Abstract
Adenosine kinase (ADK) is the major negative metabolic regulator of the endogenous neuroprotectant and homeostatic bioenergetic network regulator adenosine. We used three independent experimental approaches to determine the role of ADK as a molecular target for predicting the brain's susceptibility to ischemic stroke. First, when subjected to a middle cerebral artery occlusion model of focal cerebral ischemia, transgenic fb-Adk-def mice, which have increased ADK expression in striatum (164%) and reduced ADK expression in cortical forebrain (65%), demonstrate increased striatal infarct volume (126%) but almost complete protection of cortex (27%) compared with wild-type (WT) controls, indicating that cerebral injury levels directly correlate to levels of ADK in the CNS. Second, we demonstrate abrogation of lipopolysaccharide (LPS)-induced ischemic preconditioning in transgenic mice with brain-wide ADK overexpression (Adk-tg), indicating that ADK activity negatively regulates LPS-induced tolerance to stroke. Third, using adeno-associated virus-based vectors that carry Adk-sense or -antisense constructs to overexpress or knockdown ADK in vivo, we demonstrate increased (126%) or decreased (51%) infarct volume, respectively, 4 weeks after injection into the striatum of WT mice. Together, our data define ADK as a possible therapeutic target for modulating the degree of stroke-induced brain injury.
Keywords: adenosine kinase, gene therapy, neuroprotection, stroke, transgenic mice
Introduction
Despite intensive research into the development of neuroprotective drugs, to date no clinically viable pharmacological therapy exists to prevent neuronal injury after stroke (Wahlgren and Ahmed, 2004). Therefore, endogenous neuroprotective mechanisms are of critical importance (Dirnagl et al, 2009) and might be exploited as alternative therapeutic approaches. Several endogenous neuroprotectant mechanisms are known, including the phenomenon of ischemic tolerance, in which a ‘preconditioning' event (e.g., a mild stroke, or a challenge with an endotoxin, such as lipopolysaccharide (LPS)) protects the brain against a subsequent stroke (Dirnagl et al, 2009). Although effective in blunting the deleterious effects of a stroke, the underlying mechanisms of ischemic preconditioning are only partly understood. Epigenetic mechanisms might be involved in this phenomenon (Stenzel-Poore et al, 2003), as direct neuroprotective mediators have been identified by genomic and proteomic analyses (Dhodda et al, 2004).
The purine ribonucleoside adenosine is an endogenous neuroprotectant of the brain, which is uniquely poised to provide homeostatic bioenergetic network regulation due to its direct biochemical and pharmacological interactions with energy homeostasis, nucleic acid metabolism, methylation status, and adenosine receptor dependent signaling pathways (Boison et al, 2002; Cunha, 2005; Fredholm, 2007; Newby et al, 1985; Ribeiro et al, 2003; Stone et al, 2009). As a bioenergetic regulator, adenosine can directly affect the equilibrium of enzymatic pathways including transmethylation reactions (e.g., of DNA methylation) (Boison et al, 2002). Because of its ability to affect basic biochemistry (i.e., adenosine receptor independent effects) as well as specific pathways linked to adenosine receptors, adenosine is strategically positioned to affect several molecular pathways synergistically and thereby to provide homeostatic control of whole networks. In brain, synaptic levels of adenosine are largely regulated by an astrocyte-based adenosine cycle (Boison et al, 2010). Astrocytes express two types of equilibrative nucleoside transporters, and reuptake of adenosine is driven by intracellular phosphorylation of adenosine into 5′-adenosine monophosphate via adenosine kinase (ADK) (Boison et al, 2010).
Brain injury is characterized by an acute surge of adenosine, which likely constitutes an endogenous protective response of the brain (Clark et al, 1997). Specifically, adenosine levels have been shown to increase rapidly in the infarcted hemisphere following the induction of middle cerebral artery occlusion (MCAO) (Melani et al, 1999). This increase in adenosine may act as an acute neuroprotectant, as well as an inducer of delayed neuroprotection. Strikingly, the key regulatory enzyme of adenosine, ADK, is also endogenously regulated after stroke, and its expression is decreased following onset of injury thus potentiating the adenosine surge (Pignataro et al, 2008). Thus, expression levels of ADK might have a crucial role in determining the brain's susceptibility to stroke-induced injury. Indeed, transgenic mice overexpressing ADK are highly susceptible to stroke-induced brain injury (Pignataro et al, 2007). We therefore hypothesized that experimental or therapeutic manipulations that reduce ADK expression would confer a neuroprotective phenotype to the brain similar to ischemic preconditioning. This finding would be highly meaningful, as modulation of brain ADK levels may be a viable target for therapeutic manipulation in clinical stroke. We further hypothesized that ischemic preconditioning would be abrogated under conditions of increased adenosine clearance, such as ADK overexpression, as an acute surge of adenosine may serve as a key initiator of delayed ischemic tolerance.
To address our research hypotheses we pursued three independent experimental approaches:
(1) Using a transgenic mouse model with reduced ADK in forebrain (fb-Adk-def), we demonstrate almost complete protection of cortical structures from the deleterious effects of 60 minutes of MCAO. (2) Using transgenic mice with brain-wide overexpression of ADK (Adk-tg), we demonstrate abrogation of ischemic preconditioning. (3) Using an adeno-associated virus (AAV)-based gene therapy approach, we demonstrate that knockdown of ADK can protect the brain from the effects of a stroke. Together, our data define a novel role for ADK as a critical molecular determinant of injury severity after stroke; in addition, our data imply that a knockdown of ADK in the brain may lead to a state of permanent ischemic tolerance.
Materials and methods
Animals
All animal procedures were conducted in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care in accordance with protocols approved by the Legacy Institutional Animal Care and Use Committee and the principles outlined by the National Institute of Health. Male C57BL/6 wild-type (WT) mice (Charles River, Wilmington, MA, USA) and fb-Adk-def and Adk-tg mutants of the same genetic background weighing 25 to 30 g were housed under diurnal lighting conditions (12-hour/12-hour cycle). The fb-Adk-def mutant line was recently created by cross-breeding Emx1-Cre-Tg3 mice expressing Cre-recombinase in neurons and astrocytes of the telencephalon with ADK transgenic mice (‘Adk-tg mice') carrying a loxP-flanked ADK transgene (Adktm1−/−:TgUbiAdk) in an otherwise lethal ADK knockout (Adktm1−/−) background (Li et al, 2008). The resulting Adktm1−/−:Tg(UbiAdk):Emx1-Cre-Tg3 mice (referred to as ‘fb-Adk-def mice') are triple mutants homozygous for the deletion of the endogenous Adk gene, homozygous for the Adk-transgene, and heterozygous for Cre. These animals have a forebrain-selective reduction of ADK in cortical and hippocampal regions, while ADK continues to be overexpressed in striatum in analogy with the Adk-tg mice. Mutant animals for this study were generated by breeding Adk-tg mice with fb-Adk-def mice, resulting in both genotypes as littermates in a 1:1 ratio.
Focal Ischemia–Reperfusion Model
Transient focal ischemia (30 or 60 minutes) was induced by occlusion of the MCA in mice anesthetized using 1.5% isoflurane, 70% N2O, and 28.5% O2. Ischemia was induced by introducing a coated filament (6.0; Doccol, Redlands, CA, USA) from the external carotid artery into the internal carotid and advancing it into the arterial Circle of Willis, thereby occluding the MCA. The suture was maintained intraluminally for 30 or 60 minutes, and was then removed to restore blood flow. Regional cerebral blood flow was monitored by transcranial laser Doppler flowmetry (Transonic System Inc., Ithaca, NY, USA) throughout surgery to confirm occlusion of the MCA. Mouse body temperature was maintained at 36°C±0.5°C with a thermostat-controlled heating pad (Harvard Apparatus, Holliston, MA, USA). All surgical procedures were performed under an operating stereomicroscope.
Lipopolysaccharide Preconditioning
Lipopolysaccharide preconditioning was conducted as previously described (Rosenzweig et al, 2004) with minor modifications. Briefly, adult male WT C57BL/6 mice and Adk-tg mutants received an intraperitoneal injection of phenol-extracted LPS (0.2 mg/kg, in a volume of 0.1 mL/10 g bodyweight) from Escherichia coli 055:B5 (L-2880; Sigma, St Louis, MO, USA). Control mice received an intraperitoneal injection of sterile saline of the same volume. Three days after injection, all mice were challenged with either 30 or 60 minutes of MCAO. After 23 hours of reperfusion, mice were killed for histologic evaluation of infarct volume.
Determination of the Infarct Volume
To quantify infarct volume, two methods were used: 2,3,5-triphenyltetrazolium hydrochloride (TTC) staining and Nissl staining. Mice subjected to MCAO were killed 23 hours after ischemia. For TTC staining, perfused mouse brains were removed and placed into a mouse brain mold calibrated for dissection of 1 mm slices at regular intervals. Six adjacent 1 mm thick coronal sections were stained by immersion in 2% (w/v) TTC. For Nissl staining, mouse brains were first processed as described in the Immunohistochemistry section below and then subjected to a standard Nissl staining procedure (Li et al, 2008). Infarct volume was calculated according to published procedures (Rosenzweig et al, 2004). Cortical and striatal infarct volumes were calculated separately by subtracting undamaged areas of the ipsilateral hemisphere from the contralateral nonischemic hemisphere. Total infarct volume was calculated by summing area of infarct from six sections and multiplying by slice thickness. Percent infarct was calculated as described previously; results are reported as indirect infarct volume (Pignataro et al, 2007).
Intrastriatal Delivery of AAV8
Generation of AAV-based vectors targeting ADK was performed as described (Theofilas et al, 2011). Briefly, constructs expressing the cytoplasmic isoform of ADK in either sense, to overexpress ADK (‘ADK-SS'), or antisense orientation, to knockdown ADK (‘ADK-AS'), under the control of the gfaABC1D promoter (Lee et al, 2008). To express the constructs selectively in astrocytes, either ADK-SS or ADK-AS were packaged into AAV serotype 8-based vectors. Control vectors contained either an empty vector backbone (‘AAV-null') or expressed a green fluorescent protein under the control of the same promoter (‘AAV-GFP'). To manipulate local ADK expression, intrastriatal injections of ADK-SS, ADK-AS, AAV-null, and AAV-GFP were performed in adult male WT C57BL/6 mice under general anesthesia (1.5% isoflurane, 28.5% O2, and 70% N2O) using stereotactic procedures (AP=1.0 mm; ML=−1.6 mm; DV=−3.4 mm). Viral particles were unilaterally injected using a 5-μL Hamilton syringe with a 34-gauge stainless steel injector (Plastics One, Roanoke, VA, USA) in 2 μL of concentrated viral solutions (1 × 1012 genomic particles/mL) at a rate of 0.5 μL/min. The needle was left in place for an additional 3 minutes after injection to minimize reflux. Four weeks after virus injection, animals were subjected to MCAO followed by histologic analysis. To evaluate efficiency of virus delivery, AAV-GFP was coinjected with ADK-SS or ADK-AS at a 1:1 (v/v) ratio in another set of animals, which were also killed at 4 weeks for histologic evaluation.
Immunohistochemistry
To determine changes in the pattern of ADK expression, naive fb-Adk-def and WT mice as well as virus-injected WT mice that were not subjected to MCAO were killed for immunohistochemical analysis as previously described (Studer et al, 2006) with slight modification. Mice were transcardially perfused with saline followed by 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde, cryoprotected in 10% dimethylsulfoxide in phosphate-buffered saline (v/v) overnight, and then sectioned into 40 μm coronal sections using a vibratome (VT 1000 S, Leica, Bannockburn, IL, USA). For the detection of ADK, brain slices were incubated overnight at 4°C with primary anti-ADK diluted 1:5,000 in Tris-Triton, pH 7.4 with 2% normal serum, and 0.2% Triton X-100. Sections were then washed in TBS plus 0.05% Triton X-100, pH 7.4. For immunofluorescence staining, this was followed by a 30-minute incubation at room temperature in donkey anti-mouse secondary antibody, conjugated to Cy3 (1:300) (Jackson ImmunoResearch, West Grove, PA, USA). The sections were then washed and mounted on gelatin-coated slides and coverslipped with Dako fluorescent mounting medium (Carpenteria, CA, USA). For Immunoperoxidase staining, after incubation with primary anti-ADK antiserum, slices were incubated for 30 minutes in a biotinylated goat anti-rabbit secondary antibody (1:300). After washing, brain slices were incubated with avidin–biotin enzyme complex (Vectastain Elite Kit; Vector Labs, Burlingame, CA, USA) for 20 to 30 minutes, followed by incubation in hydrogen peroxide and 3,3′-diaminobenzidine hydrochloride (Sigma). The sections were mounted on gelatin-coated slides, air-dried, dehydrated, and cover slipped (Studer et al, 2006). Digital images of ADK immunohistochemistry were acquired using a Zeiss AxioPlan inverted microscope equipped with an AxioCam 1Cc1 camera (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA); all images were acquired under identical conditions and all image processing was applied identically across different experimental groups.
Western Blot Analysis
To quantify ADK expression, the striatum or cortex of naive adult fb-Adk-def mutants and WT mice (n=5 per genotype), or AAV8-virus-injected WT mice (n=4 per AAV8-virus subtype) were processed for aqueous protein extraction as described (Theofilas et al, 2011). Cell extracts were standardized to 40 μg protein per lane, and electrophoresed in a 10% Tris-glycine gel. After transfer, membranes were incubated in polyclonal rabbit antiserum against ADK (1:5,000), followed by incubation with peroxidase-conjugated anti-rabbit antibody (#7074, 1:8,000, Cell Signaling, Boston, MA, USA). Immunoblots were quantified using a Kodak Scientific Imaging System (v3.6.5.k2, Kodak, Rochester, NY, USA). To normalize ADK immunoreactivity to protein loading, a mouse monoclonal anti-α-tubulin antibody (# sc-8035, 1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used to reprobe the same blot and the OD ratio of ADK to α-tubulin was calculated.
In Vivo Biosensor Measurements
The adenosine biosensors used in this study were microelectrodes covered with an enzyme matrix containing adenosine deaminase and xanthine oxidase. Enzymatic degradation of adenosine creates an electrochemical gradient that can be quantified using a potentiostat. Selectivity for adenosine is controlled for by including inosine sensors that do not contain adenosine deaminase. Subtraction of the ‘inosine' signal (background) from the ‘adenosine' signal is used to calculate the tone of adenosine. This method has been described in detail elsewhere (Etherington et al, 2009). Adenosine (#SA-1003-05) and inosine (#SA-1004-05) microelectrode biosensors were purchased from Sarissa Biomedical (Coventry, UK). Before use, the sensors were rehydrated in buffer A (2 mmol/L NaH2PO4 buffer, pH 7.4, 100 mmol/L NaCl, 1 mmol/L MgCl2, 2 mmol/L glycerol) overnight and precalibrated with 40 μmol/L adenosine or inosine in Buffer A, according to the manufacturer's protocol. To measure in vivo adenosine levels, mice were affixed in a stereotactic frame under anesthesia (1.5% isoflurane, 70% N2O, and 28.5% O2). A 15-mm skin incision was made along the sagittal suture to expose the skull of the animals, and a hole was drilled through the skull with the center corresponding to the following coordinates: AP −1.00 mm, ML +1.60 mm. After breaching the dura, a minireservoir was created around the hole using a silicon gasket (diameter of 8 mm) affixed to the skull using Loctite glue (#01-06966, Henkel Co., Düsseldorf, Germany) (Figure 2B). The minireservoir was continuously perfused with HEPES-buffered saline (HBSS; containing 140 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 3 mmol/L KCl, 10 mmol/L HEPES, and 10 mmol/L glucose, 7.4 pH, sterile filtered) throughout the experiment. Precalibration to determine sensor response was performed by adding solutions of adenosine, inosine, and serotonin to the minireservoir (Figure 2B). The biosensor was mounted on a stereotaxic manipulator and inserted into the brain at the following coordinates: AP=−1.00 mm, ML=+1.60 mm, and DV=−1.50 mm, and AP=−1.00 mm, ML=+1.60 mm, and DV=−3.40 mm, for cortical and striatal adenosine quantifications, respectively. Sensor signals were amplified using a Duostat ME 200+ Amplifier (Sarissa, UK), digitized (PowerLab, AD Instruments, Colorado Springs, CO, USA), and recorded using LabChart Pro (AD Instruments). Adenosine tone was calculated using averages after currents had stabilized (typically 35 minutes after probe insertion into brain). Following removal from the brain sensors were allowed to equilibrate in HBSS (Figure 2A). The difference between the in-brain and in-HBSS measurements corresponds to local adenosine (or inosine) tone. Inosine sensors were used to normalize adenosine levels following the same procedure. After each measurement, the sensor was recalibrated using 10 μmol/L adenosine or 10 μmol/L inosine, as appropriate, in HBSS with 1 μmol/L NaH2PO4, and the inosine signal was subtracted from the adenosine signal to calculate the adenosine tone. Data from fb-Adk-def mice (n=3) were normalized against data derived from WT mice (n=3) using the same approach.
Statistical Analysis
Values are expressed as means±s.e.m. Statistical analysis was performed with one-way analysis of variance followed by post hoc analysis or Student's t-test. P<0.05 and P<0.01 were accepted as statistical significance.
Results
Adenosine Kinase Expression Levels in fb-Adk-def Mice Determine the Tone of Ambient Adenosine
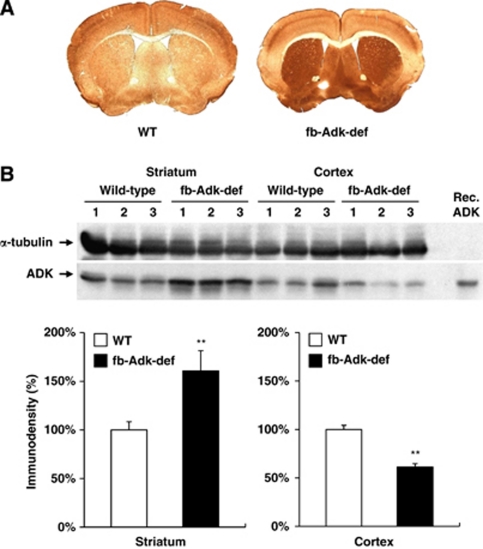
To determine whether experimental manipulation of ADK expression affects the tone of ambient adenosine, we made use of transgenic mice with a dichotomous expression pattern of ADK in brain: fb-Adk-def mice are triple mutants based on (1) systemic deletion of the endogenous (subject to endogenous regulation) Adk-gene, (2) expression of a ubiquitously (not subject to endogenous regulation) expressed loxP-flanked Adk-transgene leading to brain-wide overexpression of ADK, and (3) expression of Cre-recombinase under the control of an Emx1 promoter leading to forebrain-selective reduction of ADK (Li et al, 2008). To assess the consequences of this genetic manipulation on ADK expression levels within the striatum and cerebral cortex, we first performed ADK immunohistochemistry on brains from naive adult male fb-Adk-def or WT mice. As expected, fb-Adk-def mice had significantly reduced cortical ADK expression compared with WT mice, whereas expression levels of striatal ADK remained high as seen in Adk-tg mice (Figure 1A). The regional changes in ADK expression were further characterized by Western blot analysis performed using microdissected brain regions, that is cortex and striatum, derived from fb-Adk-def and WT mice (Figure 1B, upper panel). Quantification of the Western blot by densitometry confirmed the immunohistochemical findings of reduced ADK levels in the cortex of fb-Adk-def mice (64.6%±4.6%, n=10, P<0.01) and increased ADK levels in the striatum (163.6%±6.4%, n=10, P<0.01; Figure 1B, lower panel), compared with WT controls.
Figure 1.
Differential expression pattern of striatal and cortical adenosine kinase (ADK) in fb-Adk-def mice. Regional expression of ADK was evaluated in the brain of naive wild-type (WT) and fb-Adk-def mice. (A) Representative immunohistochemical staining with ADK primary antibody in WT (left) and fb-Adk-def (right) mice. (B) (Top) Representative Western blot of ADK from the striatum or cortex of adult WT and fb-Adk-def mutant mice. (Bottom) Quantitative analysis of striatal (left panel) and cortical (right panel) ADK levels based on two replicates of Western blots performed with samples from n=5 animals from each genotype. ADK levels were first normalized for loading using a α-tubulin standard. ADK levels are shown as relative to striatal or cortical ADK levels in WT mice (set as 100%). Data are displayed as mean±s.e.m. **P<0.01 paired comparisons t-test.
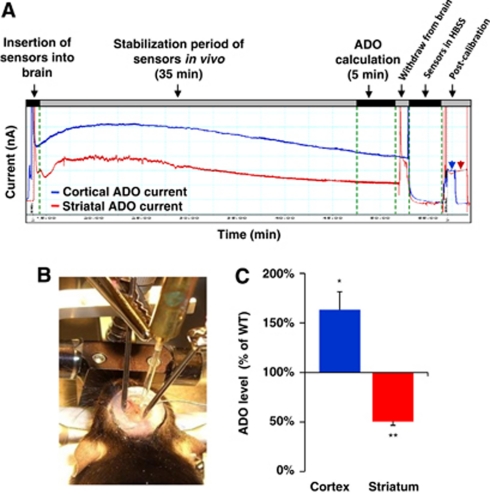
Since ADK is the key adenosine metabolizing enzyme in the adult mouse brain, any change in ADK expression is expected to result in changes in ambient adenosine. To demonstrate changes in ambient adenosine as a result of the genetic manipulation in fb-Adk-def mutants, we used adenosine microelectrode biosensors (Dale et al, 2005) to measure regional adenosine levels in vivo (Figures 2A and 2B). Corresponding to the regional overexpression of ADK, striatal adenosine levels in fb-Adk-def mice were significantly lower (50.4%±3.9%) than in the striatum of WT mice (n=3, P<0.01). Conversely, cortical adenosine levels in fb-Adk-def mice were significantly higher (163.3%±17%) than in WT mice (n=3, P<0.05; Figure 2C), in line with reduced ADK expression in cerebral cortex. To control for the specificity of the adenosine-biosensor measurements to adenosine, we performed analogous inosine-biosensor recordings and found no differences between cortical and striatal inosine levels in the fb-Adk-def mutants (data not shown). To exclude systematic errors by surgery-induced influences on the adenosine tone, all data were normalized and presented as relative changes compared with WT. Our data demonstrate that regionally restricted changes in ADK expression translate into significant changes in ambient adenosine levels.
Figure 2.
Regional adenosine kinase (ADK) manipulation causes focal changes in adenosine levels. The adenosine and inosine biosensors were used for in vivo real-time determination of adenosine levels in the striatum of fb-Adk-def mutants. Fb-Adk-def mutant mice have increased adenosine in the cortex, and reduced adenosine in the striatum when compared with wild-type (WT) mice. (A) Representative traces of the real-time adenosine measurements and postcalibration. Biosensors were precalibrated with adenosine, inosine, and serotonin then inserted into the cortex or striatum for real-time measurement. After removal, biosensors were postcalibrated with adenosine. (B) Biosensors were mounted on a stereotaxic manipulator and in vivo measurements were performed in a minireservoir that was continuously perfused with HEPES-buffered saline. (C) Statistic analysis of cortical and striatal adenosine (ADO) levels in fb-Adk-def mutants versus WT mice (n=3, per genotype). *P<0.05 and **P<0.01 versus cortex or striatum in WT mice, respectively.
Cortical Reduction of Adenosine Kinase Provides Regional Protection from Stroke
To test whether the genetic reduction of ADK in the cortex of fb-Adk-def mice could confer resistance to stroke-induced brain injury, we subjected fb-Adk-def mice to a paradigm of MCAO that modeled transient focal ischemia. Adult male WT (n=10) and fb-Adk-def mice (overexpression of ADK in striatum, but reduced expression of ADK in cortex; Figure 1) (n=11) were subjected to 60 minutes of MCAO followed by 23 hours of reperfusion. In contrast to the lethal outcome of 60 minutes of MCAO in Adk-tg mice (which globally overexpress ADK in the brain) (Pignataro et al, 2007), all WT and fb-Adk-def mice survived until sacrifice, 23 hours after injury (data not shown). Thus, the regional reduction of ADK in the cortex of an ADK-overexpressing brain is sufficient to prevent a lethal outcome after focal ischemia.
To investigate whether the local reduction of ADK in the cortex of fb-Adk-def mice provides regional or global protection against ischemic neuronal cell death, we evaluated the infarct volume of the cortex and striatum separately by 2,3,5-triphenyltetrazolium chloride (TTC) staining of brain sections after 23 hours of reperfusion. In WT mice, the indirect striatal and cortical infarct volumes were 56.1%±4.7% and 68.3%±4.4%, respectively, as compared with the volume of the contralateral hemisphere (n=10, WT mice; Figure 3). In contrast, fb-Adk-def mice had significant increase in (69.3%±4.5%) indirect infarct volume in striatum (n=11, P<0.05 versus WT; Figure 3), which equated to a 126% increase in infarct size relative to the WT controls. Most strikingly, in the cortex the indirect infarct volume in fb-Adk-def mice was reduced to 18.5%±2.5% (n=11, P<0.01, versus WT; Figure 3), a 73% reduction in cortical infarct volume relative to WT controls. These data indicate that a regional downregulation of ADK in the cortex provides localized protection against ischemic injury, even when in direct proximity to an area (striatum) with increased ADK and increased injury. Additionally, protection of the cortex in fb-Adk-def mice was found to be sufficient to prevent the lethal outcome seen after ischemia associated with global ADK overexpression in Adk-tg mice.
Figure 3.
Local downregulation of adenosine kinase (ADK) attenuates ischemic neuronal injury in the cortex. After 60 minutes of middle cerebral artery occlusion (MCAO) and 23 hours of reperfusion, mice were killed and the brains were 2,3,5-triphenyltetrazolium chloride (TTC) stained to evaluate ischemic damage. Healthy tissue stains pink, while damaged tissue remains white. (A) Representative TTC staining of wild-type (WT, left) and fb-Adk-def (right) mice. The white arrow indicates the cortical region protected against MCAO, while the yellow arrow indicates the region of exacerbated damage in the striatum of fb-Adk-def mice. (B) Quantification of indirect infarct volume in the cortex (left panel) and striatum (right panel) of WT (white bars, n=10) and fb-Adk-def mutant (black bars, n=11) mice. Data are displayed as mean±s.e.m. *P<0.05 and **P<0.01, versus WT mice.
Adenosine Kinase Activity Negatively Regulates Lipopolysaccharide-Induced Stroke Tolerance
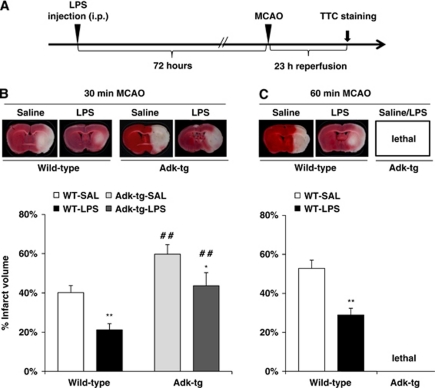
To assess whether ADK activity modulates ischemic tolerance, Adk-tg mutants, which have brain-wide overexpression of ADK, and WT mice were subjected to a single systemic injection of LPS, a known preconditioning agent, 3 days before MCAO (Figure 4A). In the absence of LPS preconditioning, saline-injected Adk-tg mice demonstrated significantly enlarged (59.8%±4.9%) infarct volume following 30 minutes of MCAO, compared with WT controls (40.2%±3.5%, P<0.05, n=8/group; Figure 4B). In WT mice, LPS preconditioning provided protection against stroke following either 30 or 60 minutes of MCAO by significantly reducing infarct volume (30 minutes of MCAO: 21.2%±3.2% versus 40.2%±3.5% of saline control; and 60 minutes of MCAO: 28.9%±3.4% versus 52.8%±4.2% of saline control, P<0.01, n=8/group; Figures 4B and 4C). The level of protection afforded by LPS preconditioning in WT mice in this study is consistent with previous studies (Rosenzweig et al, 2004). Interestingly, LPS-mediated ischemic tolerance was blunted by ADK overexpression in Adk-tg mice. This falls in line with our hypothesis that an acute surge of adenosine is required to initiate preconditioning as previous work using real-time biosensor measurements in vivo have shown levels of adenosine increase significantly following systemic administration of LPS (Gourine et al, 2007). Likewise, a recent clinical study has demonstrated increases in circulating adenosine following LPS administration to human subjects (Ramakers et al, 2011). In addition, we have demonstrated previously that adenosine levels in brain rise as a consequence of ischemic preconditioning (Pignataro et al, 2008). Lipopolysaccharide-preconditioned Adk-tg mice subjected to 30 minutes of MCAO had only a moderate decrease in infarct volume (43.6%±6.7%, versus 59.8%±4.9% of control, P<0.05, n=7 to 8/group; Figure 4B); and 60 minutes of MCAO was found to be lethal in Adk-tg mice with or without LPS preconditioning (Figure 4C). Together, these data demonstrate that increased ADK activity exacerbates stroke-related injury and attenuates the endogenous neuroprotective mechanism of LPS-induced ischemic preconditioning.
Figure 4.
Increased adenosine kinase (ADK) prevents lipopolysaccharide (LPS)-induced ischemic tolerance in Adk-tg mutants. (A) Schematic illustration of the treatment paradigm for LPS preconditioning. Three days post-LPS (0.2 mg/kg, intraperitoneally) mice received either 30 or 60 minutes of middle cerebral artery occlusion (MCAO). Animals were killed after 23 hours of reperfusion and the brains were collected for 2,3,5-triphenyltetrazolium chloride (TTC) staining. (B) (Top) Representative TTC staining from wild-type (WT) and Adk-tg mice injected with LPS (0.2 mg/kg, intraperitoneally). Mice were given 30 minutes MCAO followed by 23 hours of reperfusion. (Bottom) Quantification of indirect infarct volume in WT and Adk-tg mice injected with either LPS (0.2 mg/kg, intraperitoneally) or saline (SAL) 72 hours before 30 minutes MCAO and 23 hours of reperfusion (n=7 to 8/per group). (C) (Top) Representative TTC staining from WT mice injected with LPS (0.2 mg/kg, intraperitoneally) 72 hours before 60 minutes MCAO and 23 hours of reperfusion. (Bottom) Quantification of indirect infarct volume (n=7 to 8/per group). Data are shown as mean±s.e.m. *P<0.05, **P<0.01 LPS versus saline-treated groups. ##P<0.01 Adk-tg versus WT.
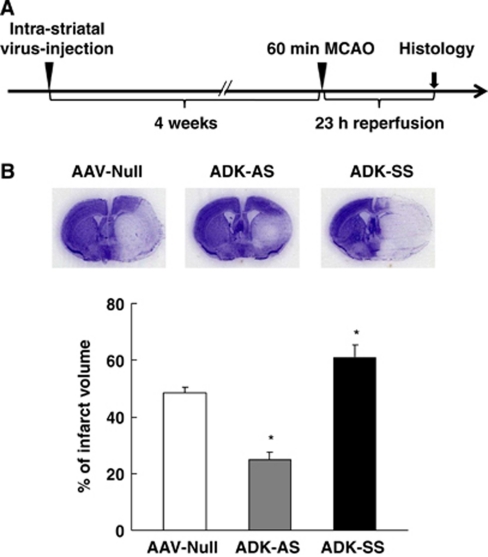
Modulation of Stroke Susceptibility by Regional Overexpression or Knockdown of Adenosine Kinase with an Adeno-Associated Virus-Based Vector System
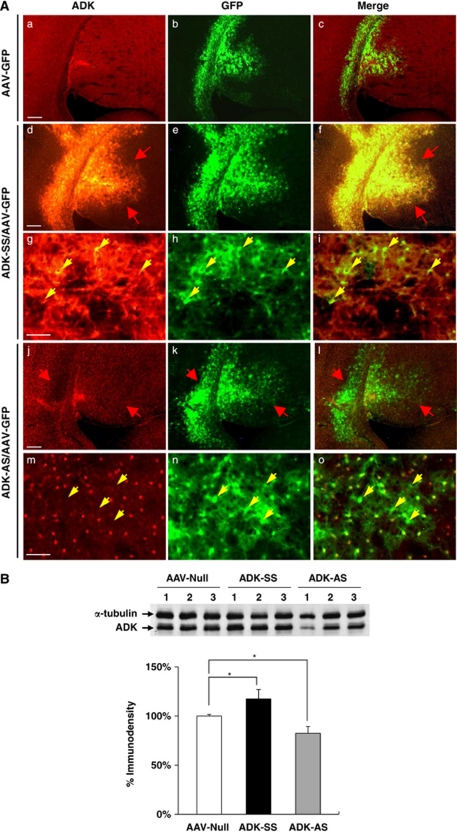
To determine if selective targeting of ADK expression has therapeutic potential in reducing stroke-induced brain injury, we constructed two novel AAV8-based vectors expressing ADK in either a sense (AAV8-pGfa-Adk-SS, ‘ADK-SS') or antisense (AAV8-pGfa-Adk-AS, ‘ADK-AS') orientation under the control of an astrocyte-specific gfaABC1D promoter (Lee et al, 2008). First, to evaluate the regional pattern of ADK expression following viral manipulation, ADK-SS or ADK-AS was unilaterally coinjected with an AAV-GFP reporter virus (1:1 volume) into the striatum of naive C57BL/6 mice. Four weeks after intrastriatal injection, the ADK-SS/AAV-GFP-injected mice were characterized by a robust increase in ADK expression (Figures 5Ad–5Af), compared with basal ADK levels observed in mice that received only AAV-GFP injection (Figures 5Aa–5Ac). At higher magnification, the ADK-SS/AAV-GFP-injected striatum showed cellular features indicative of ADK overexpression that colocalized with, and was confined to AAV-GFP-infected cells (Figures 5Ag–5Ai, arrows). Conversely, the ADK-AS/AAV-GFP-injected mice demonstrated a moderate decrease in ADK expression (Figures 5Aj–5Al), as compared with endogenous ADK levels in the AAV-GFP-injected striatum (Figure 5Aa). Abrogation of ADK expression was most prominent in GFP-expressing cells (Figures 5Am–5Ao, arrows).
Figure 5.
Modulation of adenosine kinase (ADK) expression with an adeno-associated virus (AAV)-based vector system. (A) Immunohistofluorescence of ADK (red) and green fluorescent protein (GFP) (green) in wild-type (WT) mice injected with AAV-GFP (a–c), or coinjection of AAV-GFP with either ADK-SS (d–i), or ADK-AS (j–o). (a–c) Representative immunohistofluorescence showing basal ADK levels and AAV-virus expression pattern in AAV-GFP-injected WT mice. (d–f) ADK-SS/AAV-GFP coinjection causes a robust increase in ADK immunoreactivity (red arrows). (g–i) Higher magnification images of the ADK-SS/AAV-GFP coinjection site shows that ADK colocalizes with AAV-GFP-infected cells (yellow arrows). (j–l) ADK-AS/AAV-GFP coinjection in WT mice causes a decrease in ADK immunoreactivity (red arrows). (m–o) Higher magnification images of the ADK-AS/AAV-GFP coinjection site show that cells lacking ADK colocalize with AAV-GFP-infected cells (yellow arrows). (B) (Top) Representative Western blot of ADK from adult WT mice injected with AAV-null, ADK-SS, or ADK-AS virus. (Bottom) Quantitative analysis of ADK levels based on two separate Western blots performed with samples from n=4 animals for each injection type. Protein loading was normalized to the α-tubulin standard before intergroup comparison. Values are displayed as relative to ADK protein levels in AAV-null (set as 100%) brain. Data represent the mean±s.e.m., n=4. *P<0.05 versus AAV-null group.
Regional changes in ADK expression were quantified by Western blot analysis using the entire striatum from WT mice injected with ADK-AS, ADK-SS, or AAV-null viruses (n=4, each group). Four weeks after intrastriatal injection of virus we observed a 17% decrease and 18% increase in striatal ADK expression levels in mice receiving ADK-AS or ADK-SS, respectively (P<0.05 versus the AAV-null control; Figure 5B). Together, these data demonstrate that our novel AAV8-Gfap-Adk-SS and AAV8-Gfap-Adk-AS viral vectors are effective in modulating ADK expression levels in vivo.
AAV8-Based Knockdown of Adenosine Kinase Reduces Ischemic Brain Injury
To assess whether AAV8-based overexpression of ADK renders the brain more vulnerable to cerebral ischemic injury and to evaluate whether viral downregulation of ADK is effective in protecting the brain against injury induced by stroke, we subjected ADK-SS and ADK-AS-injected mice (n=7 to 8/group) to 60 minutes of MCAO. Brain infarct volumes were evaluated after 23 hours of ischemia–reperfusion (Figure 6A). As expected, the ADK-AS and ADK-SS viruses had opposing effects on the degree of cerebral ischemic injury. Compared with the AAV-null virus-injected mice, which had an infarct volume of 48.6%±2% (n=8), the ADK-SS virus-injected mice displayed a significantly enlarged infarct volume of 61.8%±4.4% (n=7, P<0.05; Figure 6B). This is equivalent to a 25.9% increase relative to the AAV-null controls. More importantly, brain injury in ADK-AS virus-injected mice was significantly attenuated, resulting in a final infarct volume of 24.8%±2.9% (n=7, P<0.05), a 48.8% reduction compared with the null-virus-injected mice (Figure 6B). These data indicate that viral-mediated downregulation of ADK protects the brain against ischemic injury whereas upregulation of ADK exacerbates stroke-induced neuronal cell death. Taken together, these results provide proof of the concept that therapeutic manipulations that decrease levels of ADK in the brain can protect the brain from ischemic injury.
Figure 6.
Modification of infarct volume by viral overexpression or underexpression of adenosine kinase (ADK). (A) Schematic illustration of the treatment paradigm for wild-type (WT) mice given intrastriatal virus injection (adeno-associated virus AAV-null, ADK-AS, or ADK-SS) followed by 60 minutes middle cerebral artery occlusion (MCAO) (4 weeks after virus injection) followed by 23 hours of reperfusion. (B) (Top) Representative Nissl staining of brains from WT mice injected with AAV-null, ADK-AS, or ADK-SS 4 weeks prior to 60 minutes MCAO and 23 hours of reperfusion. Mice that received ADK-AS virus injections had smaller infarcts and mice who received ADK-SS virus had larger infarct volumes when compared with mice that received an injection of AAV-null virus. (Bottom) Quantification of indirect infarct volume in WT mice injected with AAV-null, ADK-AS, or ADK-SS virus (n=7 to 8/group) before 60 minutes MCAO followed by 23 hours of reperfusion. Data are shown as mean±s.e.m. *P<0.05, versus AAV-null virus-injected controls.
Discussion
Acute brain injury can result in neuroprotection and tolerance to subsequent injury (Dirnagl et al, 2009). However, the molecular effectors of this endogenous neuroprotection are incompletely known. Acute brain injury, in particular after trauma, stroke, or seizures, is associated with a surge of the brain's endogenous neuroprotectant adenosine (Clark et al, 1997; Pignataro et al, 2008), which can (1) increase the acute neuroprotective capacity of the brain and (2) trigger downstream events that create a state of delayed protection that can protect the brain from subsequent injury. As increases in brain adenosine, in response to changes in ADK expression, have been shown to be both neuroprotective and antiepileptic in acute seizure models (Li et al, 2008), ADK expression levels may also determine the degree of neuroprotection and tolerance in ischemia. Thus, the adenosine-ADK system may be a candidate as an endogenous effector of acute and delayed neuroprotection (tolerance).
Homeostatic Bioenergetic Network Regulation Through Adenosine Kinase
In contrast to conventional pharmacotherapeutic approaches that aim to achieve specificity by selective targeting of specific molecular pathways, our goal here was to broadly affect homeostatic bioenergetic network regulation by modulating the availability of adenosine through ADK manipulation. Biochemically, adenosine links energy homeostasis with nucleic acid metabolism (Newby et al, 1985) and is an important feedback regulator of transmethylation reactions (Boison et al, 2002; Studer et al, 2006), including DNA methylation, and thus ideally poised to regulate homeostatic networks through bioenergetic and epigenetic mechanisms. For example, adenosine, as degradation product of ATP is intricately linked to mitochondrial bioenergetics (Masino and Geiger, 2008) and the activity of ADK is regulated by ATP, ADP, 5′-adenosine monophosphate, and adenosine with the implication that ADK fulfills the role of a sensor of the bioenergetic state of a cell (Newby et al, 1985). In addition, as a regulator of major biochemical functions, changes in the tone of adenosine may lead to long-lasting epigenetic changes, since adenosine is a regulator of transmethylation reactions, including DNA methylation (Boison et al, 2002; Mato et al, 2008). Those adenosine receptor independent activities of adenosine need to be distinguished from signaling pathways that depend on stimulation of four types of G-protein-coupled adenosine receptors (A1, A2A, A2B, A3). Adenosine receptor dependent pathways are known to contribute to the protective role of adenosine in the delayed response to ischemic injury through its antiinflammatory actions (Yu et al, 2004), modulation of the neuroimmune response and role in promoting angiogenesis and tissue remodeling (Hasko et al, 2005). The role of specific adenosine receptors in the context of stroke however varies with level and duration of stimulation, and is highly affected by temporal and spatial relationships. As such, the precise timing, duration and level of stimulation needed at the receptor level to provide neuroprotection is likely to be impossible to mimic pharmacologically. For this reason, we have focused on using homeostatic bioenergetic network regulation as a novel strategy to manipulate global adenosine expression through its key regulatory enzyme, ADK. In this manner, we can optimally take advantage of synergistic homeostatic bioenergetic network effects of adenosine, mediated through the sum of all adenosine receptor dependent and independent effector systems. Using transgenic as well as gene therapy-based manipulations of ADK in mice, we were uniquely poised to study the net effects of adenosine modulation on acute and delayed neuroprotection.
Adenosine and Adenosine Kinase in Acute Neuroprotection
Adenosine has long been recognized for its potential as an acute neuroprotectant in the context of cerebral ischemic injury (Fredholm et al, 2005). Levels of adenosine are tightly regulated by physiological and pathophysiological changes that occur during the acute phase of ischemic injury, such as metabolic stress, vasodilatation or vasoconstriction, platelet aggregation (Phillis, 2004) and the release of excitatory neurotransmitters (Fredholm et al, 2005). Despite promise in rodent models of stroke (Kitagawa et al, 2002), the direct administration of adenosine has not been translated to clinical therapy as it has a short physiological half-life and causes many unwanted central and peripheral side effects, including suppression of cardiac function, sedation, and renal impairment. For these reasons, we focused on the key adenosine removing enzyme in brain, ADK, as therapeutic target to reduce ischemic neuronal cell death. Pharmacological inhibition of ADK has indeed been shown to reduce infarct volume after modeled stroke (Pignataro et al, 2007), but the systemic use of ADK inhibitors is not a therapeutic option due to widespread cardiovascular side effects and liver toxicity (Boison et al, 2002; Fredholm et al, 2005). Recent work in our laboratory has shown that acute downregulation of endogenous ADK in response to stroke might be an innate neuroprotective mechanism aimed at potentiating ambient levels of adenosine (Pignataro et al, 2008).
Using complementary transgene and gene therapy-based approaches, we demonstrate here that the degree of acute brain injury directly depends on expression levels of ADK and the resulting tone in ambient adenosine. Most importantly, in fb-Adk-def mice that have increased ADK in forebrain and decreased ADK in striatum ambient levels of adenosine directly correspond to ADK expression levels (Figure 2), and the regional susceptibility to stroke-induced brain injury is governed by the regional expression profile of ADK (Figures 1A and 3A). These findings indicate that adenosine-dependent acute neuroprotection depends on local rather than systemic responses.
Adenosine and Adenosine Kinase in Ischemic Tolerance
The brain has evolved endogenous mechanisms to regulate, limit, and repair damage in response to injury. The phenomenon of ischemic tolerance takes advantage of these feedback mechanisms to confer a state of decreased susceptibility to ischemic injury. By applying a noxious stimulus at just below the level of inducing damage, natural protective responses can be elicited before a larger subsequent, otherwise injurious, stimulus. Ischemic tolerance can be divided into two broad categories: acute preconditioning, which develops over minutes or hours, and delayed preconditioning, which takes 24 to 72 hours to develop and involves gene regulation and protein synthesis (Stenzel-Poore et al, 2003). The role of adenosine in ischemic preconditioning has been studied extensively in the heart; however, less is known about its role in cerebral ischemia. In acute cerebral preconditioning, the adenosine system is a well-accepted candidate to the development of ischemic tolerance (Li et al, 2009; Nakamura et al, 2002).
Data from the current study show that adenosine has a novel role in promoting delayed ischemic tolerance. If adenosine is a required component of biochemical and physiological pathways leading to tolerance, then abrogation of the adenosine response by increasing the metabolic clearance of adenosine should abrogate the phenomenon of ischemic tolerance elicited by delayed preconditioning. Here, we clearly demonstrate that transgenic animals with increased metabolic adenosine clearance due to overexpression of ADK throughout the brain (Adk-tg mice), remain susceptible to ischemic injury despite a standard preconditioning treatment with LPS. Our current results, using transgenic animals with increased adenosine clearance due to overexpression of ADK in brain (Adk-tg) illustrate the essential role of adenosine in the CNS in LPS-induced delayed-type preconditioning. Further, it is tempting to deduce, particularly in light of our current data showing that animals with increased adenosine clearance cannot be preconditioned, that an adenosine signal—triggered by a preconditioning stimulus—is needed for the development of ischemic tolerance.
Therapeutic Adenosine Augmentation
The question remains as to whether the acute or long-term effects of adenosine dominate in the phenomenon of ischemic tolerance. Acutely, adenosine has been shown to exert neuroprotective effects via signaling through inhibitory A1 receptors; however, mice lacking A1 receptors do not show increased susceptibility to ischemic damage (Olsson et al, 2004). In line with this, mice globally lacking excitatory A2A receptor have reduced susceptibility to stroke (Chen et al, 1999). Further, systemic, but not central, administration of A2A receptor antagonists have been shown to reduce infarct volume in several in vivo models of ischemic injury (Von Lubitz et al, 1995). This protection was shown to be largely dependent on A2A receptors present on bone marrow-derived cells (Yu et al, 2004). Paradoxically, systemic activation of A2A receptors also led to protection against cerebral ischemic damage (Von Lubitz et al, 1995). These partly contradictory findings suggest that adenosine receptor independent mechanisms might also contribute to neuroprotection. Thus, the global net effect of homeostatic network regulation determines the susceptibility to neuronal cell death in stroke. Therefore, we did not aim to investigate specific pathways or molecular components of downstream effector systems, but elected to augment the natural production of adenosine in response to injury by decreasing the abundance of its key regulatory enzyme ADK. The novel therapeutic concept of decreasing ADK without drastically affecting its temporal regulation may be the most practical immediate therapeutic approach to harnessing the adenosine system for ischemic neuroprotection. We have previously suggested that polymer-based, stem cell-based, and gene therapy-based adenosine augmentation therapies might uniquely be suited to suppress seizures in epilepsy (Boison, 2009; Wilz et al, 2008).
Though we have shown viral knockdown of ADK in vivo to be a successful neuroprotective strategy that induces a state of permanent tolerance to ischemia, at this time we do not propose the use of gene therapy as prophylactic to treat patients at risk of cerebral ischemia. Rather, in lieu of the development of more targeted pharmacological approaches, it may be possible to pursue other, less invasive, methods to raise basal levels of adenosine in the brain of patients and thereby mimic the features of ADK downregulation. One such possibility might be the ketogenic diet, which is currently used to treat intractable epilepsy and other neurologic disorders (Kossoff et al, 2009), and has recently been shown to increase A1R activation in the brain (Masino et al, 2011). In summary, modulation of adenosine levels via its key regulatory enzyme ADK may be used therapeutically to mimic essential features of preconditioning, resulting in a phenotype of ischemic tolerance in patients at risk of cerebral ischemic injury.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors declare no conflict of interest.
Footnotes
This work has been supported by grants NS057538, NS061844, and NS058780 from the National Institute of Neurological Disorders and Stroke.
References
- Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009;85:131–141. doi: 10.1016/j.eplepsyres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB. Adenosine signalling and function in glial cells. Cell Death Differ. 2010;17:1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Scheurer L, Zumsteg V, Rülicke T, Litynski P, Fowler B, Brandner S, Mohler H. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99:6985–6990. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RS, Carcillo JA, Kochanek PM, Obrist WD, Jackson EK, Mi Z, Wisneiwski SR, Bell MJ, Marion DW.1997Cerebrospinal fluid adenosine concentration and uncoupling of cerebral blood flow and oxidative metabolism after severe head injury in humans Neurosurgery 411284–1292.discussion 1292–1293 [DOI] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Hatz S, Tian FM, Llaudet E. Listening to the brain: microelectrode biosensors for neurochemicals. Trends Biotechnol. 2005;23:420–428. doi: 10.1016/j.tibtech.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Dale N, Llaudet E, Poputnikov DM, Spyer KM, Gourine VN. Release of ATP in the central nervous system during systemic inflammation: real-time measurement in the hypothalamus of conscious rabbits. J Physiol. 2007;585:305–316. doi: 10.1113/jphysiol.2007.143933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Mori A, Shimada J, Mitsumoto Y, Kikuchi T. Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol Res. 2002;24:317–323. doi: 10.1179/016164102101199819. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic diets: an update for child neurologists. J Child Neurol. 2009;24:979–988. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dai S, An J, Xiong R, Li P, Chen X, Zhao Y, Liu P, Wang H, Zhu P, Chen J, Zhou Y. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp Neurol. 2009;215:69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets. Trends Neurosci. 2008;31:273–278. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Fredholm B, Geiger J, Aronica E, Boison D.2011The antiepileptic effect of a ketogenic diet is mediated by adenosine A1 receptors(under review) [DOI] [PMC free article] [PubMed]
- Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Ann Rev Nutr. 2008;28:273–293. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- Melani A, Pantoni L, Corsi C, Bianchi L, Monopoli A, Bertorelli R, Pepeu G, Pedata F.1999Striatal outflow of adenosine, excitatory amino acids, gamma-aminobutyric acid, and taurine in awake freely moving rats after middle cerebral artery occlusion: correlations with neurological deficit and histopathological damage Stroke 302448–2454.discussion 2455 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nakakimura K, Matsumoto M, Sakabe T. Rapid tolerance to focal cerebral ischemia in rats is attenuated by adenosine A1 receptor antagonist. J Cereb Blood Flow Metab. 2002;22:161–170. doi: 10.1097/00004647-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Newby AC, Worku Y, Holmquist CA. Adenosine formation. Evidence for a direct biochemical link with energy metabolism. Adv Myocardiol. 1985;6:273–284. [PubMed] [Google Scholar]
- Olsson T, Cronberg T, Rytter A, Asztely F, Fredholm BB, Smith ML, Wieloch T. Deletion of the adenosine A1 receptor gene does not alter neuronal damage following ischaemia in vivo or in vitro. Eur J Neurosci. 2004;20:1197–1204. doi: 10.1111/j.1460-9568.2004.03564.x. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004;16:237–270. doi: 10.1615/critrevneurobiol.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Ramakers BP, Riksen NP, van den Broek P, Franke B, Peters WH, van der Hoeven JG, Smits P, Pickkers P. Circulating adenosine increases during human experimental endotoxemia but blockade of its receptor does not influence the immune response and subsequent organ injury. Crit Care. 2011;15:R3. doi: 10.1186/cc9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM, de Mendonca A. Participation of adenosine receptors in neuroprotection. Drug News Perspect. 2003;16:80–86. doi: 10.1358/dnp.2003.16.2.740246. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009;193:535–587. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Theofilas P, Brar S, Stewart K-A, Shen H-Y, Sandau US, Poulsen DJ, Boison D.2011Adenosine kinase as a target for therapeutic antisense strategies in epilepsy Epilepsiae-pub ahead of print 28 January 2011; doi: 10.1111/j.1528-1167.2010.02947.x [DOI] [PMC free article] [PubMed]
- Von Lubitz DK, Lin RC, Jacobson KA. Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol. 1995;287:295–302. doi: 10.1016/0014-2999(95)00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies—the need for new approaches. Cerebrovasc Dis. 2004;17 (Suppl 1:153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]