −0.05) | .009 |
| Model 4: urinary MEOHP levels | −0.17 (−0.30 to −0.03) | .02 |
| Model 5: urinary MECPP levels | −0.16 (−0.31 to −0.01) | .04 |
| Model 6: sum of urinary metabolite levels | −0.18 (−0.32 to −0.03) | .02 |
Natural logarithms of personal air DEHP concentrations or urinary metabolite concentrations were entered as the independent variable in parallel, multivariate, linear regression models (models 1–6); the dependent variable was gestational age (in weeks). All models controlled for maternal ethnicity (African American versus Dominican or other Hispanic), maternal age, maternal prepregnancy weight and height, active smoking during pregnancy, prenatal asthma, diabetes, and hypertension (yes or no), planned cesarean section (yes or no), and premature rupture of membranes (yes or no).
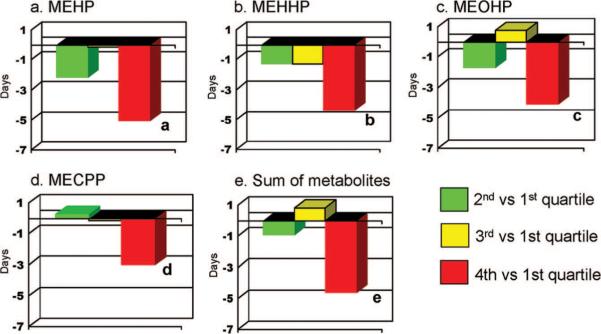
Figure 1 shows the differences in gestational ages in days for subjects with DEHP urinary metabolite concentrations in the second, third, or fourth versus first quartile. In all cases, gestational ages were significantly lower among subjects with concentrations in the fourth quartile, compared with the first quartile. For MEHP, the difference averaged 5.0 days (95% CI: 2.1– 8.0 days; P=.001). Gestational age among subjects with concentrations in the fourth quartile also was less in comparison with subjects with concentrations in the second and third quartiles, and the difference was statistically significant for most metabolites (data not shown). There was no significant difference in gestational age for any of the urinary metabolites among subjects with concentrations in the first, second, or third quartile (data not shown).
FIGURE 1.
Differences in gestational ages in days between subjects with concentrations in the second, third, and fourth quartiles, compared with the first quartile (as reference), for MEHP (a), MEHHP (b), MEOHP (c), MECPP (d), and the sum of metabolites (e).
DISCUSSION
The present study found a significant inverse association between DEHP metabolite levels in maternal, third-trimester, spot urine samples and gestational age at delivery. An inverse association also was seen between gestational age and DEHP concentrations in maternal personal air samples, but the association was not statistically significant. This was not unexpected; although inhalation is one route of exposure, diet is presumed to be the major route.33,34 Therefore, the biomarker is likely to provide a better indication of cumulative exposure. Only 1 previous study showed an inverse association between prenatal DEHP exposure and gestational age; gestational age was significantly lower among infants with MEHP levels in cord blood above versus below the LOD.9 Two additional studies measured DEHP metabolites in maternal, second- or third-trimester, spot urine samples in the same CDC laboratory as that used in the current study. However, findings were inconsistent with those presented here. In one study, involving 404 subjects from multiple racial/ethnic groups residing in New York City, gestational age increased by 1.1 days (95% CI: 0.1–2.0 days) for each 1-logarithmic unit increase in MEHP levels but was not associated with oxidative metabolite levels.15 In the other study, involving 284 subjects from 4 study sites (California, Iowa, Minnesota, and Missouri) enrolled as part of the Study for Future Families, a 1-logarithmic unit increase in urinary metabolite (MEHP, MEOHP, and MEHHP) concentrations was associated with an increase in gestational age of 1.1 to 1.3 days (95% CI: 0.7–2.5 days).16 However, when analyses were stratified according to geographic site, gestational age increased with higher metabolite concentrations in 3 sites but decreased in Minnesota.
Reasons for the discrepancies in these research findings might include differences in study design, exposure levels, race/ethnicity, underlying characteristics of the populations, or uncontrolled confounding. Differences in exposure levels might be particularly relevant, because dose-response curves for endocrine disruptors, including DEHP, frequently are nonlinear and can reverse at higher doses, with U-shaped or inverted U-shaped curves being seen.35–37 Exposures seem to have been considerably higher in the study by Latini et al,9 relative to the other cohorts (eg, the study by Wolff et al15). Differences in exposures might explain some of the discrepancies in results across the 4 sites in the study by Adibi et al.16 Exposure differences also might explain discrepancies between the study by Adibi et al16 and our findings, because our metabolite concentrations were considerably higher. The geometric means for MEOHP and MEHHP differed by almost twofold, and the 95% concentration level differed by more than twofold. This may be particularly relevant because we saw associations principally among subjects with exposure levels in the highest quartile. However, metabolite concentrations in our study were similar to those seen in the study by Wolff et al15; both studies were conducted in New York City, although there were racial/ethnic differences in the cohorts. It should be noted that the association between urinary MEHP concentrations and gestational age seen in our cohort was somewhat stronger among African American subjects than among Dominican subjects although exposure levels were similar, which suggests that effects may differ according to race/ethnicity.
Measurement errors in BMI and weight gain during pregnancy might result in residual confounding, which might be contributing to the discrepancies in the research findings. Recent research indicated that DEHP exposure might be associated with adiposity,17,38 and gestational weight gain can affect the risk of preterm birth.39 Although we assessed prepregnancy weight, height, BMI, and weight gain during pregnancy, our anthropometric measures, as well as those used in the study by Wolff et al,15 were based on maternal self-reports and might be subject to incorrect recall. Data on maternal BMI, height, and weight gain during pregnancy were not collected in the study by Adibi et al.16
Within-subject variability in urinary metabolite concentrations also might contribute to exposure misclassification, although this likely would be non-differential with respect to gestational age and would bias results toward the null hypothesis. In our study, urinary metabolite concentrations were adjusted with respect to specific gravity; in the studies by Adibi et al16 and Wolff et al,15 creatinine levels were used to control for dilution. Research findings from the CCCEH cohort suggest that variability in phthalate metabolite concentrations in repeat urine samples collected over the final 6 weeks of pregnancy are reduced when urinary concentrations are adjusted with respect to specific gravity versus creatinine levels.11 Finally, differences in race/ethnicity, education, and maternal age across the 3 studies might be proxies for other unmeasured effect modifiers, such as nutrition, coexposures, or other lifestyle factors.16 We are currently evaluating the relationship between prenatal phthalate exposures and expression of genes, including cyclooxygenase 2, PPAR-γ, and related genes, in placental tissue from the current cohort, which may provide insights into mechanisms through which prenatal DEHP exposure could alter the timing of labor.
CONCLUSIONS
Given discrepancies between our findings and previous research, additional research is warranted, especially because the magnitude of the effects seen here might be associated with adverse health effects in newborns. This would be particularly true if prenatal DEHP exposure shifted the gestational age distribution, resulting in a greater proportion of infants delivered prematurely, because premature delivery is a cause of morbidity and death.40 Because we did not collect our DEHP exposure measures until the third trimester, our cohort was predominately term by design. Therefore, we were unable to assess the associations between prenatal DEHP exposure and prematurity. However, evidence suggests that poor health and developmental outcomes in infancy and early childhood may not be limited to infants born premature. Among infants born at 37 to 38 weeks (early term), there is now evidence of more-subtle neurodevelopmental problems, including increased risk of learning disabilities and compromised school performance, compared with children born at 39 to 40 completed gestational weeks.41 Furthermore, among term births (>37 weeks), slightly shortened gestation has been associated with compromised health in later life, including susceptibility to depressive symptoms42 and death resulting from cerebrovascular disease.43 It should be noted that the proportion of births with slightly shortened gestation (37–39 weeks) has increased by 19.4% among black, white, and Hispanic groups in the past decade.44 In light of these research findings, the associations between prenatal DEHP exposure and shortened gestation seen here warrant follow-up study.
WHAT'S KNOWN ON THIS SUBJECT
Preliminary epidemiological results suggest that prenatal DEHP exposure may modulate the timing of labor. However, findings have been inconsistent, with both positive and negative associations between prenatal DEHP exposure and gestational age being seen.
WHAT THIS STUDY ADDS
Prenatal DEHP exposure was associated with shorter gestation. Gestational age decreased 1.1 days for each 1-logarithmic unit increase in maternal urine MEHP and averaged 5.0 days less among subjects with the highest concentrations. Results were similar for other DEHP metabolites.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Environmental Health Sciences (grants P50 ES09600, RO1 ES013543, RO1 ES08977, and RO1 ES11158), US Environmental Protection Agency (grants R827027 and R82860901), Irving General Clinical Research Center (grant RR00645), Bauman Family Foundation, Gladys and Roland Harriman Foundation, Hansen Foundation, W. Alton Jones Foundation, New York Community Trust, Educational Foundation of America, New York Times Company Foundation, Rockefeller Financial Services, Horace W. Smith Foundation, Beldon Fund, John Merck Fund, New York Community Trust, and V. Kann Rasmussen Foundation.
We gratefully acknowledge the technical assistance of Manori Silva, Jack Reidy, Ella Samandar, and Jim Preau in measuring the urinary concentrations of phthalate metabolites.
ABBREVIATIONS
- DEHP
di(2-ethylhexyl)phthalate
- MEHP
mono-(2-ethylhexyl)phthalate
- MEOHP
mono-(2-ethyl-5-oxohexyl)phthalate
- MEHHP
mono-(2-ethyl-5-hydroxyhexyl)phthalate
- MECPP
mono-(2-ethyl-5-carboxypentyl)phthalate
- CI
confidence interval
- CDC
Centers for Disease Control and Prevention
- CCCEH
Columbia Center for Children's Environmental Health
- PPAR-γ
peroxisome proliferator-activated receptor γ
- LOD
limit of detection
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
The findings expressed in this article are the opinions of the authors and do not necessarily reflect the official opinions of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Agency for Toxic Substances and Disease Registry . Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP) Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2002. [Accessed October 26, 2009]. Available at: www.atsdr.cdc.gov/toxprofiles/tp9.html. [PubMed] [Google Scholar]
- 2.Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361(1–2):20–29. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210(5):623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Sathyanarayana S. Phthalates and children's health. Curr Probl Pediatr Adolesc Health Care. 2008;38(2):34–49. doi: 10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Silva MJ, Reidy JA, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112(3):327–330. doi: 10.1289/ehp.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blount BC, Milgram KE, Silva MJ, et al. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72(17):4127–4134. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- 7.Singh AR, Lawrence WH, Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci. 1975;64(8):1347–1350. doi: 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- 8.Mose T, Knudsen LE, Hedegaard M, Mortensen GK. Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system. Int J Toxicol. 2007;26(3):221–229. doi: 10.1080/10915810701352721. [DOI] [PubMed] [Google Scholar]
- 9.Latini G, De Felice C, Presta G, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111(14):1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC. Association between prenatal exposure to phthalates and the health of newborns. Environ Int. 2009;35(1):14–20. doi: 10.1016/j.envint.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Adibi JJ, Whyatt RM, Williams PL, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116(4):467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva MJ, Samandar E, Preau JL, Jr, Needham LL, Calafat AM. Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology. 2006;219(1–3):22–32. doi: 10.1016/j.tox.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam. 2001;18(12):1068–1074. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- 14.Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79(7):367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- 15.Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposure and birth outcomes. Environ Health Perspect. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adibi JJ, Hauser R, Williams PL, et al. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multi-center pregnancy cohort. Am J Epidemiol. 2009;169(8):1015–1024. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feige JN, Gelman L, Rossi D, et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor γ modulator that promotes adipogenesis. J Biol Chem. 2007;282(26):19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 18.Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPARα and PPARγ by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201(1–2):133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 19.Hurst CH, Waxman DJ. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Cook TJ, Knipp GT. Effects of di-(2-ethylhexyl)-phthalate (DEHP) and its metabolites on fatty acid homeostasis regulating proteins in rat placental HRP-1 trophoblast cells. Toxicol Sci. 2005;84(2):287–300. doi: 10.1093/toxsci/kfi083. [DOI] [PubMed] [Google Scholar]
- 21.Borel V, Gallot D, Marceau G, Sapin V, Blanchon L. Placental implications of peroxisome proliferator-activated receptors in gestation and parturition. PPAR Res. 2008;2008:758562. doi: 10.1155/2008/758562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaiff WT, Barak Y, Sadovsky Y. The pleio-tropic function of PPAR γ in the placenta. Mol Cell Endocrinol. 2006;249(1–2):10–15. doi: 10.1016/j.mce.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Meade EA, McIntyre TM, Zimmerman GA, Prescott SM. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem. 1999;274(12):8328–8334. doi: 10.1074/jbc.274.12.8328. [DOI] [PubMed] [Google Scholar]
- 24.Gourlay T, Samartzis I, Stefanou D, Taylor K. Inflammatory response of rat and human neutrophils exposed to di-(2-ethyl-hexyl)-phthalate-plasticized polyvinyl chloride. Artif Organs. 2003;27(3):256–260. doi: 10.1046/j.1525-1594.2003.07107.x. [DOI] [PubMed] [Google Scholar]
- 25.Onorato TM, Brown PW, Morris PL. Mono-(2-ethylhexyl) phthalate increases spermatocyte mitochondrial peroxiredoxin 3 and cyclooxygenase 2. J Androl. 2008;29(3):293–303. doi: 10.2164/jandrol.107.003335. [DOI] [PubMed] [Google Scholar]
- 26.Latini G, Massaro M, De Felice C. Prenatal exposure to phthalates and intrauterine inflammation: a unifying hypothesis. Toxicol Sci. 2005;85(1):743. doi: 10.1093/toxsci/kfi131. [DOI] [PubMed] [Google Scholar]
- 27.Latini G, Del Vecchio A, Massaro M, Verrotti A, De Felice C. In utero exposure to phthalates and fetal development. Curr Med Chem. 2006;13(21):2527–2534. doi: 10.2174/092986706778201666. [DOI] [PubMed] [Google Scholar]
- 28.Whyatt RM, Barr DB, Camann DE, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111(5):749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perera FP, Rauh V, Tsai WY, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111(2):201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- 32.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavlock R, Boekelheide K, Chapin R, et al. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2002;16(5):529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 34.Kavlock R, Barr D, Boekelheide K, et al. NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2006;22(3):291–399. doi: 10.1016/j.reprotox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures, part I: mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): nonmonotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227(3):185–192. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Ge RS, Chen GR, Dong Q, et al. Biphasic effects of postnatal exposure to diethylhexyl-phthalate on the timing of puberty in male rats. J Androl. 2007;28(4):513–520. doi: 10.2164/jandrol.106.001909. [DOI] [PubMed] [Google Scholar]
- 38.Grün F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8(2):161–171. doi: 10.1007/s11154-007-9049-x. [DOI] [PubMed] [Google Scholar]
- 39.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008;(168):1–223. [PMC free article] [PubMed] [Google Scholar]
- 40.Institute of Medicine . Preterm Birth: Causes, Consequences, and Prevention. National Academies Press; Washington, DC: 2007. [PubMed] [Google Scholar]
- 41.Kirkegaard I, Obel C, Hedegaard M, Henriksen TB. Gestational age and birth weight in relation to school performance of 10-year-old children: a follow-up study of children born after 32 completed weeks. Pediatrics. 2006;118(4):1600–1606. doi: 10.1542/peds.2005-2700. [DOI] [PubMed] [Google Scholar]
- 42.Raïkkönen K, Pesonen AK, Kajantie E, et al. Length of gestation and depressive symptoms at age 60 years. Br J Psychiatry. 2007;190:469–474. doi: 10.1192/bjp.bp.106.022145. [DOI] [PubMed] [Google Scholar]
- 43.Koupil I, Leon DA, Lithell HO. Length of gestation is associated with mortality from cerebrovascular disease. J Epidemiol Community Health. 2005;59(6):473–474. doi: 10.1136/jech.2004.026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among US singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30(1):8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]