Abstract
Capillary-tissue exchange of inert hydrophilic solutes in the heart occurs through aqueous channels, the clefts between endothelial cells (ECs). For adenosine (and other vasoactive agents and substrates), there is also transport across the plasmalemma of the ECs. The multiple-indicator dilution technique comparing tracer adenosine flux with that of 9-β-d-arabinofuranosylhypoxanthine (an analog that is not transported by the nucleoside carrier) can be used to estimate the conductance of the facilitated transport mechanism, which is equivalent to a permeability-surface area product. Analysis by using a model of exchanges among capillary, EC, interstitium, and myocardial cells suggests that the abluminal surface of the ECs is also highly permeable to adenosine. The inference is that ECs may be an important component of a system for adenosine exchange and regulation in the heart.
Now that is it well recognized that endothelial cells (ECs) cannot be regarded as passive cellophane membranes, studies of transcapillary exchange have taken on new interest. We now consider escape from the plasma space across the capillary wall to be accomplished via transport along aqueous pathways (endothelial cell [EC] clefts or possibly chains of linked vesicles traversing the ECs), and across the luminal plasmalemma of the ECs in parallel. For lipid-soluble substances this last route might simply be passive diffusional transport across the lipid bilayer, but for a large variety of hydrophilic solutes, one can expect something more complex. A short list of possibilities includes facilitated or carrier-mediated transport across the luminal endothelial surface, cotransport or counter transport with other substances, and surface binding whether followed or not by engulfment of a surface membrane fragment into the cell.
A problem in estimating the rates of entry of substances into ECs is that the cells are small, occupying only about 1.0–1.5% of even a very richly vascularized organ such as the heart. This means that tracer entering ECs will reflux back into capillary plasma rapidly unless there is some mechanism either for retention within the ECs or for its transport out the other side of the ECs across the antiluminal plasmalemma, into the interstitial fluid space, followed by retention in parenchymal cells of the organ. With such small distances and volumes, the rate constants for exchange can be very high, particularly for solutes such as hormones and substrates. The situation therefore requires careful, relatively complete mathematical modeling to evaluate parameters defining rates of exchange.
The processes involved in the delivery of substrate into and transformation within cells include local capillary flows and their regional heterogeneities, traversal of three membranes (two endothelial membranes and the plasmalemma of parenchymal cells), distribution through cellular and interstitial spaces, and chemical reactions inside or outside the cells.
Experimentally, the situation requires the acquisition of quite precise data. When tracers are injected into the inflow of an organ, the influences of the transendothelial transport can be observed by comparisons with reference tracers, which are unable to traverse the endothelial membrane. The ideal technique for getting at the permeability characteristics of the luminal plasmalemma of the ECs is the multiple-indicator dilution technique. In this approach a set of solutes of different molecular characteristics are injected simultaneously into the inflow to the organ while a set of samples from the outflow is collected in rapid sequence. In this paper we shall outline the virtues and limitations of this multiple-indicator dilution technique for obtaining estimates of EC characteristics.
CONFIGURATION OF A MODEL FOR ENDOTHELIAL UPTAKE OR TRANSPORT
To deal with the complexities involved, the modeling needs to be approached on two levels, a single capillary level wherein the exchanges between blood, ECs, interstitium, and parenchymal cells are described quantitatively, and a whole-organ model that accounts for the heterogeneity of flow. The latter would not be needed if one could obtain data from single capillary-tissue units. Single capillaries can be isolated in tissues such as mesentery. However, the use of such preparations introduces difficulties. Collecting samples large enough for estimating radioactivity of multiple tracers, avoiding local damage to the capillary under study, and so on, mean that whole-organ experiments are often more practical and closer to the physiological situation, even though somewhat more complicated. The fact that there has been much experience in multicapillary whole-organ analysis (3) is most helpful. Because capillaries in the heart, brain, kidney, and liver are long compared with distances between the capillaries, the capillary-tissue exchange units are defined in terms of partial differential equations accounting for spatial distribution between inflow and outflow from a capillary. In the past such partial differential equations have been tedious to compute (4, 7); the development of a special numerical technique has been essential for obtaining solutions within practical computation times (5). The analytical solution for the four-region system of capillary plasma, ECs, interstitium, and parenchymal cells has been worked out by Wang (see ref 2), but there are problems in computing the analytical solution, and our numerical method is more accurate and 1000 times faster to compute.
The model of Bassingthwaighte and Wang also accounts for varied intracellular volumes of distribution (apparent partition coefficients) and for consumption in all regions. The models can (mathematically) but should not, for physical reasons, be reduced to lumped compartmental models because of the rapid kinetics and the axial distribution of the permeation process along the length of the capillary. This is a critical difference from compartmental modeling, which can neither describe the outflow dilution curves nor provide reasonable estimates of parameters.
Dispersion in arteries and veins must be accounted for. It is not sufficient to regard an organ as represented by a single arterial pathway, a single capillary-tissue unit, and a single vein, even though the descriptions of the transport through each of these may be correct. The problem is the heterogeneity of flows. The heterogeneity can often be adequately described by using simple expressions for delay and dispersion along arteries and veins and by using a set of parallel capillary-tissue exchange units having different flows. The capillary flows are defined by densities of local deposition of microspheres, or of a molecular microsphere, desmethylimipramine (10). Because the instantaneous extraction of an intravascular solute is dependent on the local flow, a larger fraction is extracted at low flows than at high flows, but less total solute is delivered and extracted than in high-flow regions.
However, when the heterogeneity is great, low-flow regions show preferentially late arrival times at the outflow (a not unexpected situation), and the extraction by the tissue is very high, the fitting of multiple sets of indicator dilution curves requires further refinement of the model of the vascular arrangements. The fitting requires the recognition of a hierarchy of arterial and venous vessels. Instead of considering the arterial inflow to consist of one rather dispersive artery, it appears more appropriate to consider a more complex system. The first large artery has relative dispersion of about 0.2, as described for human arteries (1). It leads to a group of smaller arteries of differing flows, all having the same relative dispersion but various mean transit times. These supply the capillary-tissue units, whose flows are defined by the microsphere distributions. The venous system is configured similarly to the arterial system.
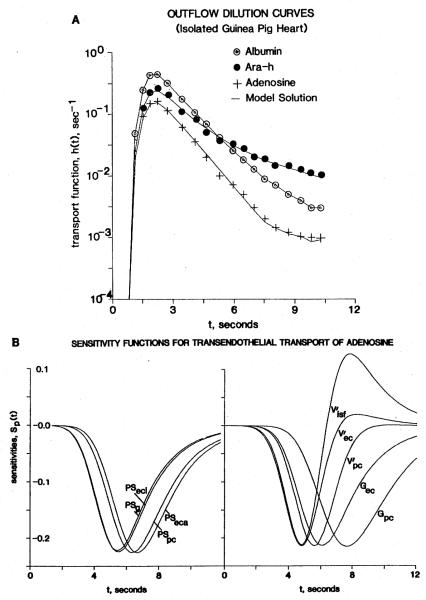
An example of how this configuration is used for analysis of adenosine transport across ECs follows. Coronary sinus outflow dilution curves for albumin (the intravascular reference), 9-β-d-arabinofuranosylhypoxanthine (araH, an inert, nontransported, nonmetabolized analog to adenosine), and adenosine itself are accurately fitted with the multicomponent model, as illustrated in Fig. 1A. The experimental data, composed of the multiple-indicator dilution curves for albumin, araH, and adenosine, and the density functions of regional flows, proved a demanding test of the form of the model. The main difficulty has not been accounting for the ECs: the capillary-tissue exchange modeling and the heterogeneity of capillary flows can be well described but parameters cannot be adjusted to fit these data. Achieving good fits of model to data has required taking more details of intravascular largevessel transport into account, as described above. In addition, the degree of heterogeneity must be accounted for properly. If narrower ranges of heterogeneity are used than those defined by the microsphere deposition densities, then the tail of the model curve for adenosine tends to be higher than the actual dilution curve. To obtain a compensatory reduction in the height of the tail of the model curve, it would be necessary to artifactually and erroneously increase the parameter values for cellular uptake and for the intracellular volumes of distribution.
Figure 1.
A) Coronary outflow dilution curves obtained from an isolated perfused guinea pig heart after a pulse injection of [14C]adenosine and two reference tracers, 131I-labeled albumin (remaining intravascular) and araH (remaining extracellular), at a flow Fs of 8.0 ml/(g · min). The model solutions (continuous lines) fitted to the data (symbols) describe the flow heterogeneity (dispersion relative to the mean = 46%, approximated by using seven parallel paths), the permeation of adenosine and araH through clefts between ECs (PSg = 2.27 ml/(g · min)), and entry into interstitial space ( = 0.2 ml/g). Other parameters for adenosine are: endothelial luminal surface PSecl = 6.0 ml/(g · min); abluminal surface PSeca = 41 ml/(g · min); intraendothelial consumption Gec = 116 ml/(g · min); parenchymal cell (myocyte) PSpc = 4.7 ml/(g · min); volume = 0.3 ml/g; and myocyte consumption Gpc = 4.8 ml/g. B) Sensitivity functions for the parameters. The following scaling factors multiplied the Sp(t) values for the illustration: for PSg 0.5, PSecl 0.45, PSeca 177.5, PSpc 47.0, 81.0, 275.0, 8200.0, Gec 210.0, Gpc 2550.0.
Figure 1B shows sensitivity functions for each of the conductances and volumes within the capillary-tissue exchange unit. The symbols are those defined by Bassingthwaighte et al. (2) as follows: PSg is the capillary permeability-surface area product for the clefts or gaps between ECs, PSecl is that for the luminal surface of the ECs, and PSeca is that for the abluminal or basement membrane surface. The total conductance from the luminal side of the capillary wall is therefore PSc = PSg + PSecl, this sum governing the initial instantaneous extractions. Because the sensitivities to PSg and PSecl are indistinguishable, PSg for araH set the value for PSg for adenosine (the two have the same molecular weight) but PSecl = 0 for araH. The volumes of plasma, interstitial fluid (ISF), EC, and parenchymal cell spaces are indicated by the V’s with subscripts isf, ec, and pc. Intracellular consumption is expressed as a first-order reaction for a tracer in the presence of steady-state concentrations of the nontracer solutes governing the reaction rates, which is a clearance G (and appropriate subscript) with the units milliliters per gram per minute (the same units as for flow, or PS). The sensitivities are highest to those parameters that are close to the vascular space, but lower to those that are farther away. This is to say that PSpc and Gpc are not so well revealed because other features (e.g., PSg and V′isf) limit the exchange between the effluent blood and the sarcolemma (for PSpc) or the intracellular reaction Gpc. Thus the sensitivity to Gpc or V′pc is considerably less than that for PSg or PSecl. A factor to be considered is that the sensitivity to any particular parameter is dependent on the settings of other values. For example, when Gec or V′ec is high, the sensitivity to PSeca diminishes. Sensitivity to PSeca is also reduced by a high value of Gec: the amount of tracer passing through ECs to the interstitium is reduced by uptake as nucleotide within ECs. Dilution in a large endothelial volume of distribution V′ec, as if the membrane transport process were highly concentrating within the ECs, has a similar effect; however, experiments by others suggest that free adenosine is not concentrated in cells, but merely equilibrated.
PROPOSED UTILITY OF MODELS INCORPORATING ECs
The first use is simply to estimate capillary permeability-surface area products and to distinguish between the rate of permeation through the clefts compared to that through the EC luminal surface. This is what we have accomplished thus far.
A second is to determine the mechanism of the transendothelial permeation. For adenosine this route of permeation is blocked by the presence of dipyridamole, which indicates a competition by the drug for the transport site. In the presence of 10 μM dipyridamole, the adenosine outflow dilution curve becomes almost exactly superimposed on that for the araH, which demonstrates complete block of the transporter. By performing a set of experiments at various concentrations of dipyridamole, the fractional reduction in number of transport sites available to the tracer adenosine can be estimated. A curve of PSecl vs. concentration of blocker there by provides an estimate of KI, for the blocker; this can be done for a range of non tracer adenosine concentrations as well as for any competitor. The shape of the resultant curve is an indication of the mechanism; for example, a curve more steep in its central portion than that for a Michaelis-Menten relationship would suggest that the number of molecules per transport site is greater than one.
A third and most important use of such modeling is to incorporate the values obtained from the tracer exchange studies into models of metabolic and regulatory systems. By using sets of similar models for adenosine, inosine, hypoxanthine, and uric acid, and obtaining data on each of these substances in the venous outflow (within the ISF and the total within the tissue itself), one can characterize with much greater precision the metabolism of adenosine. For this to be applied to the in vivo situation, data on deamination rates at various concentrations of diaminase and uptake into red cells should be brought into the modeling as well. The role of adenosine as a controller of the state of vasodilatation (6, 9) can be examined further with such models. One can use the numbers obtained via tracer experiments to predict the estimates of interstitial or lymphatic (or pericardial sac) concentrations of adenosine after an increase in intracellular production rate. For example, it is clear that the capillary barrier is a substantial barrier to adenosine washout from the interstitium, so that an increase in adenosine production after inotropic stimulation would result in an interstitial concentration considerably higher than that in the capillary effluent. This would minimize the loss of adenine nucleotide from the heart and would serve as the first component of a system for adenosine salvaging. Depending on the magnitude of this difference, and on the role of the antiluminal surface of the ECs in adenosine transport, it may be that interstitial adenosine reaches levels not predicted from the capillary-venous outflow, and which, as suggested by Hanley et al. (8), would be high enough to relax smooth muscle cells.
A particular area of future study must be in the kinetics of exchange of the adenine base among adenosine, inosine, hypoxanthine, and adenine nucleotides. The indicator dilution technique has the potential to aid in the elucidation of these processes in vivo. However, the most important data with those with not only outflow information but also measurements of the tissue concentrations of the individual products or metabolites as a function of time. Only by combinations of techniques will the normal in vivo kinetics become well understood.
SUMMARY
A capillary exchange model for adenosine is proposed. Along an axially distributed unit, it accounts for exchanges among blood, endothelial cells, interstitium, and parenchymal cells. For the modeling of indicator dilution curves a carefully constructed model describing the heterogeneity of transport through these units and the larger conduit vessels has required substantial evolution. The model is regarded as potentially useful for understanding adenosine metabolism and the regulation of its concentrations in the separate spaces in the myocardium.
Acknowledgments
The efforts of Jean-Vi Lenthe and Geraldine Crooker in the preparation of this manuscript and of Marta Chaloupka in the preparation of the figure are greatly appreciated.
Footnotes
From the Symposium Capillary Endothelium: A Metabolic Barrier for Solute Transport presented by The American Physiological Society at the 68th Annual Meeting of the Federation of American Societies for Experimental Biology, St. Louis, Missouri, April 3, 1984. Accepted for publication September 13, 1984.
Research supported by National Institutes of Health grants HL 19139 and RR 01243.
REFERENCES
- 1.Bassingthwaighte JB. Plasma indicator dispersion in arteries of the human leg. Circ. Res. 1966;19:332–346. doi: 10.1161/01.res.19.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassingthwaighte JB, Chaloupka M, Wang CY. Transport by endothelial cells in vivo: model analysis from indicator dilution after single transcapillary passage. Federation Proc. 1983;42:580. (abstr.) [Google Scholar]
- 3.Bassingthwaighte JB, Goresky CA. Modeling in the analysis of solute and water exchange in the microvasculature. In: Renkin EM, Michel CC, editors. Handbook of physiology, Section 2, The cardiovascular system, Vol. IV, Microcirculation, Part 1. Am. Physiol. Soc.; Bethesda: 1984. pp. 549–626. [Google Scholar]
- 4.Bassingthwaighte JB, Knopp TJ, Hazelrig JB. A concurrent flow model for capillary-tissue exchanges. In: Crone C, Lassen NA, editors. Capillary permeability. Munksgaard; Copenhagen: 1970. pp. 60–80. [Google Scholar]
- 5.Bassingthwaighte JB, Lenhoff AM, Stephenson JL. A slidingelement algorithm for rapid solution of spatially distributed convection-permeation models. Biophys. J. 1984;45:175a. [Google Scholar]
- 6.Feigl EO. Coronary physiology. Physiol. Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher JE. Mathematical modeling of the microcirculation. Math. Biosci. 1978;38:159–202. [Google Scholar]
- 8.Hanley F, Messina LM, Baer RW, Uhlig PN, Hoffman JIE. Direct measurement of left ventricular interstitial adenosine. Am. J. Physiol. 1983;245:H327–H335. doi: 10.1152/ajpheart.1983.245.2.H327. [DOI] [PubMed] [Google Scholar]
- 9.Knabb RM, Ely SW, Bacchus AN, Rubio R, Berne RM. Consistent parallel relationships among myocardial oxygen consumption, coronary blood flow, and pericardial infusate adenosine concentration with various interventions and β-blockade in the dog. Circ. Res. 1983;53:33–41. doi: 10.1161/01.res.53.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Little SE, Bassingthwaighte JB. Plasma-soluble marker for intraorgan regional flows. Am. J. Physiol. 1983;245:H707–H712. doi: 10.1152/ajpheart.1983.245.4.H707. [DOI] [PMC free article] [PubMed] [Google Scholar]