Abstract
A specialized brain vasculature is key for establishing and maintaining brain interstitial fluid homeostasis, which for most amino acids (AAs) are ∼10% plasma levels. Indeed, regulation of AA homeostasis seems critical for normal central nervous system functions, and disturbances in brain levels have both direct and indirect roles in several neuropathologies. One mechanism contributing to the plasma to brain AA gradients involves polarized expression of solute carrier (SLC) family transporters on blood–brain barrier (BBB) endothelial cells. Of particular interest is the localization of sodium-dependent transporters that can actively move substrates against their concentration gradient. In this study, the in vivo endothelial membrane localization of the sodium-dependent glutamine transporters Snat3 (Slc38a3) and Snat1 (Slc38a1) was investigated in the mouse brain microvasculature using immunofluorescent colocalization with cellular markers. In addition, luminal membrane expression was probed by in vivo biotinylation. A portion of both Snat3 and Snat1 vascular expressions was localized on luminal membranes. Importantly, Snat1 expression was restricted to larger cortical microvessels, whereas Snat3 was additionally expressed on BBB capillary membranes. This differential expression of system A (Snat1) versus system N (Snat3) transporters suggests distinct roles for Snats in the cerebral vasculature and is consistent with Snat3 involvement in net transendothelial BBB AA transport.
Keywords: blood–brain barrier, brain amino-acid homeostasis, brain vasculature, glutamine, solute carrier family (SLC) transporter, system A and N
Introduction
To respond to the brain's substantial nutrient demands and to provide for the rapid and effective removal of metabolic by-products and toxins, a specialized brain capillary vasculature, known as the blood–brain barrier (BBB), has developed. The BBB forms a dynamic interface between the plasma and the brain parenchyma, and owing to the large capillary surface area, is the primary site of blood–brain exchange. Anatomically, the BBB is a polarized, selective, diffusion barrier formed by capillary endothelial cells with unique characteristics including low paracellular permeability. Exchange of solutes requires movement through the two (luminal or blood facing and abluminal or brain facing) closely apposed (separated by 200 to 500 nm) and biochemically distinct BBB endothelial membranes (Pardridge, 2005). The abluminal endothelial basement membrane is periodically, but irregularly, associated with pericytes (Engelhardt and Sorokin, 2009) and is intimately associated with a surrounding cover of astrocyte endfeet. In turn, astrocytes and to some extent capillaries are associated with neurons. Communication between the endothelium and associated cells has a part in the regulation of various BBB functions, including barrier formation and angiogenesis (Virgintino et al, 2004, 2007). The entire structure is known as the ‘neurovascular unit' (Engelhardt and Sorokin, 2009).
Amino acids (AAs), some of which serve as neurotransmitters and/or as potentially rate-limiting precursors in protein, energy, and other metabolic pathways, occupy a uniquely sensitive position in the brain milieu. Consequently, homeostatic concentrations are highly controlled, and in interstitial and cerebrospinal fluid, most AAs (except glutamine) are maintained at well below plasma levels (Hawkins et al, 2006). Glutamine, which is the most abundant circulating AA in the body, is a key constituent of nitric oxide synthesis, energy supply, and nitrogen metabolism pathways. Furthermore, central nervous system replenishment of the major neuronal excitatory and inhibitory neurotransmitters, glutamate and GABA, involves transfer of glutamine from astrocytes to neurons for conversion to glutamate (the ‘glutamate–glutamine cycle'). Thus, glutamine brain interstitial fluid levels, although approaching those of plasma, are nonetheless regulated (McKenna, 2007).
In general, AA movement across membranes is mediated by specialized transporter proteins belonging to several families of the solute carrier (SLC) gene series (Hediger et al, 2004). Homeostatic brain AA concentrations are believed to be actively maintained to a large extent by polarized BBB transendothelial transport by SLC transporters (Engelhardt and Sorokin, 2009). We along with others hypothesize that asymmetric expression of AA transporters with different transport mechanisms and substrate selectivities determine the net transendothelial flux. However, until recently, it was only possible to characterize BBB AA transport in terms of transport ‘systems' described functionally by substrate and inhibitor affinities and ion dependencies. From such data, several hypothetical schemes for the expression of transport systems on luminal and abluminal BBB membranes have been suggested, e.g., the restriction of sodium (Na+)-dependent AA transport systems to abluminal membranes to operate solely for brain AA efflux (Hawkins et al, 2006). Nowadays, with expression profiling tools, molecular identity and localization of BBB transporters can be determined (Pardridge, 2005). Therefore, to elucidate the role of AA transporters in the BBB, we previously screened gene expression in highly pure mouse brain endothelial cell RNA from freshly isolated versus briefly cultured endothelial cells. We identified a subset of AA transporters with high in vivo mRNA levels that were strongly decreased by culture and were suggested to be involved in the differentiated BBB transport function (4F2hc/Slc3a2, Lat1/Slc7a5, Snat3/Slc38a3, Snat5/Slc38a5, and Cat1/Slc7a1). In contrast, culture induced a robust increase in Snat1/Slc38a1, y+Lat2/Slc7a6, and xCT/Slc7a11 mRNAs, thus indicating their potential involvement in supplying AAs for endogenous cellular metabolism (Lyck et al, 2009).
As for other barrier tissues, brain capillaries exhibit a polarized distribution of luminal and abluminal membrane proteins. Thus, a key step in delineating the mechanisms of BBB active control of brain AAs is to determine the endothelial membrane (blood/luminal or brain/abluminal) to which plasma AA transporters are localized. For example, the endothelial glucose transporter Glut1 (Slc2a1) is expressed on both membranes, but at a ∼4 times higher level in abluminal mouse brain cortical BBB membranes (Farrell and Pardridge, 1991). In this study, we determined the membrane distribution of selected AA transporters. The distribution of Na+-dependent transporters, which use the Na+ electrochemical gradient to actively transport substrates potentially against their concentration gradient, is of particular interest. Therefore, we focused on two Na+-dependent AA transporters (namely Snat1/Slc38a1 and Snat3/Slc38a3) hypothesized to have different roles in endothelial nutrient supply versus transendothelial AA transport (Lyck et al, 2009). To investigate BBB membrane localization, we examined immunofluorescent-labeled mouse brain tissue sections by confocal microscopy focusing on endothelial cell nuclear regions where luminal and abluminal membranes can be distinguished. Furthermore, an in vivo biotinylation approach was used to specifically label proteins expressed at the vascular luminal membrane surface (Roberts et al, 2008; Roesli et al, 2008). In summary, using both methods, we detected the protein expression of Snat1 and Snat3 transporters in the cerebral vasculature, with significant levels of Snat3, but not of Snat1, on BBB membranes.
Materials and methods
Animals
Female C57BL/6JOlaHsd mice were purchased from Harlan Laboratories B.V. (Venray, The Netherlands). Snat3 wild-type and knockout mice (Q263X Hybrid C3H-C57BL/6J; N-ethyl-N-nitrosurea-mutagenesis) were produced by Ingenium Pharmaceuticals AG (Martinsried, Germany) and kindly provided by CA Wagner (University of Zurich, Switzerland; EUGINDAT, EU FP6). All animal procedures were carried out according to the Swiss Animal Welfare laws and approved by the Kantonales Veterinäramt Zurich (license 119/2008).
Antibodies
The primary antibodies used were mouse-anti-glucose transporter 1 (Glut1), monoclonal ab40084 (Abcam, Cambridge, MA, USA), chicken-anti-laminin polyclonal ab14055 (Abcam), rabbit-anti-laminin polyclonal L9393 (Sigma-Aldrich, St Louis, MO, USA), rat-anti-transferrin receptor (CD71) monoclonal NB100-64979 (Novus Biologicals, Cambridge, UK), goat-anti-CD98 (4F2hc) polyclonal sc-7094 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rat-anti-CD98 (4F2hc) monoclonal 557479 (BD Biosciences, Allschwil, Switzerland); rabbit-anti-L-type AA transporter 1 (LAT1) polyclonal KE026 (CosmoBio Co., Tokyo, Japan); and mouse-anti-β-actin monoclonal A5316 (Sigma-Aldrich). Polyclonal antisera against mouse Snat1, human SNAT3, and human LAT1 were raised in rabbits using synthetic peptides of the following epitopes: mouse Snat1: MMHFKSGLELTELQNMTVC (Abgent, Bioggio, Switzerland); human SNAT3: MEIPRQTEMVELVPNGKC (Pineda, Berlin, Germany) (Busque and Wagner, 2009; Moret et al, 2007); and human LAT1: CTVLCQKLMQVVPQET (Franca et al, 2005). The secondary antibodies used were as indicated: Alexa Fluor 488 goat anti-rabbit/rat IgG (Invitrogen, Eugene, OR, USA), DyLight 549 goat anti-chicken IgY (Jackson ImmunoResearch, Suffolk, UK), Cy5 goat anti-mouse IgG (Jackson ImmunoResearch), ECL anti-mouse IgG, horseradish peroxidase-linked whole antibody (GE Healthcare, Little Chalfont Buckinghamshire, UK), anti-rabbit IgG (Fc) AP conjugate (Promega, Madison, WI, USA), anti-goat IgG AP conjugate (Promega), and streptavidin-horseradish peroxidase conjugate (GE Healthcare).
In Vivo Biotinylation
The in vivo biotinylation protocol was based on the method published by Roberts et al (2008) and Roesli et al (2006) with minor modifications. The abdominal aorta of anesthetized 5-week-old mice was clamped, and the brain perfused through the left cardiac ventricle with prewarmed (38°C) 10% (wt/vol) dextran 40 (Invitrogen, Paisley, UK) and phosphate-buffered saline (PBS) (Sigma-Aldrich, Steinheim, Germany). Drainage was from the right atrium. Perfusion was performed at 100 mm Hg to maintain BBB integrity. For in vivo biotinylation, immediately after PBS/dextran, mice were perfused with 5 mL fresh biotinylation solution (3 mg/mL EZ-Link sulfo-NHS-LC-biotin (Thermo Scientific, Rockford, IL, USA) in PBS, 10% dextran 40), incubated for 5 minutes without flow, and then quenched with 10% dextran 40, 50 mmol/L Tris, and PBS. As a negative control (nonbiotinylated), mice were perfused without biotin. The cerebellum was removed and brains were snap frozen in liquid nitrogen.
Protein Extraction and Purification of Biotinylated Proteins
Total lysate was prepared as described previously with minor modifications by Roberts et al (2008) and Roesli et al (2006). In brief, 25 mg/mL brain tissue was homogenized in lysis buffer (2% SDS, 50 mmol/L Tris, 10 mmol/L EDTA, complete EDTA-free proteinase inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) in PBS, pH 6.8) using a disperser (Polytron 2100, Kinematica AG, Littau, Switzerland). Homogenates were sonicated (Labsonic 1510, B.Braun, Bender+Hobun, Zurich, Switzerland), incubated at 95°C for 20 minutes and centrifuged at 17,000 g for 20 minutes at 25°C. Total protein concentration was determined using BCA protein assay (Thermo Scientific). NeutrAvidin-agarose resin (Thermo Scientific, 500 μL per sample) was washed in buffer A (1% Nonidet P-40, 0.1% SDS, PBS) and lysis buffer. In all, 15 mg total protein extract (total protein lysate) was incubated with Neutravidin resin for 2 hours at room temperature with mixing. The supernatant was collected (unbound protein fraction) and the Neutravidin-agarose resin was washed with buffer A and buffer B (0.1% Nonidet P-40, 1 mol/L NaCl, PBS) and resuspended in solution C (2% SDS, 30 mmol/L biotin (Sigma-Aldrich), 50 mmol/L phosphate, 100 mmol/L NaCl, 6 mol/L urea, 2 mol/L thiourea; pH ∼12) (Rybak et al, 2004), incubated for 15 minutes at room temperature, followed by 15 minutes at 96°C. The supernatant was collected and concentrated with trichloroacetic acid (final concentration 10%) overnight at 4°C. Precipitated proteins were washed 4 times with ice-cold ethanol, lyophilized, and resuspended in Laemmli buffer (Neutravidin-bound fraction).
Gel Electrophoresis and Western Blot Analysis
For SDS-PAGE, 25% of the total Neutravidin-agarose-bound protein fraction with or without in vivo biotinylation versus 20 μg of the corresponding unbound protein fraction or of total protein lysate were analyzed by western blotting on polyvinylidene fluoride membranes (Immobilon-P, Millipore, Bedford, MA, USA). Samples from at least 3 independently treated mouse brains were isolated and analyzed on 6, 7.5, or 8.5% SDS-PAGE. Immunoblotting was performed as described previously (Romeo et al, 2006). For western blot analysis, all antibodies except anti-β-actin (1:5,000) were used at 1:500 and chemiluminescence was detected with Immobilon western chemiluminescent horseradish peroxidase or AP substrate (Millipore) using a Fuji LAS-4000 camera (Bucher Biotec, Basel, Switzerland). The same membranes used to detect SLC proteins were stripped, blocked, and reprobed with streptavidin-horseradish peroxidase, followed by anti-β-actin antibody.
Protein Deglycosylation Assay
Overall, 20 μg of total liver and brain lysates, prepared as described under the section ‘Protein extraction and purification of biotinylated proteins', and 25% of total Neutravidin-agarose-bound protein fractions with and without in vivo biotinylation, were treated with N-glycosidase F (PNGase F; EC 3.5.1.52; New England Biolabs, Beverly, MA, USA). The samples were denatured at 65°C for 15 minutes in the provided buffer containing β-mercaptoethanol and SDS, incubated for 1 h at 37°C in reaction buffer with 1% Nonidet P-40 with or without added PNGase F.
Immunohistochemistry
Localization of SLC transporters was assessed by confocal microscopy of immunofluorescent colocalization with antibodies for specific cell or tissue-type markers (specifications described under the section ‘Antibodies'). Anesthetized mice were perfused through the left cardiac ventricle with PBS, pH 7.4, followed by fixative (2% paraformaldehyde, 0.2% glutaraldehyde in PBS). The brains were harvested, postfixed for 2 h, washed overnight in PBS and then stored in 0.02% paraformaldehyde at 4°C. For staining, 20-μm-thick tissue sections were prepared using a vibrating microtome (Leica VT 1000S; Leica, Heerbrugg, Switzerland). Fluorescence immunolabeling was carried out on free-floating sections at room temperature. Sections were gently agitated in 0.5% Triton X-100, PBS for 30 minutes, blocked for 10 minutes (Protein Block Serum-Free; Dako, Carpinteria, CA, USA), and incubated with primary antibodies (1:200 dilution) overnight at 4°C. Sections were then incubated with secondary antibodies, followed by 10 minutes in 4% paraformaldehyde. Nuclear counterstaining with PO-PRO-1 iodide (1:400; Invitrogen) was performed. Sections were mounted on Polysine slides (Kindler, Freiburg, Germany) in a Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Specificity of the Snat3 antibody was assessed in knockout mice and control littermates. Secondary antibodies alone did not produce significant staining (data not shown).
Data Analysis
Stained tissue sections were viewed with a Leica TCS SP2 confocal laser scanning microscope (Leica) using a 63 × objective lens (numerical aperture of 1.4, pinhole set to 1.0 airy unit) with different zoom factors (5 to 8). A sequential scan procedure was applied during image acquisition and parameters were adjusted to use the full dynamic range of the photomultipliers. Typically, stacks of 4 to 8 images (512 × 512 pixels) were taken and analyzed at 122-nm intervals through the z axis of the section. Representative images from the examined samples were chosen for figure editing. Digital images were processed using the software Imaris (Bitplane, Zurich, Switzerland), Huygens v1.2.3., or Leica Confocal Software LAS-AF (Leica).
Results
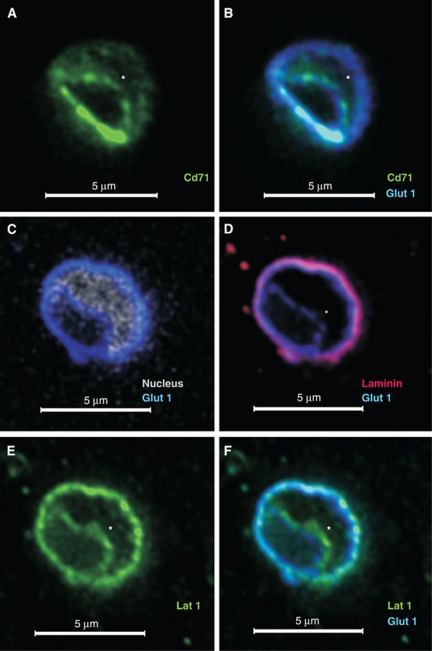
Confocal microscopy and in vivo biotinylation are complementary strategies for identification of brain microvascular luminal membrane proteins. We first confirmed that the subcellular localization of known membrane proteins could be distinguished on cortical capillaries (∼5 to 10 μm in diameter) by immunofluorescent colocalization of membrane and cellular markers. Cross-sections in which the nucleus (PO-PRO nuclear dye) separated the two endothelial membranes were examined. In brain capillaries, Glut1 is unevenly expressed on luminal and abluminal membranes (Farrell and Pardridge, 1991), whereas the transferrin receptor CD71 is restricted to the lumen (Roberts et al, 2008). As expected, staining for CD71 (Figure 1A) colocalized with luminal Glut1 (Figures 1B and 1C). Staining for the basement membrane protein laminin was used to approximately demarcate the abluminal endothelium from the brain parenchyma. Although owing to the limit of resolution of light microscopy, some laminin signal overlapped with abluminal Glut1 staining, a portion remained distinct indicating partial discrimination of abluminal Glut1 expression. Importantly, a distinct Glut1 luminal staining was evident confirming the unambiguous visualization of luminal localization in the capillary nuclear region by confocal microscopy (Figures 1C and 1D). As a further control, we tested for detection of the previously described BBB expression of the heterodimeric AA transporter Lat1-4F2hc (Slc7a5-Slc3a2) (Matsuo et al, 2000). As expected, a relatively even membrane distribution of Lat1 staining colocalizing with Glut1 on luminal and abluminal membranes was observed (Figures 1E and 1F).
Figure 1.
Localization of specific markers at the luminal and/or abluminal membrane of the brain vasculature. Freshly PFA-fixed mouse brain tissue sections stained as indicated were imaged using confocal microscopy. Representative images of ∼5-μm-diameter capillaries stained with: (A, B) anti-Cd71 (green; marker for the luminal membrane), anti-Glut1 (blue; marker for both membranes) or (C–F) with antibodies against Lat1 (green; known to be expressed on both membranes), Glut1 (blue), laminin (red; marker for extracellular matrix separating the abluminal membrane from brain cells), and the nuclear dye PO-PRO (white; separates the luminal from the abluminal membrane). The nucleus is indicated by ‘*' if PO-PRO nuclear staining is not shown. PFA, paraformaldehyde.
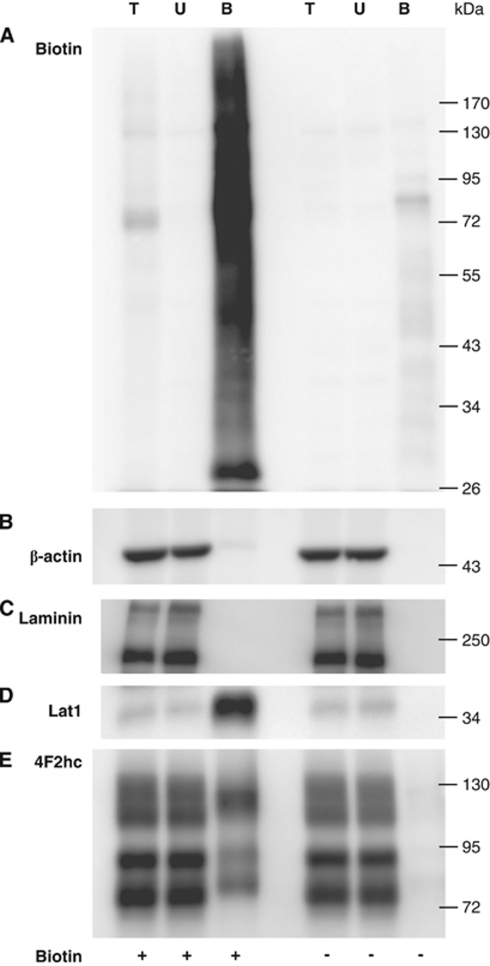
As a second independent method to probe for membrane localization, we used in vivo biotinylation to specifically label proteins expressed on the luminal brain vasculature (Roberts et al, 2008; Roesli et al, 2008). Under conditions designed to maintain BBB integrity, mice brains were perfused with PBS/dextran with and without sulfo-NHS-LC-biotin. The cerebellum was removed and the total brain lysate subjected to Neutravidin pull-down. Streptavidin blot detected a strong biotin signal in the Neutravidin-bound protein fraction from biotinylated lysates that was only weakly found or absent in the pull-down fraction from nonbiotinylated controls and in total protein lysates and unbound fractions, indicating successful exogenous biotinylation of brain proteins (Figure 2A). Furthermore, the abundant cytoskeletal protein, β-actin, and the basal membrane glycoprotein, laminin, were nearly not detected in the Neutravidin-bound fractions, but strongly present in all other fractions (Figures 2B and 2C). In addition, fluorescent Neutravidin staining of the brain tissue confirmed restriction of biotinylation to the vasculature (data not shown). These results are consistent with the restriction of the biotin labeling to luminal membrane extracellular protein domains and with negligible nonspecific protein pull-down by Neutravidin.
Figure 2.
In vivo biotinylation of mouse brain vascular luminal membrane proteins preserves BBB integrity and detects known luminal Lat1-4F2hc (Slc7a5-Slc3a2) expression. Mouse brains were perfused with PBS/dextran with and without sulfo-NHS-LC-biotin (indicated as + or − biotin). After protein extraction and pull-down, 25% of the total Neutravidin-agarose-bound protein fraction (B), 20 μg of the corresponding unbound protein fraction (U), and of the total protein lysate (T) were analyzed by western blotting. Results from representative blots probed as indicated are shown in panels: (A) streptavidin-HRP (B) β-actin (intracellular marker), (C) laminin (extraendothelial marker), (D) Lat1 (luminal expression), and (E) 4F2hc (luminal expression), indicating restriction of exogenous biotinylation to luminal membrane proteins and the detection of known luminally expressed transporters. BBB, blood–brain barrier; HRP, horseradish peroxidase; PBS, phosphate-buffered saline.
Furthermore, a strong band of the expected molecular weight (MW) for Lat1 (∼36 kDa) was observed in the Neutravidin-bound fraction from in vivo biotinylated lysates but not in the corresponding nonbiotinylated fraction (Figure 2D). Probing for 4F2hc luminal expression by in vivo biotinylation resulted in the previously reported pattern of 3 bands (Franca et al, 2005) putatively corresponding to ∼75 kDa core-glycosylated and ∼90 kDa terminally glycosylated 4F2hc, and ∼120 kDa 4F2-Lat1 heterodimer in biotinylated but not in nonbiotinylated Neutravidin pull-downs (Figure 2E). Results from deglycosylation of the Neutravidin-bound fraction from the biotinylated brains were comparable with mouse kidney total membranes with nonglycosylated 4F2hc migrating at ∼60 kDa (data not shown). The data confirm that used in concert, confocal microscopy and in vivo biotinylation successfully identify known microvascular membrane luminal proteins.
Luminal Membrane Expression of System N, Na+-Dependent, Amino-Acid Transporter Snat3
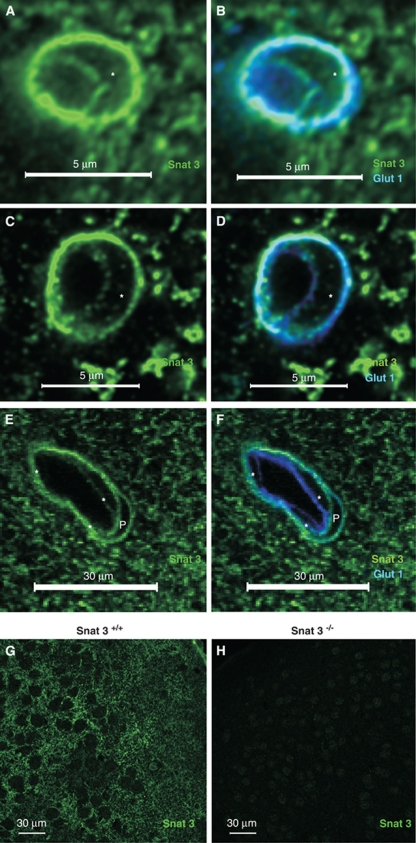
This dual strategy was then used to examine the localization of Snat3, which we hypothesize is possibly important for BBB transendothelial AA transport (Lyck et al, 2009). In the central nervous system, Snat3 has been shown to be expressed in astrocytes but not in neurons or oligodendrocytes (Boulland et al, 2003). Labeling of adult mouse brain tissue sections revealed Snat3 colocalization with Glut1 on capillaries (Figures 3A and 3B). Snat3 antibody specificity was tested by comparing immunofluorescence results from Snat3 wild-type (Snat3+/+), heterozygous (Snat3+/−), and homozygous knockout (Snat3−/−) mice. As Snat3−/− mice generally die before weaning, comparative staining was carried out using tissue obtained from postnatal day 10 mice. As for wild-type mice (Figures 3A, 3B, and 3G), Snat3+/− mice showed robust Snat3 expression in capillaries (Figures 3C and 3D), as well as, in larger microvessels consisting of multiple endothelial cells and associated pericytes (Figures 3E and 3F). However, specific Snat3 staining was not observed in the corresponding knockout tissue (Figure 3H), indicating the Snat3 labeling is specific.
Figure 3.
Snat3 is localized on both BBB endothelial membranes. (A, B) Representative confocal image of a capillary from fresh fixed mouse brain sections stained with anti-Snat3 (green) and anti-Glut1 (blue), and the nucleus is indicated by ‘*', showing colocalization of Snat3 with Glut1 on both BBB membranes in adult (5-week-old) wild-type mice. (C–F) As Snat3−/− mice generally die before weaning, the analysis was carried out using tissue obtained from postnatal day 10 mice. Representative confocal images of tissue sections from 10-day-old heterozygous (Snat3+/−) mice showing (panels C and D) a capillary and (panels E and F) a large microvessel stained with antibodies against Snat3 (green), Glut1 (blue), or nuclear dye, PO-PRO (white), as indicated, verifying the expression of Snat3 in cortical microvessels of different sizes. Endothelial nuclei were indicated by ‘*' and the pericyte nucleus was designated by ‘P'. (G, H) Representative overview confocal images stained with Snat3 antibody (green) verifies expression of Snat3 in brain parenchyma from 10 day wild-type (Snat3+/+; panel G) but not knockout (Snat3−/−; panel H) mice. BBB, blood–brain barrier.
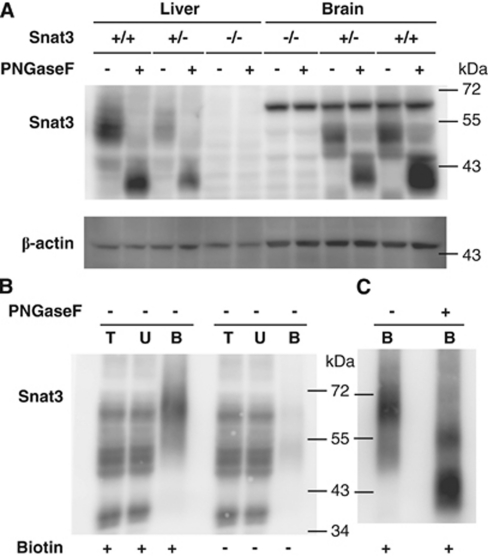
Consistent with antibody specificity for Snat3, western blots of total brain and liver lysates detected specific bands present in Snat3+/+ and Snat3+/− but not in Snat3−/− mice (Figure 4A). In addition, although the banding pattern of glycosylated Snat3 in the liver varies from that of the brain, in all cases, deglycosylation with PNGaseF resulted in a band with the expected apparent MW for nonglycosylated Snat3 of ∼42 kDa (Figure 4A). Figure 4B shows that after in vivo biotinylation, western blot detected a putative Snat3 band with an apparent MW of ∼65 kDa in the Neutravidin-bound fraction but not in the corresponding fraction from nonbiotinylated mice. As for Snat3 total lysates, deglycosylation of the Neutravidin-bound fraction resulted in a shift from ∼65 to ∼42 kDa (Figure 4C). Both the Snat3 knockout and deglycosylation data support the interpretation that the ∼65-kDa band in the Neutravidin-bound fraction is glycosylated luminal Snat3 (Figure 4B). Taken together, the immunofluorescence and in vivo biotinylation data show luminal, and are consistent with abluminal, Snat3 localization in BBB capillaries.
Figure 4.
Comparison of Snat3 signal in the brain parenchyma and vasculature of Snat3+/+ and Snat3−/− mice confirms specificity of antibody and luminal localization of Snat3 along the vascular tree. (A) Representative western blot of mouse brain (Brain) and total liver (Liver) lysates from 10-day-old wild-type (+/+), heterozygous (+/−), or Snat3 knockout (−/−) mice probed with anti-Snat3 antibody (upper panel) or β-actin (lower panel). In (+/+) and (+/−) mice, Snat3 bands display tissue-specific patterns that after digestion with PNGaseF uniformly migrate around ∼42 kDa, verifying the specificity of Snat3 signal. (B) In vivo biotinylation of mouse brain vascular lumen with subsequent purification of biotinylated proteins confirms luminal localization of Snat3. A representative western blot of total protein lysate (T; 20 μg), unbound protein (U; 20 μg), and Neutravidin-bound (B; 25% of the total) fractions prepared from mice perfused±sulfo-NHS-LC-biotin (Biotin) is shown. (C) Luminal expressed Snat3 represents glycosylated Snat3, as indicated by the anti-Snat3 blot results of the Neutravidin-bound (B) fraction after PNGaseF digestion.
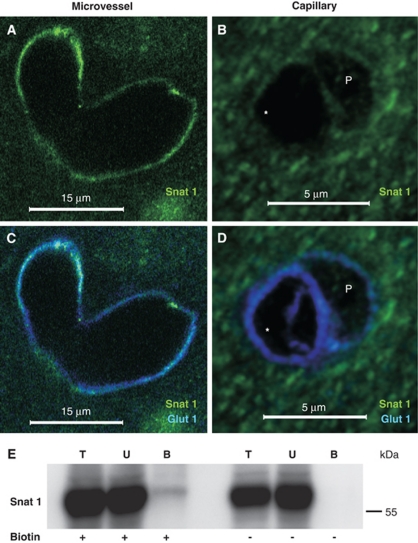
Localization of System A, Na+-Dependent, Amino-Acid Transporter Snat1 on Large but not Capillary Microvessels
Snat1 is highly expressed in the cortex primarily in neurons but also to a lesser extent in astrocytic processes (Melone et al, 2004). On the basis of our previous RNA study, we expected a low expression in the vasculature (Lyck et al, 2009). However, Figure 5 shows a representative staining in which vascular Snat1 can be seen colocalized with Glut1 in two larger (>15 μm diameter) cortical microvessels at a branch point (Figures 5A and 5C). In contrast, a specific Snat1 signal was not evident in capillaries. For example, Figures 5B and 5D show representative staining of a cross-section in the nuclear region of a capillary (∼5 μm diameter) endothelial cell and an associated pericyte, both of which are demarcated by Glut1 (Cornford and Hyman, 2005) but where Snat1 staining is not above background. Western blot after in vivo biotinylation resulted in strong reactivity in all (±biotinylation) total and unbound lysates (as expected from its high brain expression), but only a weak band corresponding to the expected MW of Snat1 (∼58 kDa) in the Neutravidin-bound fraction from biotinylated but not from nonbiotinylated lysates (Figure 5E). Taken together, the data are consistent with endothelial expression of Snat1 on larger microvessels but not the BBB vasculature, and support luminal localization for a portion of the vascular Snat1.
Figure 5.
Snat1 is localized on the luminal membrane of brain vasculature, but not on BBB capillaries. Fresh PFA-fixed mouse brain tissue sections were imaged by confocal microscopy after staining with anti-Snat1 (green) and anti-Glut1 (blue) antibody; the endothelial nucleus is indicated as ‘*' and pericyte as ‘P'. Representative images of (A, C) the junction between two larger microvessels, (>15 μm) revealing Snat1 expression, and (B, D) a capillary (∼5 μm) on which Snat1 expression is below the detection limit, are shown. (E) Mice were in vivo perfused±sulfo-NHS-LC-biotin (Biotin). Western blot analysis of total protein lysate (T; 20 μg), unbound protein (U; 20 μg), and Neutravidin-bound (B; 25% of the total) luminal fractions indicates luminal expression of Snat1. BBB, blood–brain barrier; PFA, paraformaldehyde.
Discussion
Confocal microscopy and in vivo biotinylation data for two Na+-dependent transporters, Snat3 and Snat1, reveal a partially overlapping cortical vascular expression. Snat3 was found to be expressed both luminally and abluminally in young and adult mice on larger microvessels and BBB capillaries, whereas a specific signal for Snat1 was observed only in the larger microvessels. Although subpial arterioles and venules (10 to 100 μm diameter) along with capillaries (4 to 10 μm diameter) are found, only capillaries express the cohort of transport proteins required for vectorial BBB flux. Conversely, cultured endothelial cells from large non-BBB microvessels exhibit relatively rapid proliferation (Ge et al, 2005). Taken together with our previous gene expression study showing differential mRNA expression and regulation, our current localization data underscore the potential differing contributions of Snat3 and Snat1 to BBB transendothelial transport versus endogenous endothelial cellular metabolism, respectively.
Potential Roles of Snat1 and Snat3 in the Cerebral Vasculature
Mechanistically, Snat1 is a ‘system A', Na+-dependent, pH-sensitive transporter for a broad range of small aliphatic AAs (such as alanine, serine, proline, asparagine, glutamine, histidine), whereas Snat3 is a system N, Na+-co- and H+-anti-porter with a preference for glutamine, histine, and asparagine. An important distinction between the two transporters arises from the 1:1 coupling by Snat1 of AA transport to the Na+ electrochemical gradient. Consequentially, Snat1 mediates essentially unidirectional AA transport, whereas under appropriate conditions, Snat3 flux is reversible (Broer et al, 2002; Mackenzie and Erickson, 2004). For example, Chaudhry et al observed that Na+, glutamine, and H+ concentration gradients can influence Snat3 transport direction. They calculated ∼400 μmol/L extracellular glutamine as a threshold between uptake (>400 μmol/L) and efflux in a cell model system (Chaudhry et al, 1999). Glutamine levels in the plasma (∼0.5 to 0.9 mmol/L) and cerebrospinal fluid (∼0.2 to 0.5 mmol/L) vary within this range; consequently, Snat3 may mediate a regulated and reversible transport of glutamine (Broer and Brookes, 2001). Furthermore, intracellular stores of glutamine, which range from 2 to 20 mmol/L, are considerably higher than extracellular levels. By harnessing the Na+ electrochemical gradient, Snat1 is potentially capable of aiding endothelial filling of intracellular glutamine against its concentration gradient. In addition, the nearly complete investment of capillaries with astrocyte endfeet has been suggested to likely facilitate transfer of AAs between astrocytes and the BBB endothelium (Broer and Brookes, 2001). This suggests that as for astrocytic Snat3 and neuronal Snat1, which potentially cooperate in transferring glutamine from astrocytes to neurons (Mackenzie and Erickson, 2004), BBB-expressed Snat3 may participate in the endothelial shuttling of glutamine either directly or through astrocytes to and from the brain parenchyma.
Snat3: Luminal Na+-Dependent Glutamine Blood–Brain Barrier Transporter?
Despite extensive study, the nature and role of luminal cerebral vascular AA transport remain somewhat unclear; this is especially true for Na+-dependent transport. Both functional transport and the expression of a marker (γ-glutaminyl transferase) for luminal Na+-dependent AA transport has been detected. In addition, early in vivo studies by Oldendorf and others (reviewed in the study by Smith et al (1987)) measured a low but significant brain uptake for glutamine that was attributed to a neutral AA carrier system. Later, Ennis et al (1998) showed a Na+-dependent, pH-sensitive glutamine luminal transport that was fully inhibited by histidine but not by cystine (system A or ASC inhibitors) or 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) (system L inhibitor) and therefore identified as system N. In addition to our present report showing Snat3 protein expression, our previous study additionally found a robust and regulated gene expression of the other system N transporter Snat5 in BBB endothelial cells. Furthermore, a recent report by Agarwal et al (2010) found Snat5 to be the most ‘BBB-selective marker' in a comparison of membrane proteins from the liver, kidney, heart, and lung vasculatures. The other transporters for glutamine reported to be expressed in cerebral vessels are Lat1 (system L), Asct2 (system ASC), and y+Lat2 (system y+) (Lyck et al, 2009; O'Kane et al, 2004), all of which are inhibited by one of the compounds mentioned above (Ennis et al, 1998) and mediate obligatory exchange of AA substrates (Verrey et al, 2004). Therefore, only Snat3 and Snat5 are the known molecular candidates for mediating the reported system N luminal BBB glutamine transport. The current in vivo demonstration of Snat3 BBB luminal expression lends strong support to the function of Snat3 in mediating at least a portion of the system N, Na+-dependent luminal glutamine transport observed in the BBB.
Acknowledgments
The authors thank CA Wagner, supported by funding from SNF grant 3100A0-122217/1, for the generous use of Snat3 knockout mice.
The authors declare no conflict of interest.
Footnotes
This study was supported by research funding from SNF grant 31-130471 to François Verrey, and the Center for Microscopy and Image Analysis, University of Zurich, Switzerland.
References
- Agarwal N, Lippmann ES, Shusta EV. Identification and expression profiling of blood-brain barrier membrane proteins. J Neurochem. 2010;112:625–635. doi: 10.1111/j.1471-4159.2009.06481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulland JL, Rafiki A, Levy LM, Storm-Mathisen J, Chaudhry FA. Highly differential expression of SN1, a bidirectional glutamine transporter, in astroglia and endothelium in the developing rat brain. Glia. 2003;41:260–275. doi: 10.1002/glia.10188. [DOI] [PubMed] [Google Scholar]
- Broer A, Albers A, Setiawan I, Edwards RH, Chaudhry FA, Lang F, Wagner CA, Broer S. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J Physiol. 2002;539:3–14. doi: 10.1113/jphysiol.2001.013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Busque SM, Wagner CA. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am J Physiol Renal Physiol. 2009;297:F440–F450. doi: 10.1152/ajprenal.90318.2008. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Krizaj D, Barber D, Storm-Mathisen J, Copenhagen DR, Edwards RH. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell. 1999;99:769–780. doi: 10.1016/s0092-8674(00)81674-8. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Hyman S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx. 2005;2:27–43. doi: 10.1602/neurorx.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Kawai N, Ren XD, Abdelkarim GE, Keep RF. Glutamine uptake at the blood-brain barrier is mediated by N-system transport. J Neurochem. 1998;71:2565–2573. doi: 10.1046/j.1471-4159.1998.71062565.x. [DOI] [PubMed] [Google Scholar]
- Farrell CL, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci USA. 1991;88:5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca R, Veljkovic E, Walter S, Wagner CA, Verrey F. Heterodimeric amino acid transporter glycoprotein domains determining functional subunit association. Biochem J. 2005;388:435–443. doi: 10.1042/BJ20050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Song L, Pachter JS. Where is the blood-brain barrier. Really. J Neurosci Res. 2005;79:421–427. doi: 10.1002/jnr.20313. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, O'Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr. 2006;136:218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Lyck R, Ruderisch N, Moll AG, Steiner O, Cohen CD, Engelhardt B, Makrides V, Verrey F. Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J Cereb Blood Flow Metab. 2009;29:1491–1502. doi: 10.1038/jcbfm.2009.72. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Tsukada S, Nakata T, Chairoungdua A, Kim DK, Cha SH, Inatomi J, Yorifuji H, Fukuda J, Endou H, Kanai Y. Expression of a system L neutral amino acid transporter at the blood-brain barrier. Neuroreport. 2000;11:3507–3511. doi: 10.1097/00001756-200011090-00021. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Melone M, Quagliano F, Barbaresi P, Varoqui H, Erickson JD, Conti F. Localization of the glutamine transporter SNAT1 in rat cerebral cortex and neighboring structures, with a note on its localization in human cortex. Cereb Cortex. 2004;14:562–574. doi: 10.1093/cercor/bhh018. [DOI] [PubMed] [Google Scholar]
- Moret C, Dave MH, Schulz N, Jiang JX, Verrey F, Wagner CA. Regulation of renal amino acid transporters during metabolic acidosis. Am J Physiol Renal Physiol. 2007;292:F555–F566. doi: 10.1152/ajprenal.00113.2006. [DOI] [PubMed] [Google Scholar]
- O'Kane RL, Vina JR, Simpson I, Hawkins RA. Na+ -dependent neutral amino acid transporters A, ASC, and N of the blood-brain barrier: mechanisms for neutral amino acid removal. Am J Physiol Endocrinol Metab. 2004;287:E622–E629. doi: 10.1152/ajpendo.00187.2004. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Molecular biology of the blood-brain barrier. Mol Biotechnol. 2005;30:57–70. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Roesli C, Neri D, Rybak JN. In vivo protein biotinylation and sample preparation for the proteomic identification of organ- and disease-specific antigens accessible from the vasculature. Nat Protoc. 2006;1:192–199. doi: 10.1038/nprot.2006.29. [DOI] [PubMed] [Google Scholar]
- Roesli C, Mumprecht V, Neri D, Detmar M. Identification of the surface-accessible, lineage-specific vascular proteome by two-dimensional peptide mapping. FASEB J. 2008;22:1933–1944. doi: 10.1096/fj.07-100529. [DOI] [PubMed] [Google Scholar]
- Romeo E, Dave MH, Bacic D, Ristic Z, Camargo SM, Loffing J, Wagner CA, Verrey F. Luminal kidney and intestine SLC6 amino acid transporters of B0AT-cluster and their tissue distribution in Mus musculus. Am J Physiol Renal Physiol. 2006;290:F376–FF83. doi: 10.1152/ajprenal.00286.2005. [DOI] [PubMed] [Google Scholar]
- Rybak JN, Scheurer SB, Neri D, Elia G. Purification of biotinylated proteins on streptavidin resin: a protocol for quantitative elution. Proteomics. 2004;4:2296–2299. doi: 10.1002/pmic.200300780. [DOI] [PubMed] [Google Scholar]
- Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Errede M, Robertson D, Capobianco C, Girolamo F, Vimercati A, Bertossi M, Roncali L. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol. 2004;122:51–59. doi: 10.1007/s00418-004-0665-1. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]