Abstract
Cortical spreading depression (CSD) is associated with mitochondrial depolarization, increasing intracellular Ca2+, and the release of free fatty acids, which favor opening of the mitochondrial permeability transition pore (mPTP) and activation of calcineurin (CaN). Here, we test the hypothesis that cyclosporine A (CsA), which blocks both mPTP and CaN, ameliorates the persistent reduction of cerebral blood flow (CBF), impaired vascular reactivity, and a persistent rise in the cerebral metabolic rate of oxygen (CMRO2) following CSD. In addition to CsA, we used the specific mPTP blocker NIM811 and the specific CaN blocker FK506. Cortical spreading depression was induced in rat frontal cortex. Electrocortical activity was recorded by glass microelectrodes, CBF by laser Doppler flowmetry, and tissue oxygen tension with polarographic microelectrodes. Electrocortical activity, basal CBF, CMRO2, and neurovascular and neurometabolic coupling were unaffected by all three drugs under control conditions. NIM811 augmented the rise in CBF observed during CSD. Cyclosporine A and FK506 ameliorated the persistent decrease in CBF after CSD. All three drugs prevented disruption of neurovascular coupling after CSD; the rise in CMRO2 was unchanged. Our data suggest that blockade of mPTP formation and CaN activation may prevent persistent CBF reduction and vascular dysfunction after CSD.
Keywords: cerebral blood flow, migraine, mitochondria, spreading depression, traumatic brain injury
Introduction
This is a study of the effects that blocking the mitochondrial permeability transition pore (mPTP) and calcineurin (CaN) have on the physiological, neurovascular, and metabolic responses to cortical spreading depression (CSD). Cortical spreading depression is a propagating depolarization wave that causes dramatic failure of brain ion homeostasis and affects neurons and astrocytes, resulting in huge, but spontaneously reversible, changes in energy consumption and cerebral blood flow (CBF). Hyperemia is observed during CSD; in the wake of CSD, oligemia is accompanied by impaired neurovascular coupling (Chang et al, 2010; Piilgaard and Lauritzen, 2009) and the basal cerebral metabolic rate of oxygen (CMRO2) is concurrently elevated for up to 2 hours (Piilgaard and Lauritzen, 2009). In this paper we use the terms neurovascular and neurometabolic coupling to denote mechanisms that increase CBF or CMRO2 in active regions in response to functional activation (Attwell et al, 2010).
Cortical spreading depression is an essential mechanism of migraine with aura (Lauritzen, 1994) and the persistent decrease in CBF and impaired neurovascular coupling after CSD observed in rats is also observed in patients with migraine (Hadjikhani et al, 2001; Lauritzen, 1994). Recent data suggest that CSD and periinfarct depolarizations occur at a high frequency in the cerebral cortex of patients with acute traumatic brain injury (Strong et al, 2002), intracerebral hemorrhage, or subarachnoid hemorrhage (Dreier et al, 2006). The pathological correlate of CSD, spreading ischemia, has been recorded in patients with subarachnoid hemorrhage (Dreier et al, 2009). Therefore, we considered it relevant to look for a new therapeutic principle that could ameliorate the persistent impairment of cortical blood vessel control after CSD.
During CSD, Ca2+ accumulates in neurons and astrocytes (Basarsky et al, 1998; Chuquet et al, 2007) while free fatty acids (Lauritzen et al, 1990) are released, and neuronal mitochondria depolarize (Zhou et al, 2010). This combination of events favors formation of the mPTP, an important mechanism of mitochondrial dysfunction and apoptosis (Friberg and Wieloch, 2002), and activation of CaN (Sierra-Paredes and Sierra-Marcuno, 2008); however, it is unknown whether these pathways are involved in CSD. Calcineurin is a phosphatase that abounds in cortical neurons and astrocytes (Gabbott and Bacon, 1997). Calcineurin-mediated mechanisms modulate several brain processes, including γ-aminobutyric acid and glutamate receptor channels (Sierra-Paredes and Sierra-Marcuno, 2008), phosphorylation of nitric oxide synthase (NOS) (which is essential for neurovascular coupling) (Park et al, 2008), and mitochondrial fission under conditions of persistently rising cytosolic Ca2+ (Cereghetti et al, 2008). Blockade of both mPTP and CaN before, during, or following episodes of brain ischemia can rescue mitochondrial function, O2 metabolism, and tissue integrity, suggesting that these signaling pathways contribute importantly to impairment of function in acute models of brain diseases (Friberg and Wieloch, 2002; Sullivan et al, 2005). We tested the hypothesis that mPTP and CaN are involved in CSD, and that blockade of either or both pathways affects acute and subacute changes in neurovascular and neurometabolic coupling accompanying the event.
Materials and methods
All experiments were conducted in full compliance with the guidelines set forth in the European Council's Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes, and were approved by the Danish National Ethics Committee.
Sixty-six male Wistar rats (317±3.6 g) were anesthetized with Isoflurane (Abbott, Copenhagen, Denmark; induction: 5%, surgery: 2%) while ventilated with O2-enriched air. The head was placed in a stereotactic head holder. Lidocaine (5 mg/mL) was used at the contact spots for the ear pins. A femoral artery catheter was inserted to measure arterial blood pressure and take blood–gas samples. A femoral vein catheter was inserted for saline infusion. The trachea was cannulated and the rats were ventilated and maintained throughout the experiment. Blood–gas analysis was performed hourly to assure physiological conditions (ABL 715, Radiometer, Brønshøj, Denmark). Body temperature was maintained at 36°C to 37°C using a custom-made heating pad. Three craniotomies were performed with a dental drill; two over homologous regions of the somatosensory cortex, and one over the frontal cortex on the registration side to elicit CSD (Figure 1A). Wells were made around each craniotomy using 5% agar in Ringer solution. The dura was carefully removed under a microscope. Any visible sign of brain damage or bleeding excluded the rat from the protocol. During and after removal of the dura, the craniotomy sites were continuously superfused with artificial cerebrospinal fluid (aCSF—composition in mmol/L: 2.0 glucose, 126.0 NaCl, 2.8 KCl, 22.0 NaHCO3, 1.45 CaCl2, 1.0 Na2HPO4, and 0.876 MgCl2). After surgery, the anesthesia was changed to α-chloralose (1,2-0-[2,2,2-trichloro-ethylidene]-[α]--gluco-furanose) HBC (2-hydroxypropyl-β-cyclodextrin) complex (Sigma-Aldrich, Brøndby, Denmark), dissolved in saline (0.5 g/mL) (bolus: 1.6 mL/kg intravenously, continuous infusion: 1.1 mL/kg per hour intravenously). This anesthetic regime is considered ideal for studies of neurovascular and neurometabolic coupling. The level of anesthesia was checked by observing arterial blood pressure during stimulation, as well as by tail pinch. Experiments were performed after a postoperative recovery period of at least 30 minutes to obtain a stable level of anesthesia, a stable CBF baseline, and an arterial blood pressure of 90 to 120 mm Hg, not varying >10% in the same animal. Arterial blood pressure remained constant during CSD and electrical stimulation. Therefore, the CBF increases noted were not due to fluctuations in blood pressure. The blood pressure signals were A/D converted, amplified, and sampled via the 1401-plus interface (Cambridge Electronic Design (CED), Cambridge, UK) connected to a PC running Spike2 software (CED). At the end of the experiment, the heart was stopped by an intravenous bolus injection of air and the zero level of the recording parameters was recorded. In all experiments described, the recording electrodes and laser Doppler probe were placed in a region devoid of large vessels (>100 μm) as close as possible to each other at coordinates 3 mm posterior to Bregma and 6 mm lateral to the midline, corresponding to the barrel cortex. To ensure that recordings were in the barrel cortex, initial stimulation of the contralateral infraorbital nerve was performed and the area of recordings was approved by the presence of large and robust local field potential (LFP) and CBF responses.
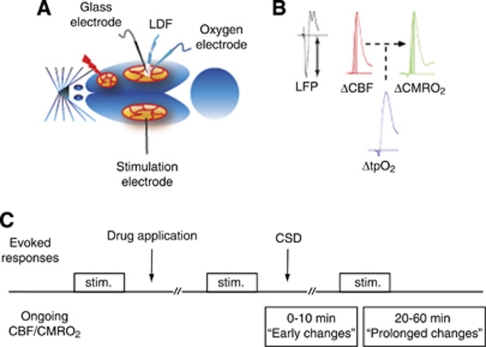
Figure 1.
Experimental design. (A) Rat brain viewed from above. Cortical spreading depression (CSD) was elicited in the right frontal cortex with a brief needle stab, and propagated into the right somatosensory cortex monitored by a glass microelectrode recording electrocorticographic (ECoG) activity. Using the same microelectrode, we recorded local field potentials (LFPs) evoked by a bipolar stimulation electrode placed in the contralateral somatosensory cortex. Baseline and evoked changes in cerebral blood flow (CBF) were recorded by laser Doppler flowmetry (LDF), while tissue oxygenation (tpO2) was recorded using a Clark-type polarographic glass microelectrode. (B) Cerebral metabolic rate of oxygen (CMRO2) was calculated offline from simultaneous measurements of CBF and tpO2. Evoked CBF, tpO2, and CMRO2 responses were defined as area under curve (AUC) from stimulation onset to peak value. Evoked LFP responses were defined by their peak amplitude. (C) The experimental protocol started with recordings of evoked responses (trains lasting 1 second of 1, 6, and 20 Hz stimulation, repeated three times). Topical application of drug (cyclosporine A (CsA), FK506, NIM811, or FK506+NIM811) followed. Incubation lasted for minimum 30 minutes before repeating evoked responses as stated above. CSD was elicited and between 20 to 60 minutes after CSD-evoked responses were repeated again. Changes in ongoing CBF and CMRO2 were evaluated both during the hyperemia phase (‘early changes') and during the oligemia phase (‘prolonged changes'). The effects of vasoconstrictor U46619 were tested in separate experiments (protocol not shown in this figure).
Evoked Responses—Transcallosal Fiber Stimulation
A coated bipolar stainless steel electrode (SNEX 200, contact separation: 0.25 mm; RMI, Woodland Hills, CA, USA) was lowered 0.5 mm into the left somatosensory cortex 3.0 mm posterior to the bregma and 6.0 mm lateral to the midline using stereotactic instruments (Figure 1A). Direct current stimulation (ISO-flex; A.M.P.I., Jerusalem, Israel) was given as square wave pulses (100 microseconds, 1.5 mA) at 1, 6, and 20 Hz in trains lasting for 1 second. Each frequency was run three times with interstimulus intervals of 0.5 to 1.5 minutes, depending on the stimulation frequency, to allow the reestablishment of a stable baseline between stimulations. Cortical stimulation could potentially elicit episodes of CSD on the stimulated side. To circumvent this problem, we applied the N-methyl--aspartate receptor (NMDAR) antagonist 5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten (MK801) to the stimulated (left) cortex at a concentration of 1 mmol/L, which prevented the occurrence of CSD (Hoffmeyer et al, 2007;Lauritzen and Hansen, 1992). This procedure ensured reproducible evoked responses of tissue partial pressure of oxygen (tpO2), LFPs, and CBF responses in the activated (right) cortex.
Electrophysiology
We used single-barreled glass microelectrodes filled with 2 mol/L saline (impedance, 2 to 3 MΩ tip, 2 μm). Extracellular LFPs were recorded with a single glass electrode at a depth of 300 to 600 μm in the right somatosensory cortex (Figure 1A). An Ag/AgCl ground electrode was placed in the neck muscle. The preamplified ( × 10) signal was A/D converted, amplified, band pass filtered at 1 to 1 kHz, and digitized using the 1401-plus hardware (CED) connected to a PC running SPIKE 2.6 software. Digital sampling rates for LFP and electrocorticogram (ECoG) were 5 kHz. DC potentials were recorded from the same glass electrode using a 0.1-Hz low pass filter.
Induction of Cortical Spreading Depression
Cortical spreading depression was induced with a brief needle stab in the frontal cortex of the right hemisphere.
Tissue Partial Pressure of Oxygen Measurements
We used a modified Clark-type polarographic oxygen microelectrode (OX-10, Unisense A/S, Aarhus, Denmark) with a guard cathode for tpO2 measurements. The advantages of this electrode type are its small tip size (10 μm in this study) and its built-in guard cathode, which removes all oxygen from the electrolyte reservoir.
This setup enabled us to measure tpO2 over time (and among different treatment conditions) with excellent long-term stability (signal drift, 0% to 0.5% per hour). The field of sensitivity is a sphere of two times the tip diameter. The electrodes used in this study were constructed so that 90% of the response time was <1 second and the stirring sensitivity was nearly negligible at <0.8%. Oxygen microelectrodes respond linearly to changes in oxygen concentration. Calibration of each electrode was performed before and after each experiment, both in air-saturated saline and in oxygen-free solution consisting of 0.1 mol/L (+)-sodium -ascorbate and 0.1 mol/L NaOH dissolved in saline. Mean tpO2 under control conditions was 18.5±3.1 mm Hg (mean±s.e.m.; n=29). The oxygen electrode was vertically inserted stepwise into the somatosensory cortex and positioned at the same cortical depth as the glass microelectrode (300 to 600 μm). The distance between the two electrodes was <200 μm. Both electrodes and the laser Doppler flowmetry probe recorded from the same depth and were kept in the same position throughout the experiment. No linear drifts in baseline occurred during the experiments. The oxygen electrodes were connected to a high-impedance Picoamperometer (PA 2000, Unisense A/S) that measured the currents across the oxygen electrodes. Signals were A/D converted and recorded at a sampling rate of 10 Hz (Power 1401 and Spike 2.6; CED).
Laser Doppler Flowmetry
Cerebral blood flow was continuously recorded using laser Doppler flowmetry. The laser Doppler flowmetry probe was at a fixed position at 0.3 mm above the pial surface in a region devoid of large blood vessels (wavelength, 780 nm; fiber separation, 250 μm; Periflux 4001 Master, Perimed, Jarfälla, Sweden). With this wavelength and fiber separation, changes in CBF were recorded to a cortical depth of 1 mm. After stable baseline recordings had been obtained, the probe was left for the duration of the experiment. The signal was A/D converted and recorded using the CED 1401-plus interface and CED Spike 2.6 software (10 Hz digital sampling rate). The laser Doppler flowmetry monitor displays blood flow readings in arbitrary units that do not allow for measurement of CBF in terms of absolute values, but the method is valid in determining relative changes of CBF during moderate flow increases.
Laser Speckle Imaging of Cerebral Blood Flow
Cerebral blood flow changes were monitored using a Full-Field Laser Perfusion Imager (MOOR Instruments, Millwey, UK) in experiments investigating vasoconstriction due to application of the thromboxane A2 analogue U46619.
Calculation of Cerebral Metabolic Rate of Oxygen
Cerebral metabolic rate of oxygen was calculated offline from simultaneously obtained recordings of tpO2 and CBF. Baseline values of tpO2 and CBF were taken as the mean of a 1-second period obtained before onset of stimulation. These values were then combined with reported values for CBF and CMRO2 from the literature (53 mL/100 g per minute and 219 μmol/100 g per minute, respectively) to calculate the corresponding L. Standard values used in calculations were: P50 (36 mm Hg), h (2.7), and Ca (8 μmol/mL). This corresponds to an L value of 4.15 μmol/100 g per minute per mm Hg. The relationship between the three variables is
 |
where P50 is the half-saturation tension of the oxygen–hemoglobin dissociation curve, h is the Hill coefficient of the same dissociation curve, Ca is the arterial oxygen concentration, and L is the effective diffusion coefficient of oxygen in brain tissue.
Drugs
MK801 is an NMDAR (ionotropic glutamate receptor) antagonist. Stock solution was made in distilled water. For topical application, stock solution was further diluted in aCSF to a final MK801 concentration of 1 mmol/L. MK801 was supplied in all experiments where evoked responses were examined, and was applied to the hemisphere where the stimulation electrode was placed (left hemisphere). MK801 was applied 40 minutes before insertion of the stimulation electrode. This procedure prevented unwanted episodes of CSD due to insertion of the stimulation electrode.
Cyclosporine A (CsA) (Sigma-Aldrich) is a fungal metabolite that blocks the activity of CaN (protein phosphatase 2B) via formation of the CsA–cyclophilin complex. Cyclosporine A also blocks the formation of mPTP via interaction with cyclophilin-D. Cyclosporine A was first dissolved in DMSO (dimethyl sulfoxide), and then a stock solution was made by dissolving further in saline. For topical application, the stock solution was dissolved in aCSF. The final concentration of CsA was 0.1 μmol/L (DMSO concentration was <0.01%). This concentration has previously proven effective in blocking the opening of the mPTP (Halestrap et al, 1997) (0.2 μmol/L—liver mitochondria) (Sullivan et al, 2005) (1 μm completely abolished mPTP in isolated cortical mitochondria).
FK506 (Sigma-Aldrich) is a potent in vitro immunosuppressant and T-cell proliferation blocker with neuroprotective and neuroregenerative properties. FK506 disrupts CaN-mediated signal transduction via interaction with FK506-binding protein-12 (FKBP12). The effect of FK506 appears in nanomolar concentrations. FK506 was initially dissolved in DMSO and further dissolved in saline. For topical application, stock solution was dissolved in aCSF. The final concentration of FK506 used in this study was 7.5 nmol/L (DMSO concentration was <0.001%).
NIM811 (Novartis, Basel, Switzerland) is a nonimmunosuppressive cyclosporine derivative that acts by specifically inhibiting opening of the mPTP in a manner comparable to CsA (Waldmeier et al, 2002). NIM811 was initially dissolved in DMSO, and stock solution was further dissolved in saline. For topical application, stock solution was dissolved in aCSF. The mPTP-blocking effect of NIM811 appears at the same concentrations as for CsA. The final concentration of NIM811 used in this study was 0.1 μmol/L (DMSO concentration was <0.01%).
U46619 is a thromboxane A2 analog used as a vasoconstrictor (Ayata et al, 2004). A U46619 stock solution was made by dissolving the agent in distilled water. For topical application, stock solution was dissolved in aCSF to a final concentration of 1 mmol/L U46619.
Data Analysis and Statistics
Simultaneously recorded LFP, CBF, and tpO2 signals were used for analysis (Figure 1C). The LFPs were averaged, and amplitudes were calculated for each stimulation period as the difference between the baseline and the first negative peak (Figure 1B) and the sum of LFP amplitudes (ΣLFP) in a stimulation train (calculated as: frequency × amplitude × stimulation time) was used for statistical testing. The evoked CBF and CMRO2 responses were calculated as the area under the curve from stimulation onset to peak value (ΔCBF unit: % × s; ΔCMRO2 unit: μmol × s/100 g per minute). Custom-made analysis programs based on Matlab 7.8 (Mathworks Inc., Natick, MA, USA) were used to calculate CMRO2. area under the curves for evoked CBF and evoked CMRO2 responses were calculated using Microsoft Office Excel 2007 (Microsoft, Seattle, WA, USA). For all analyses of evoked responses, log10-transformed values for CBF, CMRO2, and frequency were used. Post-CSD measurements of evoked responses were performed at 20 to 60 minutes after elicitation of CSD. The impact of single episodes of CSD on frequency-dependent evoked CBF/CMRO2 responses was tested in every group using the linear regression with random effects procedure (proc mixed in SAS). The model included animal, frequency (6 and 20 Hz), CSD (pre/post), and interactions between frequency and CSD as explaining parameters. The impact of single episodes of CSD on ΣLFP was tested in every group using the linear regression with random effects procedure (proc mixed in SAS). The model included animal, frequency (1, 6, and 20 Hz), CSD (pre/post), and interaction between frequency and CSD as explaining parameters. Electrocorticogram power analysis was performed by analyzing 30 seconds epochs taken just before recording evoked responses. Difference in ECoG power (unit: %) between pre- and post-CSD in the different groups was tested using analysis of variance. Post hoc comparisons of groups were performed using Bonferroni correction. Evaluation of changes in ongoing CBF and CMRO2 (unit: %) during and in the wake of CSD was performed using a 30-second running average window for smoothing of data. Immediate changes (‘early responses') in CBF and CMRO2 were defined as maximum values within the first 10 minutes after CSD. Prolonged changes in CBF and CMRO2 were defined as minimum values at 20 to 60 minutes after CSD. Differences between groups in ongoing CBF and CMRO2 during and in the wake of CSD were tested using the analysis of variance procedure with post hoc Bonferroni correction. The effect of vasoconstrictor U46619 was evaluated as the peak amplitude (unit: %) within 5 minutes after application of the drug using the analysis of variance procedure with post hoc Bonferroni correction. SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Values in text and figures are expressed as mean±s.e.m. Values were considered statistically significant at P<0.05. Post hoc power analysis of all analysis of variances performed are stated in text by their β values (equals 1-power).
Results
Effect of Cortical Spreading Depression on Cerebral Blood Flow and Cerebral Metabolic Rate of Oxygen Under Control Conditions and After Treatment with Cyclosporine A, FK506, and NIM811
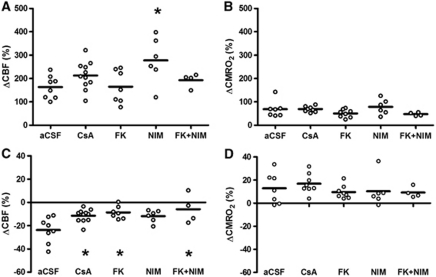
Cerebral blood flow and CMRO2 increased in all experimental groups during single episodes of CSD, as reported previously (Piilgaard and Lauritzen, 2009) (Figures 2A and 2B). We observed a statistically significant augmentation in the rise of CBF during CSD in the NIM811-treated group of 277%±40% (n=6, P=0.03), compared with the effect of CSD in the control group (163%±17% n=8). The hyperemic response remained unchanged in the other groups compared with the control group (CsA=213%±18%, n=11, P=1.00; FK506=165%±27%, n=7, P=1.00; FK506+NIM811=193%±15%, P=1.00). Hyperemia in the NIM811-treated group was also significantly higher than in the FK506-treated group (P=0.04). No other significant between-group differences were observed (β=0.06) (Figure 2A). Cerebral metabolic rate of oxygen during CSD increased by 69%±13% in the control group (n=7). No significant changes were evident compared with the control group (CsA=69%±4%, n=8, P=1.00; FK506=50%±6%, n=8, P=1.00; NIM811=78%±13%, n=6, P=1.00; FK506+NIM811=47%±4%, n=4, P=1.00) No other significant between-group differences were observed (β=0.52) (Figure 2B).
Figure 2.
Effects of cyclosporine A (CsA), FK506, and NIM811 on cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2) during and after cortical spreading depression (CSD). (A) CBF increase during CSD: all groups showed a pronounced rise in CBF during CSD. The increase in the NIM811 group was significantly larger than the responses in the artificial cerebrospinal fluid (aCSF) group and in the FK506 group. (B) In comparison, the rise in CMRO2 during CSD increased in all groups without significant differences between groups. (C) In the control group (aCSF), oligemia after CSD was evident. This oligemia was significantly attenuated in groups treated with CsA, FK506, or FK506+NIM811. NIM811 applied alone did not significantly change oligemia after CSD compared with the control group (aCSF). (D) In comparison, the persistent rise in CMRO2 after CSD remained elevated for the following hour without differences between groups. Statistically significant changes in comparison with the control group (aCSF) are marked with an asterisk.
Cerebral Blood Flow and Cerebral Metabolic Rate of Oxygen After Cortical Spreading Depression Under Control Conditions and After Treatment with Cyclosporine A, FK506, and NIM811
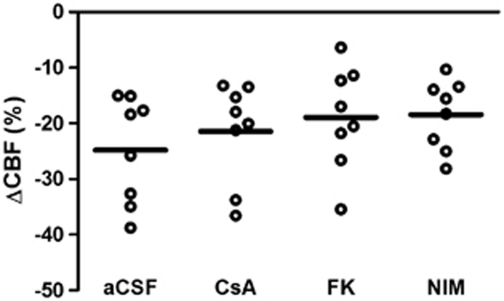
After CSD, basal CBF decreased in the control group (mean change was −24%±4% n=8). Significant amelioration of oligemia after CSD was seen after CsA treatment (−11%±2%, n=11, P=0.02), FK506 (−9%±2%, n=7, P=0.01) and FK506+NIM811 treatment (−6%±6%, n=4, P=0.01) compared with the control group. The NIM811-treated group also demonstrated a tendency toward reduction in oligemia (−12%±2%, n=6, P=0.09), but this mean change did not reach statistical significance. No other between-group differences were evident (β=0.06) (Figure 2C). After CSD, CMRO2 remained increased by 13%±5% in the control group (n=7). This rise in CMRO2 was unchanged for all treatment groups compared with the control group: CsA (17%±3%, n=11, P=1.00), FK506 (10%±2%, n=8, P=1.00), NIM811 (10%±5%, n=6, P=1.00), and FK506+NIM811 (9%±2%, n=4, P=1.00). No other between-group differences were observed (β=0.80) (Figure 2D). The findings suggest that blockade of CaN ameliorates the reduction in CBF after CSD. None of the three drugs had any effect on baseline CBF or CMRO2 under control conditions and the effect of these drugs is therefore not explained by interference with basic mechanisms of CBF control.
Effects of Treatment with Cyclosporine A, FK506, and NIM811 on Electrocorticogram Power
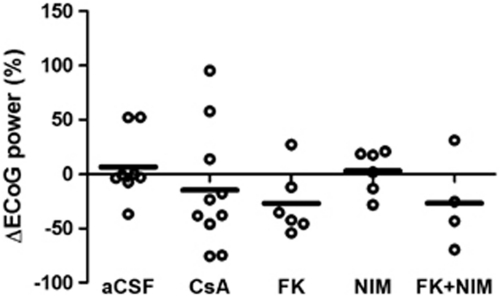
After CSD, the power in the ongoing ECoG activity in the control group was back to basal level at 20 minutes after single episodes of CSD (7%±11%, n=8). There were no significant differences in ECoG activity in the four groups compared with the control group (Figure 3): CsA (−15%±18%, n=10, P=1.00), FK506 (−27%±12%, n=6, P=1.00), NIM811 (3%±8%, n=6, P=1.00), and FK506+NIM811 (−27%±21%, n=4, P=1.00). No other between-group differences were found (β=0.73), suggesting that the drug treatments did not interfere with ongoing synaptic activity. Therefore, the effect of the three drugs on the level of CBF is not explained by fluctuations in ongoing synaptic activity.
Figure 3.
Ongoing electrocorticogram (ECoG) power at 20 minutes after cortical spreading depression (CSD) was the same for all groups. The Ongoing ECoG power at 20 minutes after CSD had returned to pre-CSD values in the control group. Additionally, no statistically significant changes were observed in the treatment groups. Therefore, the attenuation of the cerebral blood flow (CBF) reduction after CSD in the treatment groups was not explained by alterations in ongoing neuronal activity reflected in the ECoG.
Effects of Cyclosporine A, FK506, and NIM811 on Evoked Cerebral Blood Flow, Cerebral Metabolic Rate of Oxygen, and Local Field Potential Responses Under Control Conditions and After Cortical Spreading Depression
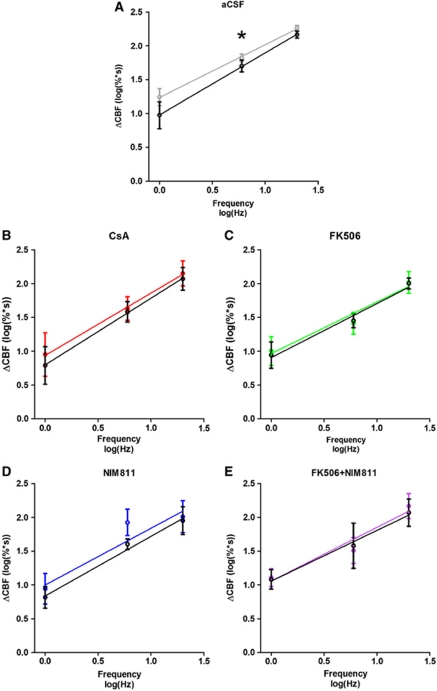
Every treatment group displayed a strong frequency dependency in the evoked responses (P<0.01) under control conditions and after CSD; however, the level of evoked CBF responses decreased after CSD in the control group (P=0.01) (Figure 4A). All three drugs ameliorated this impairment of CBF responses after CSD; no significant change in CBF response levels were evident (CsA: P=0.33; FK506: P=0.81; NIM811: P=0.18; FK506+NIM811: P=0.54) (Figures 4B–4E). In comparison, the frequency dependency of ΣLFP or CMRO2 responses remained unaffected by CSD (data not shown). In addition, none of the drugs changed the relation between stimulation frequency and ΔCMRO2 or ΣLFP after CSD, except for ΣLFP responses in the CsA group, where a decrease in level after CSD corresponded to a 9% reduction in intercept (P=0.03). In summary, CsA, FK506, and NIM811 all prevented reductions in evoked CBF responses after CSD without changing the evoked CMRO2 responses. Evoked ΣLFPs were unaffected by FK506 and NIM811, while a decreased response level after CSD was observed in the CsA-treated group.
Figure 4.
Frequency-dependent changes in evoked cerebral blood flow (CBF) responses before and after single episodes of cortical spreading depression (CSD). A strong frequency dependency was observed in each group. (A) In the control group, a significant decrease in response level was evident after CSD. A decrease in response level of CBF was ameliorated by topical application of: cyclosporine A (CsA) (B), FK506 (C), NIM811 (D), and FK506+NIM811 (E). Color code: black line in each graph indicates evoked responses in the wake of CSD.
Effects of Vasoconstrictor U46619
The observation that both CsA and FK506 ameliorated the decrease in CBF after CSD could be explained by a nonspecific effect on cerebral vasoconstriction. To test this hypothesis, we examined the effects of the three drugs on cortical vasoconstriction induced by topical application of U46619, a thromboxane A2 agonist. In the control group (aCSF), we found a reduction in CBF (−25%±3%, n=8) after topical application of U46619. No significant changes were evident in the treatment groups compared with the control group (CsA: −21%±3%, n=8, P=1.00; FK506: −19%±3%, n=8, P=1.00; NIM811: −18%±2%, n=8, P=0.91) (Figure 5). No statistically significant between-group differences were observed (β=0.78). These findings suggest that the effects of the three drugs were specific to CSD-produced vasoconstriction.
Figure 5.
Cerebral vasoconstriction induced by thromboxane A2 analog U46619 was unaffected by cyclosporine A, FK506, and NIM811. U46619 was applied topically in every group to assess the impact of the three drugs on a standard procedure to elicit vasoconstriction. None of the drugs affected vasoconstriction induced by U46619 compared with control conditions (artificial cerebrospinal fluid (aCSF)). This finding suggests that the observed effect of the three drugs on the prolonged decline in cerebral blood flow (CBF) after cortical spreading depression (CSD), and on neurovascular coupling, is not due to interference with vasoconstriction per se, but on CSD-specific mechanisms.
Discussion
We have demonstrated that CsA, FK506, and FK506+NIM811 (but not NIM811 alone) ameliorate a prolonged decrease in CBF and that all three drugs (CsA, FK506, and NIM811) ameliorate impaired neurovascular coupling after CSD without affecting any prolonged rise in CMRO2. Under control conditions, none of these drugs affected synaptic activity, basal levels of CBF and CMRO2, neurovascular and neurometabolic coupling, or the ability of the cortical blood vessels to constrict in response to a thromboxane A2 analog. Our data suggest that interference with the CaN and mPTP pathways affects neurovascular coupling only under pathological conditions. We previously reported that neurovascular coupling was impaired after CSD and that this could be explained in part by the impairment of interneuronal signaling, which occurs after CSD (Kruger et al, 1996; Piilgaard and Lauritzen, 2009). The mPTP is particularly interesting in this context since interneurons contain very high levels of cyclophilin-D and are believed to be more sensitive to mPTP formation than pyramidal cells (Hazelton et al, 2009).
Cortical spreading depression is accompanied by transient rises in intracellular Ca2+ (Chuquet et al, 2007), mitochondrial depolarization (Zhou et al, 2010), release of free fatty acids (Lauritzen et al, 1990), and depletion of ATP (Lauritzen et al, 1990). This constellation of events favors the formation of the mPTP, while rises in intracellular Ca2+ alone are sufficient to activate CaN (Friberg and Wieloch, 2002; Sierra-Paredes and Sierra-Marcuno, 2008). We assessed the involvement of mPTP and CaN pharmacologically by using CsA, which inhibits mPTP formation by binding cyclophilin-D (a protein that constitutes part of the mPTP). Cyclosporine A also binds to the catalytic subunit of CaN, thereby inhibiting its phosphatase activity. To sort out the influence of mPTP and CaN pathways separately, we used NIM811 and FK506. NIM811 is a CsA derivative that mimics the action of CsA in preventing mPTP formation and opening, but lacks the ability to inhibit CaN (Mbye et al, 2009). FK506 acts through the disruption of CaN-mediated signaling mechanisms, such as the activity of γ-aminobutyric acid and glutamate receptors (Sierra-Paredes and Sierra-Marcuno, 2008) and mitochondrial function (Cereghetti et al, 2008). Since CsA, NIM811, and FK506 have each proven to be anticonvulsive and neuroprotective in a variety of neuropathological states that are known to be accompanied by CSD, we examined whether mPTP and CaN protected against vascular dysfunction after CSD.
Neurovascular and Neurometabolic Coupling During Cortical Spreading Depression
Cortical spreading depression is accompanied by a dramatic rise in CBF (Busija et al, 2008) with different time courses and magnitudes of CBF increase in rats and mice (Ayata et al, 2004). The increase in CBF may change if multiple CSD episodes are elicited (Fabricius et al, 1995), and with the vascular supply of the tissue (Sukhotinsky et al, 2010). Release of NO has also been suggested to contribute to vasodilation during CSD (Busija et al, 2008), although this hypothesis is controversial (Fabricius et al, 1995). However, in disease states such as subarachnoid hemorrhage, the polarity of the vascular reaction during CSD may change so as to become a strong vasoconstriction that produces localized damage in the cerebral cortex (Dreier et al, 1998), which suggests that the cerebrovascular reaction is context sensitive and that mechanisms intrinsic to the brain determine the vascular signature. Interference with formation of mPTP during CSD by NIM811 was accompanied by a 70% larger rise in CBF than during control. The effect on the acute rise in CBF is relevant since CSD-induced increases in CBF may be too small to match the accompanying increase in CMRO2 (Lacombe et al, 1992; Piilgaard and Lauritzen, 2009). Registration of NADH fluorescence signals by two-photon microscopy during CSD suggests that oxidative metabolism is confined to neurons immediately adjacent to the capillaries, while metabolism is glycolytic in cortical pockets at a distance from capillaries (Takano et al, 2007). With a larger rise in CBF in rats treated with NIM811, the discordance between O2 use and supply is smaller than in control rats, and NIM811 may for that reason be beneficial for cerebrocortical function.
Ongoing Electrocorticogram Activity, Evoked Local Field Potential, and Cerebral Metabolic Rate of Oxygen
Spontaneous ECoG activity and evoked LFP responses returned to normal within 10 to 20 minutes after CSD, both in controls and in treated animals (Leao, 1944). This result suggests that neither the persistent large reduction in CBF in control animals, nor the smaller CBF reduction following treatment is explained by variations in spontaneous cortical electrical activity or evoked LFP responses. Cortical interneurons do not contribute directly to the ECoG or LFP signal due to their so-called closed field configuration (i.e., stellate interneurons do not produce extracellular currents that can be monitored by microelectrodes). Under most in vivo experimental conditions their activity can only be assessed by their impact on cortical pyramidal cells (e.g., a protracted positive potential that follows the initial negative depolarizing phase). This positive inhibitory phase is suppressed following CSD, suggesting that cortical inhibitory interneurons take longer to recover from CSD than excitatory pyramidal cells (Piilgaard and Lauritzen, 2009). Therefore, the persistent decrease in CBF after CSD cannot be predicted from the ECoG or LFP amplitude due to a sampling bias towards cortical pyramidal cells.
Neurovascular Coupling and Basal Cerebral Blood Flow in the Wake of Cortical Spreading Depression
Basal CBF and the CBF response to transcallosal fiber stimulation depends on preserved NOS and cyclooxygenase activity (Hoffmeyer et al, 2007). Transcallosal fiber stimulation activates pyramidal cells, which contains cyclooxygenase 2, and inhibitory interneurons that contain NOS or vasoactive intestinal peptide (Enager et al, 2009). Nitric oxide synthase inhibition by intravenous and topical application of NG-nitro--arginine does not change the reduction of CBF following CSD, which could suggest that this mechanism is already perturbed, while excess supply of -arginine (the substrate for NOS) prevents the development of prolonged oligemia after CSD, and animals treated with -arginine recover reduced vascular reactivity to hypercapnia after CSD much faster than control rats (Fabricius et al, 1995). The central role for NO in the protracted vascular changes following CSD is further corroborated by the observation that topical application of the NO donors or of the cell-permeable cyclic guanosine 3′,5′monophosphate (cGMP) analog 8-Br-cGMP reestablished resting CBF to values measured before CSD, and reversed CSD-induced attenuation of the cerebrovascular response to hypercapnia as well (Scheckenbach et al, 2006). These observations point to functional downregulation of the NO–cGMP pathway following CSD.
One such mechanism could be the phosphorylation of neuronal NOS, which is structurally and functionally linked to the NMDAR via PSD-95 (Brenman et al, 1996), and NMDAR activation is an integral part of CSD (Lauritzen and Hansen, 1992). The bond to the NMDAR enables neuronal NOS to be efficiently and selectively activated by the influx of Ca2+ during activity. In addition, NOS activity is controlled by phosphorylation, and while the consequences of the phosphorylation of NOS are complex (Ruiz et al, 2000), it has become clear that NOS activity in the rat primary somatosensory cortex is enhanced by phosphorylation and that this control step is necessary for full development of a hyperemic response to somatosensory stimulation (Park et al, 2008). Cyclosporine A and FK506 are both assumed to enhance NOS phosphorylation (Ruiz et al, 2000) and are expected via this mechanism to stimulate NO formation and ameliorate oligemia and the impairment of neurovascular coupling after CSD. Therefore, the beneficial effects of interference with the CaN pathway may be related to an increase in the production of the cerebrovascular modulator NO in relevant interneurons that enables pial vessels to respond to local as well as systemic vasodilators (Lindauer et al, 1999), or to interference with Ca2+-dependent dysfunction of neuronal or astrocytic mitochondrial function (Cereghetti et al, 2008). The question is whether there is a direct effect on the blood vessels as well, which exhibit reduced cerebrovascular reactivity to chemical stimuli applied directly at the vessel wall (Florence et al, 1994; Seitz et al, 2004). This possibility cannot be excluded based on the present findings.
It is noteworthy that CsA, FK506, and FK506+NIM811 all ameliorated the development of vascular dysfunction following CSD without evidence of changing normal neuronal function or neurovascular coupling itself, while NIM811 applied alone had no effect on the CBF reduction. The correlation between evoked synaptic activity and the associated CBF increases were unchanged by the three drugs, both in terms of intercept and slope, indicating normal and constant neurovascular coupling. The positive effect on basal CBF and neurovascular coupling was also not due to interference with vasoconstrictor mechanisms. The thromboxane A2 analog U46619 reduced CBF to the same degree during control experiments and when the cerebral cortex had been superfused with either of the drugs for 30 minutes before the vasoconstrictor was applied (Figure 5). Therefore, we consider the effects of these drugs to be specific to interference with CSD-related mechanisms.
In conclusion, we have provided pharmacological evidence that CsA and drugs affecting the two main CsA targets (CaN and mPTP) separately affect vascular dysfunction following CSD. We hypothesize that CSD causes transient formation of mPTP in a small population of highly Ca2+-sensitive mitochondria, most likely in interneurons, (Hazelton et al, 2009) with slow recovery of function. Activation of CaN contributes mechanistically to the development of persistent vascular changes accompanying CSD, as well. Therefore, we suggest that drugs targeting mPTP and CaN may be useful to treat vascular dysfunction in patients with migraine and acute cerebral cortical injury.
Acknowledgments
The authors thank Micael Lønstrup for expert laboratory assistance.
The authors declare no conflict of interest.
Footnotes
This study was supported by the Lundbeck Foundation via the Lundbeck Foundation Centre for Neurovascular Signalling (LUCENS), the NOVO Nordisk Foundation, the Danish Medical Research Council, the NORDEA foundation for Center for Healthy Aging, and Foundation Leducq.
References
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, Moskowitz MA. Pronounced hypoperfusion during spreading depression in mouse cortex. J Cereb Blood Flow Metab. 2004;24:1172–1182. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]
- Basarsky TA, Duffy SN, Andrew RD, MacVicar BA. Imaging spreading depression and associated intracellular calcium waves in brain slices. J Neurosci. 1998;18:7189–7199. doi: 10.1523/JNEUROSCI.18-18-07189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol. 2008;86:417–433. doi: 10.1016/j.pneurobio.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de BO, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Shook LL, Biag J, Nguyen EN, Toga AW, Charles AC, Brennan KC. Biphasic direct current shift, haemoglobin desaturation and neurovascular uncoupling in cortical spreading depression. Brain. 2010;133:996–1012. doi: 10.1093/brain/awp338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci. 2007;27:4036–4044. doi: 10.1523/JNEUROSCI.0721-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Korner K, Ebert N, Gorner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhaupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Akgoren N, Lauritzen M. Arginine-nitric oxide pathway and cerebrovascular regulation in cortical spreading depression. Am J Physiol. 1995;269:H23–H29. doi: 10.1152/ajpheart.1995.269.1.H23. [DOI] [PubMed] [Google Scholar]
- Florence G, Bonvento G, Charbonne R, Seylaz J. Spreading depression reversibly impairs autoregulation of cortical blood flow. Am J Physiol. 1994;266:R1136–R1140. doi: 10.1152/ajpregu.1994.266.4.R1136. [DOI] [PubMed] [Google Scholar]
- Friberg H, Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Calcineurin immunoreactivity in prelimbic cortex (area 32) of the rat. Brain Res. 1997;747:352–356. doi: 10.1016/s0006-8993(96)01376-5. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchez DR, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem. 1997;174:167–172. [PubMed] [Google Scholar]
- Hazelton JL, Petrasheuskaya M, Fiskum G, Kristian T. Cyclophilin D is expressed predominantly in mitochondria of gamma-aminobutyric acidergic interneurons. J Neurosci Res. 2009;87:1250–1259. doi: 10.1002/jnr.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J Cereb Blood Flow Metab. 2007;27:575–587. doi: 10.1038/sj.jcbfm.9600372. [DOI] [PubMed] [Google Scholar]
- Kruger H, Luhmann HJ, Heinemann U. Repetitive spreading depression causes selective suppression of GABAergic function. Neuroreport. 1996;7:2733–2736. doi: 10.1097/00001756-199611040-00065. [DOI] [PubMed] [Google Scholar]
- Lacombe P, Sercombe R, Correze JL, Springhetti V, Seylaz J. Spreading depression induces prolonged reduction of cortical blood flow reactivity in the rat. Exp Neurol. 1992;117:278–286. doi: 10.1016/0014-4886(92)90137-f. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J Cereb Blood Flow Metab. 1992;12:223–229. doi: 10.1038/jcbfm.1992.32. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Hansen AJ, Kronborg D, Wieloch T. Cortical spreading depression is associated with arachidonic acid accumulation and preservation of energy charge. J Cereb Blood Flow Metab. 1990;10:115–122. doi: 10.1038/jcbfm.1990.14. [DOI] [PubMed] [Google Scholar]
- Leao AAP. Spreading depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol. 1999;277:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- Mbye LH, Singh IN, Carrico KM, Saatman KE, Hall ED. Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:87–97. doi: 10.1038/jcbfm.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, Strickland S, Iadecola C. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci USA. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29:1517–1527. doi: 10.1038/jcbfm.2009.73. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Alvarez G, Ramos M, Hernandez M, Bogonez E, Satrustegui J. Cyclosporin A targets involved in protection against glutamate excitotoxicity. Eur J Pharmacol. 2000;404:29–39. doi: 10.1016/s0014-2999(00)00584-7. [DOI] [PubMed] [Google Scholar]
- Scheckenbach KEL, Dreier JP, Dirnagl U, Lindauer U. Impaired cerebrovascular reactivity after cortical spreading depression in rats: restoration by nitric oxide or cGMP. Exp Neurol. 2006;202:449–455. doi: 10.1016/j.expneurol.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Seitz I, Dirnagl U, Lindauer U. Impaired vascular reactivity of isolated rat middle cerebral artery after cortical spreading depression in vivo. J Cereb Blood Flow Metab. 2004;24:526–530. doi: 10.1097/00004647-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Sierra-Paredes G, Sierra-Marcuno G. Ascomycin and FK506: pharmacology and therapeutic potential as anticonvulsants and neuroprotectants. CNS Neurosci Ther. 2008;14:36–46. doi: 10.1111/j.1527-3458.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, Parkin MC, Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Yaseen MA, Sakadzic S, Ruvinskaya S, Sims JR, Boas DA, Moskowitz MA, Ayata C. Perfusion pressure-dependent recovery of cortical spreading depression is independent of tissue oxygenation over a wide physiologic range. J Cereb Blood Flow Metab. 2010;30:1168–1177. doi: 10.1038/jcbfm.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death. J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng WG, Lou NH, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- Zhou N, Gordon GRJ, Feighan D, MacVicar BA. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb Cortex. 2010;20:2614–2624. doi: 10.1093/cercor/bhq018. [DOI] [PubMed] [Google Scholar]