The average leukocyte telomere length of obese children is 23.9% shorter than that of normal weight children of a similar age.
Abstract
Context:
Obesity in adults is associated with shorter mean leukocyte telomere length (LTL), a marker of biological age that is also associated with age-related conditions including cardiovascular disease and type 2 diabetes. However, studies of childhood obesity and LTL have proved inconclusive.
Objective:
The objective of the study was to clarify the relationship between telomere length and childhood obesity by measuring the average LTL in a large case-control cohort.
Participants and Methods:
LTL was measured in 793 French children aged 2–17 yr (471 with early onset obesity and 322 nonobese controls) using multiplex quantitative real-time PCR. The average LTL in the two groups was compared, and the relationships between telomere length and selected anthropometric and biochemical measurements were examined.
Results:
Obese children had a mean LTL that was 23.9% shorter than that of nonobese children (P < 0.0001). Telomere length was inversely associated with age (R = −0.17, P = 0.002 in controls; R = −0.15, P = 0.001 in cases), log weight (R= −0.13, P = 0.017 in controls; R = −0.16, P = 0.0004 in cases), and height (R = −0.15, P = 0.008 in controls; R = −0.17, P = 0.0002 in cases). The mean LTL of girls and boys was not significantly different in either the cases or controls or in the group overall.
Conclusion:
Obese girls and boys have significantly shorter leukocyte telomeres than their nonobese counterparts, a finding that highlights a potentially deleterious impact of early onset obesity on future health.
Telomeres are DNA-protein complexes found at the ends of linear chromosomes, which protect chromosome ends from degradation and prevent them from being targeted by cellular DNA damage response systems. In vertebrates, each telomere is composed of variable numbers of the tandem repeat sequence (TTAGGG)n, extending to 10–15 kb in humans, associated with a protein complex (1). Telomere length is highly variable between individuals, reflecting both variation in telomere length at birth (2) and in differences in telomere attrition rate throughout life (3, 4).
In the majority of somatic cells, telomeres become progressively shorter with each cell division, a process exacerbated by oxidative stress (5, 6). An additional factor thought to be responsible for telomere attrition in leukocytes is inflammation because it increases turnover of hematopoietic stem cells (7). Short telomeres in adults are associated with cardiovascular disease (8, 9), type 2 diabetes (10–13), insulin resistance (14), impaired glucose tolerance (15), and hypertension (16). Although the causal direction of these relationships remains to be determined (17, 18), short telomeres are nonetheless a well-established marker for a range of age-related conditions.
The prevalence of obesity is rising rapidly throughout the world, with the increase in childhood obesity a particular cause for concern (19). This sharp rise is attributed to the sedentary lifestyle and high-calorie diet adopted recently by many populations. However, both common and rare genetic variants sensitize particular individuals to this obesogenic environment, predisposing to obesity (20–22).
In studies of adult subjects, shorter leukocyte telomere length (LTL) has been associated with obesity as well as smoking and chronic psychological stress (4, 23–26), suggesting that long-term exposure to a deleterious environment exacerbates telomere attrition. Indeed, there is evidence from a longitudinal study of LTL in overweight and obese women that the duration of obesity is a more important factor than weight gain or obesity status per se (26). Furthermore, weight loss is associated with longer telomeres in the rectal mucosa of obese men, although it is not known whether this association is reflected in leukocyte telomeres (27).
In contrast to adults, initial small-scale studies of telomere length and childhood obesity have produced conflicting results (28, 29). We therefore sought to clarify the relationship between LTL and early-onset obesity in a large (n = 793) case-control study of French children.
Subjects and Methods
Cohorts
Both cases and control subjects were participants in a genome-wide association study to identify loci associated with early-onset obesity (21), in which the threshold for childhood obesity was set as the 97th age- and gender-specific percentile of body mass index (BMI) from a French reference population (30). Control children were selected as having a BMI less than the 90th percentile for gender and age, the threshold for being overweight (31).
Informed consent from all participants and ethical approval were obtained as detailed previously (32, 33). Genomic leukocyte DNA was extracted from peripheral blood samples using the salting-out method (34) for the controls, and using PURE-GENE D50K DNA isolation kits (Gentra Systems, Minneapolis, MN) for the cases. In the controls, serum levels of total cholesterol and fasting glucose were enzymatically determined using the AU 640 analyzer (Olympus, High Wycombe, UK), and serum concentrations of high-density lipoprotein (HDL)-cholesterol were measured using a COBAS-Mira analyzer (Roche Diagnostics, Rotkreuz, Switzerland). Fasting glucose levels in the cases were measured using the glucose oxidase procedure. For both cases and controls, low-density lipoprotein-cholesterol concentrations were calculated using the Friedewald formula (35). In addition to the anthropometric and biochemical parameters listed in Table 1, additional data for body fat content, age of onset of obesity, and eating behaviors were available for 217 of the cases.
Table 1.
Characteristics of the study cohorts
| Nonobese (n = 322, 163 males) | Obese (n = 471, 218 males) | P | |
|---|---|---|---|
| Age (yr) | 11.91 ± 2.26 | 11.06 ± 3.12 | <0.0001 |
| Height (cm) | 149.27 ± 13.53 | 152.30 ± 16.93 | 0.004 |
| Weight (kg) | 39.98 ± 11.02 | 71.28 ± 26.79 | <0.0001 |
| BMI Z-score | −0.12 ± 0.97 | 4.24 ± 1.16 | <0.0001 |
| Systolic blood pressure (mm Hg) | 109.61 ± 9.46 | 114.20 ± 14.50 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 54.38 ± 9.92 | 68.44 ± 11.33 | <0.0001 |
| Total cholesterol (mmol/liter) | 4.87 ± 0.84 | 4.47 ± 0.80 | <0.0001 |
| HDL-cholesterol (mmol/liter) | 1.48 ± 0.37 | 1.23 ± 0.28 | <0.0001 |
| Fasting glucose (mmol/liter) | 4.90 ± 0.38 | 4.92 ± 0.48 | 0.11 |
Anthropometric and biochemical measurements are given as mean ± sd. Measurements for gender, age, height, weight, and BMI Z-score were available for all participants. Data on systolic and diastolic blood pressure were available for all nonobese and 370 of 471 obese subjects; data on fasting glucose were available for all nonobese and 418 of 471 obese subjects; data on HDL-cholesterol levels were available for all nonobese and 410 of 471 obese subjects and data on total cholesterol for all nonobese and 412 of 471 obese subjects.
Quantitative PCR
The mean LTL in genomic DNA samples prepared from peripheral blood lymphocytes was measured using multiplex quantitative real-time PCR (36). Duplicate quantitative real-time PCR reactions were carried out in a total volume of 12.5 μl, using approximately 15 ng of template DNA, with final concentrations of 1× iQ Sybr Green supermix (Bio-Rad Laboratories, Hemel Hempstead, UK), 900 nm of telg and telc primers, and 500 nm of single-copy gene primers (hbgd and hbgu) (for primer sequences, see Ref. 36). All the PCRs were carried out in white 384-well plates on a CFX384 real-time PCR detection system (Bio-Rad Laboratories). Five serial dilutions of a reference sample (leukocyte DNA from an adult female) spanning 5–50 ng were run in triplicate on each plate.
After amplification and data collection, the CFX manager software (Bio-Rad Laboratories) was used to generate standard curves from the reference DNA dilutions, one for the telomere signal and one for the single-copy gene signal. The telomere amplicon signal (T) to single-copy gene control amplicon (S) ratios for each sample were calculated as T, the amount of reference DNA that matched the experimental sample for copy number of the telomere template, divided by S, that which matched the copy number of the single-copy gene template. The mean coefficient of variation for the T/S measurements of duplicate samples was 4.5%.
Statistical analysis
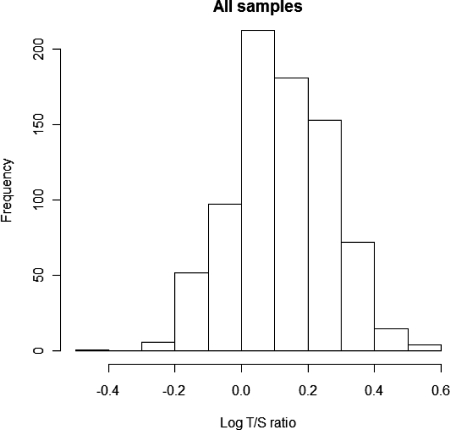
All telomere measurements (obtained as T/S ratios) were log transformed before statistical analysis to ensure normal distribution of the data, as assessed both by visual inspection of a histogram of the plotted values, and the Shapiro-Wilk test for normality (Fig. 1). Unpaired two-tailed t tests were used to evaluate differences in telomere length between groups, using homoscedastic tests in which the variances were not significantly different (as determined by performing an F test). Pearson's correlation was used to test for association with individual variables, which were also log transformed when necessary. Multiple linear regression with stepwise removal of nonsignificant explanatory variables was carried out to investigate the relative contributions of selected variables to LTL. All analyses and plots were carried out using version 2.10.1 of the R statistical package (cran.r-project.org).
Fig. 1.
Distribution of LTL for all samples (n = 793), expressed as log T/S values. Log T/S values are normally distributed (Shapiro-Wilk test for normality, P = 0.65).
Results
As expected, comparison of selected anthropometric and biochemical characteristics revealed highly significant differences between the cases and controls for several traits associated with adiposity, including circulating lipid levels and blood pressure (Table 1). There was no significant difference between the cohorts in fasting glucose levels which was therefore not included in later analyses.
Average LTL was ascertained in all 793 subjects using multiplex quantitative PCR (36). The measurements obtained using this method are expressed as T/S ratios, reflecting the telomere signal (T) relative to a single-copy gene (S), normalized to a single reference individual.
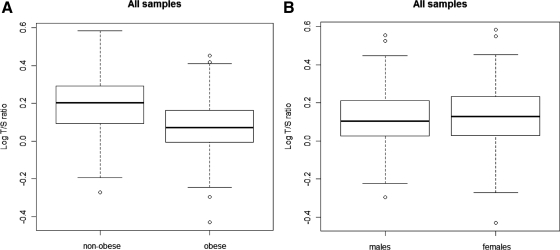
Statistical analyses were carried out after log transformation of all T/S ratios to achieve a normal distribution (Fig. 1). The mean log T/S ratio in the obese children (0.072, se = 0.006) was significantly less than that in nonobese children (0.191, se 0.008, P < 0.0001) (Fig. 2A). This difference equates to a 23.9% decrease in mean T/S ratio in obese compared with nonobese children.
Fig. 2.
Comparison of LTL distribution in nonobese and obese subjects (A) and in male and female subjects (B). The box plots indicate the maximum and minimum, the lower and upper quartiles, and the median log T/S ratio value in each group. The mean log T/S ratio values of each group are given below. A, LTL (expressed as log T/S ratio) is significantly shorter in obese compared with nonobese study subjects. The mean log T/S ratio in all nonobese samples is 0.191 (se 0.008), the mean log T/S ratio in all obese samples is 0.072 (se 0.006, P < 0.0001). B, LTL (expressed as the log T/S ratio) is not significantly different in male study subjects compared with female study subjects (the mean log T/S ratio of males = 0.113, se 0.007; the mean log T/S ratio of females = 0.126, se 0.007, P = 0.19).
To investigate a possible gender effect, girls and boys were next analyzed separately. The mean log T/S ratio in obese girls (0.081, se 0.008) was significantly shorter than that in nonobese girls (0.199, se 0.012, P < 0.0001), a difference that equates to a 23.8% decrease in the T/S ratio. Similarly, the mean log T/S ratio in obese boys (0.061, se 0.008) was significantly shorter than in nonobese boys (0.183, se 0.010, P < 0.0001), a difference that equates to a 24.5% decrease in the T/S ratio. The overall mean log T/S ratio in all girls (0.126, se 0.007) was slightly greater than that in all boys (0.113, se 0.007), but this difference was not statistically significant (P = 0.19) (Fig. 2B).
To investigate the potential influence of pubertal stage on leukocyte telomere length, the analysis was carried out separately in those aged younger and older than 9 yr. In the children older than 9 yr, the mean log T/S ratio in the obese children (0.064, se 0.007, n = 364) was significantly shorter than that in nonobese children (0.187, se 0.008, n = 291, P < 0.0001), a difference that equates to a 24.7% decrease in the T/S ratio. The results for the children younger than 9 yr were very similar to those obtained in the older children: the mean log T/S ratio in the obese children (0.100, se 0.011, n = 109) was again significantly shorter than that in nonobese children (0.227, se 0.028, n = 31, P = 0.0002), a difference that equates to a 25.4% decrease in the T/S ratio. Separate analysis of girls and boys aged under/over 9 yr revealed no statistically significant gender differences in these subgroups.
Univariate analyses were performed, within each of the obese and nonobese groups separately, to investigate the potential contribution of selected variables to variation in LTL (Table 2). Explanatory variables were log transformed where necessary to ensure normality. As expected, significant inverse associations between the log T/S ratio and age were identified in both the cases and controls. The log T/S ratio was also inversely associated with height and weight in both groups.
Table 2.
Univariate analysis of selected biochemical and anthropometric variables
| Variable | Nonobese |
Obese |
||
|---|---|---|---|---|
| R | P value | R | P value | |
| Age | −0.17 | 0.002 | −0.15 | 0.001 |
| Height | −0.15 | 0.008 | −0.17 | 0.0002 |
| Log weight | −0.13 | 0.017 | −0.16 | 0.0004 |
| BMI Z-score | 0.03 | 0.619 | −0.02 | 0.723 |
| Log systolic blood pressure | −0.01 | 0.82 | −0.08 | 0.131 |
| Log diastolic blood pressure | −0.01 | 0.849 | 0.02 | 0.739 |
| Log cholesterol | 0.10 | 0.068 | −0.07 | 0.18 |
| Log HDL-cholesterol | 0.04 | 0.499 | 0.09 | 0.071 |
Significant P values shown in bold, whereas those approaching significance are in italics. In both nonobese and obese children, age, height, and log weight are inversely associated with mean telomere length (log T/S ratio). Borderline significant associations are also detected between log T/S ratio and log cholesterol levels in the controls and log HDL-cholesterol levels in the cases.
The high level of multicolinearity between age, height, and log weight meant that it was not possible to determine their relative contributions to telomere length. Multivariate analysis using all these variables, with LTL as the dependent variable, resulted in a model with age as the sole explanatory variable: adjusting log T/S values for any of age, height, or log weight abolished associations between LTL and the other two variables. The effect sizes of each of these variables on LTL were not significantly different in cases compared with controls. No improvement in the model was achieved by including, for obese subjects, time since onset of obesity as an additional parameter, because this variable showed the same multicolinearity with age, height, and log weight.
Although not quite reaching nominal statistical significance, there were discernible trends between longer telomere length and increasing total cholesterol in the controls and with increasing HDL-cholesterol in the cases. No associations between LTL and BMI Z-score or either systolic or diastolic blood pressure were detected in either cohort (Table 2).
Analysis of additional obesity-related traits in a subset of 217 cases for which data on body fat content, age of onset of obesity, and eating behaviors revealed no significant associations with LTL that were separate from the effects of weight or height.
Discussion
We have demonstrated a highly significant inverse association between early-onset obesity and mean LTL in both boys and girls in a large case-control study of French children. Mean LTL in the obese cases is almost 25% less than in the controls, and LTL declines with increasing age, weight, and height in both groups, with comparable effect sizes for all three variables in both cases and controls.
In adulthood, shorter leukocyte telomeres are associated with BMI in women (24) and waist to hip ratio in both men and women (4). Furthermore, adipocytes from obese adults have telomeres approximately 17% shorter than those in adipocytes from nonobese adults (37). The mechanisms underlying the association between obesity and short telomeres in adults are also unknown, although an increase in oxidative stress and inflammation have both been suggested as possible explanations (23).
Previous studies investigating telomere length and childhood obesity proved inconclusive. Zannolli et al. (28) found no difference in LTL between obese and nonobese individuals in 53 Italian children, whereas Al-Attas et al. (29) investigated 148 Arab children and found that mean telomere length was shorter in obese boys compared with lean boys but found no difference between obese and lean girls. It is possible that the disparity between our findings and those of previous workers is due to undetermined genetic or nongenetic differences that exist between the populations examined. However, it is more likely that the increased statistical power afforded by the larger size of our study (n = 793), and the severe phenotype of the cases (above the 97th age and sex specific percentile of BMI), allowed us to detect a highly significant association between telomere length and obesity in both boys and girls.
Interestingly, we found no significant difference between mean LTL in girls and boys in our study, which agrees with a previous report on LTL in newborns (2). This implies that the difference observed in adulthood, men having shorter mean leukocyte telomeres than women (38), may be due to factors that exert their influence after, rather than during, early childhood.
Despite evidence that childhood obesity contributes to earlier onset of puberty (39), we found no evidence that this factor influenced our results. In the absence of data on pubertal stage, we subdivided our cohort into those younger and older than 9 yr of age. We found that shorter LTL was associated with obesity in both groups: in the children younger than 9 yr, mean telomere length in the obese children was 25.4% shorter than in the controls, whereas in the children older than 9 yr, it was 24.7% shorter.
In agreement with numerous previous studies in both adults and children, we found an inverse association between age and telomere length, within each of the case and control cohorts. However, we also detected inverse associations between LTL and height and weight. All of these variables are indicators of body size in childhood, and although it was not possible to determine the relative contribution of each, this supports the hypothesis that telomere length in childhood chronicles the expansion of the hematopoietic stem and progenitor cell populations, which in turn reflect body size (40).
The highly significant difference between telomere length in obese and nonobese children may be partly or wholly due to a general overgrowth compared with their normal-weight peers of a similar chronological age. Although the mean age of the obese children was almost 1 yr younger than the controls, they were somewhat taller and their mean weight was almost 80% greater (Table 1). Thus, the shorter mean LTL of the obese children compared with the nonobese children may simply be due to an increased rate of hematopoietic stem cell turnover. Consistent with this is the finding that no association was observed in either cohort between BMI Z-score and telomere length, indicating that the correlation is with absolute body size, rather than size relative to age- and gender-matched peers.
Although the difference in body size is the most striking phenotypic difference between our case and control cohorts, the observed association with telomere length may nonetheless reflect some other aspect of obesity, such as circulating lipid levels, or inflammation. There is evidence from a longitudinal study in adults that telomere length is positively associated with HDL-cholesterol levels (3). The authors propose that this association might be explained by the antioxidant and antiinflammatory effects of HDL-cholesterol, both of which might slow leukocyte telomere attrition rates. In our study, levels of HDL-cholesterol were significantly lower in the obese, compared with the nonobese children, and LTL in obese children showed a discernible (although not quite significant) positive correlation with HDL-cholesterol, each of which is consistent with this hypothesis. Conversely, another potential explanation for the shorter telomeres in obese children is inflammation (7), which would increase leukocyte progenitor cell turnover.
Although shorter telomeres are associated with hypertension in adults (16), we found no association between blood pressure and LTL in either the nonobese or obese cohort. This suggests that this trait is only a significant predictor of telomere length in adulthood and not childhood. However, because ours was a case-control study designed to test for differences between normal-weight and obese children, it may have been underpowered to detect associations between LTL and continuous traits such as blood pressure and circulating lipids.
In conclusion, we have demonstrated that obese children have telomeres that are substantially shorter than those of nonobese controls of comparable age. Further population-based studies in young cohorts are required to identify the obesity-related traits that explain this observation and additionally to investigate the extent to which this difference in telomere length extends into adulthood. The fact that obese children have an apparent biological age that is significantly greater than their chronological age highlights the importance of intervention and also support for these individuals at the earliest opportunity to minimize their risk of future disease.
Acknowledgments
J.L.B. is supported by Wellcome Trust fellowship Grant WT088431MA.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- HDL
- high-density lipoprotein
- LTL
- leukocyte telomere length
- S
- single-copy gene control amplicon
- T
- telomere amplicon signal.
References
- 1. de Lange T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110 [DOI] [PubMed] [Google Scholar]
- 2. Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. 2002. Telomere length in the newborn. Pediatr Res 52:377–381 [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS, Aviv A. 2009. Leukocyte telomere length is associated with HDL cholesterol levels: the Bogalusa heart study. Atherosclerosis 205:620–625 [DOI] [PubMed] [Google Scholar]
- 4. Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. 2010. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the Heart and Soul Study. PLoS ONE 5:e8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Zglinicki T. 2002. Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344 [DOI] [PubMed] [Google Scholar]
- 6. Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. 2004. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117:2417–2426 [DOI] [PubMed] [Google Scholar]
- 7. Aviv A, Valdes AM, Spector TD. 2006. Human telomere biology: the pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol 35:1424–1429 [DOI] [PubMed] [Google Scholar]
- 8. Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. 2007. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 165:14–21 [DOI] [PubMed] [Google Scholar]
- 9. Brouilette SW, Moore JS., McMahon AD., Thompson JR., Ford I, Shepherd J, Packard CJ., Samani NJ. 2007. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369:107–114 [DOI] [PubMed] [Google Scholar]
- 10. Adaikalakoteswari A, Balasubramanyam M, Mohan V. 2005. Telomere shortening occurs in Asian Indian type 2 diabetic patients. Diabet Med 22:1151–1156 [DOI] [PubMed] [Google Scholar]
- 11. Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. 2006. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 29:283–289 [DOI] [PubMed] [Google Scholar]
- 12. Boehm BO, Möller P, Högel J, Winkelmann BR, Renner W, Rosinger S, Seelhorst U, Wellnitz B, März W, Melzner J, Brüderlein S. 2008. Lymphocytes of type 2 diabetic women carry a high load of stable chromosomal aberrations. Diabetes 57:2950–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salpea KD, Talmud PJ, Cooper JA, Maubaret CG, Stephens JW, Abelak K, Humphries SE. 2010. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 209:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. 2005. Rise in insulin resistance is associated with escalated telomere attrition. Circulation 111:2171–2177 [DOI] [PubMed] [Google Scholar]
- 15. Adaikalakoteswari A, Balasubramanyam M, Ravikumar R, Deepa R, Mohan V. 2007. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis 195:83–89 [DOI] [PubMed] [Google Scholar]
- 16. Yang Z, Huang X, Jiang H, Zhang Y, Liu H, Qin C, Eisner GM, Jose PA, Jose P, Rudolph L, Ju Z. 2009. Short telomeres and prognosis of hypertension in a Chinese population. Hypertension 53:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Testa R, Ceriello A. 2007. Pathogenetic loop between diabetes and cell senescence. Diabetes Care 30:2974–2975 [DOI] [PubMed] [Google Scholar]
- 18. Aviv A. 2009. Leukocyte telomere length, hypertension, and atherosclerosis: are there potential mechanistic explanations? Hypertension 53:590–591 [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization 2006. World Health Organization: obesity and overweight fact sheet 2006. Geneva: World Health Organization [Google Scholar]
- 20. Walley AJ, Asher JE, Froguel P. 2009. The genetic contribution to non-syndromic human obesity. Nat Rev Genet 10:431–442 [DOI] [PubMed] [Google Scholar]
- 21. Meyre D, Delplanque J, Chèvre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proença C, Gaget S, Körner A, Kovacs P, Kiess W, Tichet J, Marre M, Hartikainen AL, Horber F, Potoczna N, Hercberg S, Levy-Marchal C, Pattou F, Heude B, Tauber M, McCarthy MI, Blakemore AI, Montpetit A, Polychronakos C, Weill J, Coin LJ, Asher J, Elliott P, Järvelin MR, Visvikis-Siest S, Balkau B, Sladek R, Balding D, Walley A, Dina C, Froguel P. 2009. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 41:157–159 [DOI] [PubMed] [Google Scholar]
- 22. Walters RG, Jacquemont S, Valsesia A, de Smith AJ, Martinet D, Andersson J, Falchi M, Chen F, Andrieux J, Lobbens S, Delobel B, Stutzmann F, El-Sayed Moustafa JS, Chèvre JC, Lecoeur C, Vatin V, Bouquillon S, Buxton JL, Boute O, Holder-Espinasse M, Cuisset JM, Lemaitre MP, Ambresin AE, Brioschi A, Gaillard M, Giusti V, et al. 2010. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature 463:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. 2005. Obesity, cigarette smoking, and telomere length in women. Lancet 366:662–664 [DOI] [PubMed] [Google Scholar]
- 24. Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. 2009. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet 5:e1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. 2004. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 101:17312–17315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, Sandler DP. 2009. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev 18:816–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Callaghan NJ, Clifton PM, Noakes M, Fenech M. 2009. Weight loss in obese men is associated with increased telomere length and decreased abasic sites in rectal mucosa. Rejuvenation Res 12:169–176 [DOI] [PubMed] [Google Scholar]
- 28. Zannolli R, Mohn A, Buoni S, Pietrobelli A, Messina M, Chiarelli F, Miracco C. 2008. Telomere length and obesity. Acta Paediatr 97:952–954 [DOI] [PubMed] [Google Scholar]
- 29. Al-Attas O, Al-Daghri N, Bamakhramah A, Shaun Sabico S, McTernan P, Huang TT. 2010. Telomere length in relation to insulin resistance, inflammation and obesity among Arab youth. Acta Paediatr 99:896–899 [DOI] [PubMed] [Google Scholar]
- 30. Rolland-Cachera MF, Deheger M, Bellisle F. 2001. Definition actuelle et evolution de la frequence de l‘obesite’ chez l'enfant. Cahiers de Nutrition et de Diététique 36:108 [Google Scholar]
- 31. Poskitt EM. 1995. Defining childhood obesity: the relative body mass index (BMI). Acta Paediatr 84:961–963 [DOI] [PubMed] [Google Scholar]
- 32. Visvikis-Siest S, Siest GR. 2008. The STANISLAS cohort: a 10-year follow-up of supposed healthy families. Gene-environment interactions, reference values and evaluation of biomarkers in prevention of cardiovascular diseases. Clin Chem Lab Med 46:733–747 [DOI] [PubMed] [Google Scholar]
- 33. Meyre D, Boutin P, Tounian A, Deweirder M, Aout M, Jouret B, Heude B, Weill J, Tauber M, Tounian P, Froguel P. 2005. Is Glutamate decarboxylase 2 (GAD2) a genetic link between low birth weight and subsequent development of obesity in children? J Clin Endocrinol Metab 90:2384–2390 [DOI] [PubMed] [Google Scholar]
- 34. Miller SA, Dykes DD, Polesky HF. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- 36. Cawthon RM. 2009. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moreno-Navarrete JM, Ortega F, Sabater M, Ricart W, Fernandez-Real JM. 2010. Telomere length of subcutaneous adipose tissue cells is shorter in obese and formerly obese subjects. Int J Obes 34:1345–1348 [DOI] [PubMed] [Google Scholar]
- 38. Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. 2001. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37:381–385 [DOI] [PubMed] [Google Scholar]
- 39. Aksglaede L, Juul A, Olsen LW, Sorensen TI. 2009. Age at puberty and the emerging obesity epidemic. PLoS ONE 4:e8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sidorov I, Kimura M, Yashin A, Aviv A. 2009. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol 37:514–524 [DOI] [PubMed] [Google Scholar]