Abstract
Neuro-oxidative-nitrosative stress may prove the molecular basis underlying brain dysfunction in sepsis. In the current review, we describe how sepsis-induced reactive oxygen and nitrogen species (ROS/RNS) trigger lipid peroxidation chain reactions throughout the cerebrovasculature and surrounding brain parenchyma, due to failure of the local antioxidant systems. ROS/RNS cause structural membrane damage, induce inflammation, and scavenge nitric oxide (NO) to yield peroxynitrite (ONOO−). This activates the inducible NO synthase, which further compounds ONOO− formation. ROS/RNS cause mitochondrial dysfunction by inhibiting the mitochondrial electron transport chain and uncoupling oxidative phosphorylation, which ultimately leads to neuronal bioenergetic failure. Furthermore, in certain ‘at risk' areas of the brain, free radicals may induce neuronal apoptosis. In the present review, we define a role for ROS/RNS-mediated neuronal bioenergetic failure and apoptosis as a primary mechanism underlying sepsis-associated encephalopathy and, in sepsis survivors, permanent cognitive deficits.
Keywords: apoptosis, cognitive dysfunction, inflammation, reactive oxygen-nitrogen species, sepsis-associated encephalopathy
Introduction
Sepsis, the systemic inflammatory response to infection (Bone, 1996), is a frequent cause of death, both in the general population and in patients in the intensive care unit (Vincent et al, 2006). In the latter setting, organ dysfunction, including acute brain dysfunction, termed sepsis-associated encephalopathy (SAE) or sepsis-associated delirium, occurs in up to two thirds of patients (Vincent et al, 2006). SAE may be the first symptom of sepsis, and is characterized by confusion, agitation, and impaired consciousness (Young et al, 1990; Bolton et al, 1993; Ebersoldt et al, 2007, Iacobone et al, 2009). These symptoms may be an independent predictor of death (Sprung et al, 1990; Ebersoldt et al, 2007). Furthermore, encephalopathy in critically ill patients may predict long-term cognitive function after discharge from the intensive care unit (Girard et al, 2010). Accordingly, clinical and experimental studies suggest that up to 60% of sepsis survivors exhibit permanent cognitive deficits (Iwashyna et al, 2010), notably memory loss, and impaired learning capability (Hopkins et al, 1999, 2004, 2005; Barichello et al, 2005; Semmler et al, 2007).
As of now, the underlying mechanisms of SAE and the associated neurologic sequelae remain unresolved. However, as will be outlined in the present review, emerging evidence points toward a role for neuro-oxidative-nitrosative stress, that is, potentially damaging redox processes that involve the formation of reactive oxygen and nitrogen species (ROS/RNS) within the central nervous system. ROS include not only oxygen-centered free radicals, for example the superoxide anion (O2•−) and hydroxyl radicals (•OH), but also certain nonradical derivatives of oxygen, such as hydrogen peroxide (H2O2), whereas nitric oxide (NO), peroxynitrite (ONOO−), and nitrogen dioxide (NO2•) are examples of RNS (Halliwell, 2006). The brain is constantly exposed to relatively high levels of ROS and RNS, mainly due to a high metabolic rate of oxygen, which is associated with a high rate of O2•− leakage from the mitochondrial electron transport chain (Halliwell, 2006). The abundance of eicosapentaenoic and docosahexaenoic polyunsaturated fatty-acid side chains in neuronal cell membranes, which are readily oxidized, force the limited antioxidant defenses to work at near-maximal levels, even in the healthy state (Halliwell, 2006).
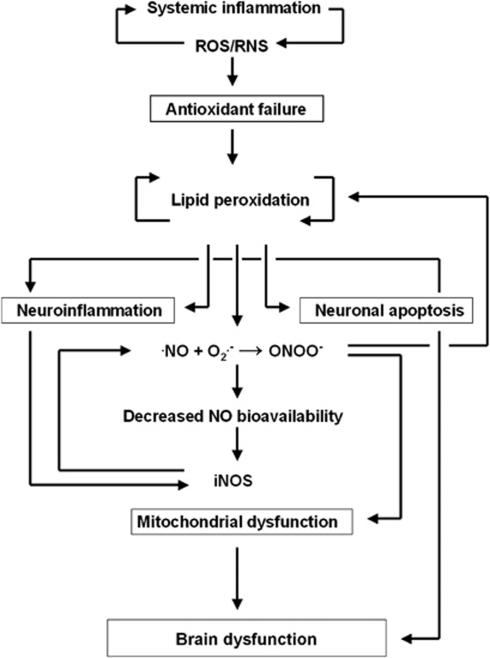
Based on the data from various experimental models of sepsis in animals, as well as data from clinical studies, the present review will focus on the potential role of neuro-oxidative-nitrosative stress in the pathophysiology of sepsis. We propose a model, which comprises six principal components that follow in succession and are unified through the focal generation of ROS/RNS: (1) antioxidant failure, (2) lipid peroxidation, (3) inflammation, (4) NO depletion, (5) mitochondrial dysfunction, and (6) apoptosis. According to this model, ROS/RNS generated during the systemic inflammatory response initiate lipid peroxidation in the cerebrovasculature and brain parenchyma, due to failure of the antioxidant systems of the brain (Figure 1). Lipid peroxidation chain reactions generate further free radicals within the brain; the free radicals induce focal inflammation, decrease the vascular bioavailability of NO, and prompt neuronal bioenergetic failure through mechanisms established vide infra. This impairs cerebral oxidative metabolism and causes an acute suppression of global cerebral function, which then gives rise to the symptoms characteristic of SAE. Free radicals further induce neuronal apoptosis in certain brain regions ‘at risk,' and thus cause cognitive deficits that may persist after clinical recovery from sepsis.
Figure 1.
A hypothetical model of brain dysfunction in sepsis. Reactive oxygen and nitrogen species (ROS/RNS), generated during the systemic inflammatory response, trigger lipid peroxidation chain reactions, due to the limited antioxidant capacity of the brain. Free radical-mediated structural membrane damage induces neuroinflammation; furthermore, free radicals (such as superoxide anion, O2•−) deplete ambient nitric oxide (•NO) in the cerebrovascular bed to form peroxynitrite (ONOO−). The consequently reduced vascular bioavailability of NO acts in concert with the local inflammatory response to increase the expression of the inducible NO synthase (iNOS). ONOO− irreversibly inhibits the mitochondrial electron transport chain, which increases the mitochondrial release of free radicals. Free radical-mediated uncoupling of oxidative phosphorylation compounds the mitochondrial dysfunction, and renders the neuron in a state of ‘cytopathic hypoxia,' thus leading to neuronal bioenergetic failure. Furthermore, free radicals trigger apoptosis by altering the intracellular Ca2+ homeostasis in the brain areas ‘at risk,' such as the cerebral cortex and hippocampus, which exacerbates the local inflammatory response further.
We hypothesize that this model provides the molecular basis for the brain dysfunction characteristic of sepsis. In the following, we review the current evidence that forms the basis of the six principal components of the model as proposed.
Antioxidant failure
In the healthy state, free radical formation is counteracted by a variety of antioxidant systems that include the enzymes superoxide dismutase, catalase and glutathione peroxidase, as well as nonenzymatic, chain-breaking antioxidants, such as ascorbate (vitamin C) and α-tocopherol (vitamin E) (Buettner, 1993; Halliwell, 2006).
Superoxide Dismutase, Catalase, and Glutathione Peroxidase
Superoxide dismutase activity has been reported to increase in the brains of experimentally septic rats (Barichello et al, 2006; Ninkovic et al, 2006). This enzyme dismutates O2•− to H2O2 and oxygen (Halliwell, 2006). In contrast to O2•−, H2O2 diffuses readily across membranes into the extracellular compartment, in which it may decompose to •OH. The latter has the highest known one-electron potential (E°′), which places it at the top of the so-called ‘league of reactivity' (Buettner, 1993) (Table 1), that is, it is thermodynamically capable of abstracting a hydrogen atom from any other biomolecule. Catalase and glutathione peroxidase prevent the formation of •OH by reducing H2O2 to water and oxygen at the expense of glutathione (Halliwell, 2006). Thus, an increase in superoxide dismutase may be associated with an increase in oxidative stress, unless it is accompanied by a concurrent increase in catalase and glutathione peroxidase. Increased levels of brain superoxide dismutase without a proportional increase in catalase activity have thus been suggested to be a characteristic of brain regions that are susceptible to free radical-mediated structural membrane damage in sepsis (Barichello et al, 2006). Since catalase is not present in the brain mitochondria, where most of the H2O2 is generated (Halliwell, 2006), the relative increase in mitochondrial glutathione peroxidase activity may prove a more accurate marker. Accordingly, melatonin, which increases the activity of mitochondrial glutathione peroxidase, has been demonstrated to reduce neuronal damage in experimentally septic rats (Sener et al, 2005b).
Table 1. Reactivity of selected reactive oxygen and nitrogen species (ROS/RNS) and antioxidants of relevance in neuro-oxidative-nitrosative stress.
| Couple | E°′ (mV) | |
|---|---|---|
| Hydroxyl radical | [•OH, H+/H2O] | +2,310 |
| Peroxynitrite/nitrogen dioxide | [ONOO−, 2 H+/NO2•, H2O] | +1,400 |
| Nitrogen dioxide/nitrite | [NO2•/NO2−] | +990 |
| Lipid alkylperoxyl radical/lipid hydroxyperoxide | [L-OO•, H+/L-OOH] | +750–1,400 |
| Superoxide anion/hydrogen peroxide | [O2•−, 2 H+/H2O2] | +940 |
| Lipid pentadienyl radical/bisallylic hydrogen group | [L•, H+/L-H] | +600 |
| Nitric oxide/nitroxyl anion | [•NO/NO−] | +390 |
| Tocopheroxyl radical/α-tocopherol | [αT•, H+/αTH] | +500 |
| Hydrogen peroxide/hydroxyl anion | [H2O2, H+/H2O, •OH] | +320 |
| Ascorbate/ascorbyl radical | [AH/H+, A•−] | +282 |
A higher one-electron reduction potential (E°′) indicates higher reactivity; hence, a compound is thermodynamically capable of abstracting an electron from any biomolecule with a lower E°′. The reported E°′ values have been obtained from Stanbury (1989); Koppenol et al (1992); Buettner (1993); and Bartberger et al (2002).
Ascorbate and α-Tocopherol
The aqueous-phase antioxidant ascorbate prevents lipid peroxidation chain reactions by undergoing oxidation to yield the ascorbyl radical, a relatively inert intermediate that can donate an electron to any oxidizing species, given the low E°′ of the ascorbate-ascorbyl radical couple (Table 1). Also, ascorbate recycles the tocopheroxyl radical back to the fully functional lipid-soluble α-tocopherol isomer; α-tocopherol acts as an antioxidant by chain-breaking lipid peroxides at the membrane-water interface in a manner similar to ascorbate (Sharma and Buettner, 1993).
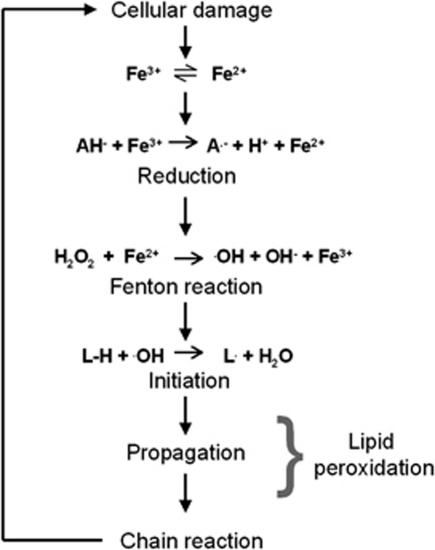
In a study of patients with SAE, cerebrospinal fluid and plasma ascorbate levels were found to be lower than in nonseptic controls, whereas α-tocopherol levels were not affected (Voigt et al, 2002). This was interpreted to reflect ascorbate oxidation at rates that overwhelmed the capacity of the brain cells to regenerate ascorbate (Voigt et al, 2002). Since ascorbate was not found to be entirely depleted from the cerebrospinal fluid, it is, however, probably not the lack of antioxidant protection from ascorbate that facilitates neuro-oxidative-nitrosative stress in sepsis; neuro-oxidative-nitrosative stress is more likely to involve the often overlooked ability of ascorbate to catalyze, as opposed to quench, lipid-derived free radicals by reducing ferric (Fe3+) to ferrous (Fe2+) iron (Bailey, 2005) (Figure 2). Although the brain is rich in nonheme iron (Haacke et al, 2005), the potential for these reactions to occur is remote in healthy conditions, since iron is sequestered in noncatalytic protein-bound forms, such as ferritin (Haacke et al, 2005). In the context of sepsis, however, degenerating neurons and other cells could potentially release Fe2+ to the extracellular space, and thus enable ascorbate to catalyze the formation of •OH through the Fenton reaction (Figure 2). A similar molecular concept has been demonstrated in patients undergoing lower limb vascular surgery with obligatory ischemia-reperfusion, due to cross-clamping, which increases the extracellular availability of Fe2+ (Bailey et al, 2006). The patients were randomized to ascorbate prophylaxis or placebo in a double-blinded, randomized manner. During lower limb ischemia, the patients receiving ascorbate exhibited a release of lipid hydroperoxide across the ischemic vascular bed, and the systemic levels of lipid-derived alkoxyl and alkyl radicals increased during reperfusion (Bailey et al, 2006). Analogous mechanisms may contribute to oxidative-nitrosative stress upon ischemia-reperfusion of the cerebrovascular bed, as indicated by findings from patients undergoing carotid endarterectomy (Bailey et al, 2007).
Figure 2.
Ascorbate: the phantom menace? Catalytically active ferric (Fe3+) and ferrous (Fe2+) iron may be unlocked and released to the extracellular space upon cellular damage. Because of its relatively low one-electron reduction potential (E°′=+282 mV), ascorbate (AH−) is capable of donating an electron to Fe3+ thereby reducing it to Fe2+, which in turn reacts with hydrogen peroxide (H2O2) to form the hydroxyl radical (•OH) through the Fenton reaction. •OH may consequently initiate lipid peroxidation chain reactions and cause structural membrane damage throughout the cerebrovasculature and brain parenchyma. A•−, ascorbyl radical; L•, lipid pentadienyl radical; OH−, hydroxide.
The concept of ascorbate as a prooxidant does, however, remain highly controversial, and there is evidence indicating that ascorbate retains its potent antioxidant properties in plasma in vivo, even in the presence of iron overload (Chen et al, 2000). Therefore, the relevance of these iron-dependent mechanisms in the brain in sepsis remains to be elucidated, but is supported by findings from septic rats, in which treatment with the glutathione precursor N-acetylcysteine only improved long-term cognitive outcome when combined with the iron-chelating agent desferrioxamine, although neither antioxidant had a significant effect when administered alone (Barichello et al, 2007).
We propose that failure of the antioxidant defenses in the brain is an early event in sepsis that facilitates oxidative-nitrosative stress.
Lipid peroxidation
The inadequacy of the antioxidant response renders the brain susceptible to oxidation in sepsis. Oxidative stress is initially promoted by phagocytic cells, such as neutrophils, which generate disproportionately large amounts of ROS/RNS upon an infectious insult; this mechanism probably serves to eradicate invading pathogens (Martins et al, 2003). Furthermore, the invading pathogen may release endo- and exotoxins, including prooxidants such as H2O2 (Bermpohl et al, 2005), which cause cellular damage and facilitate the formation of ROS/RNS. ROS/RNS are thermodynamically poised to cause cell membrane damage throughout the cerebrovasculature and brain parenchyma (Boueiz and Hassoun, 2009; Pun et al, 2009), due to the relatively low E°′ of the bisallylic hydrogen group (L-H) in polyunsaturated fatty-acid side chains (Table 1). Any oxidizing species with a higher E°′ than L-H can thus initiate lipid peroxidation; ·OH is used as an initiator in this example:
The resultant pentadienyl radical (L·) reacts rapidly with oxygen (k=3 × 108/M s) to form an alkylperoxyl radical (L-OO•):
L-OO• has a higher E°′ than does L-H (Table 1), thus enabling it to propagate the reaction to yield lipid hydroxyperoxide (L-OOH) and additional L•:
The L• may then generate more L-OO•; in effect, a lipid peroxidation chain reaction ensues, in which surrounding membrane lipids are oxidized. This increases membrane fluidity and triggers inactivation of membrane-bound proteins, thus impairing membrane integrity and function (Halliwell, 2006). Through these mechanisms, free radicals may contribute to the widespread blood-brain barrier damage that has been demonstrated in experimental studies of sepsis (Eckman et al, 1958; Clawson et al, 1966; du Moulin et al, 1985; Esen et al, 2005). Accordingly, treatment with β-glucan has been found to reduce vasogenic edema and hemorrhage in the brains of experimentally septic rats (Sener et al, 2005a), presumably by preventing blood-brain barrier disruption. β-glucan is an antioxidant derived from cell walls of fungi and some cereal plants; it is unknown how it exerts its antioxidant effects, but it is thought to bind to the plasma membrane lipid bilayer and specifically inhibit lipid peroxidation in a manner similar to that of α-tocopherol (Babincova et al, 2002).
Until now, no studies have directly demonstrated lipid peroxidation in sepsis, either in the cerebrovascular endothelium, or in the brain parenchyma. There is, however, some indirect evidence of its presence, since lipid peroxides degrade to reactive aldehyde products, such as malondialdehyde. Malondialdehyde belongs to the group of thiobarbituric acid reactive substances (TBARS), meaning that it is capable of reacting with thiobarbituric acid to yield a fluorescent product than can be detected ex vivo (Moore and Roberts, 1998). The concentration of TBARS increases in cerebrospinal fluid of patients with sepsis (Takezawa et al, 1983) and in the brain homogenates of septic animals (Takeda et al, 1986; Abd El-Gawad and Khalifa, 2001; Sener et al, 2005a, 2005b; Goraca and Aslanowicz-Antkowiak, 2009). Elevated TBARS levels have furthermore been demonstrated in specific brain regions, that is, the hippocampus, the cerebral cortex, and the cerebellum, as early as 6 hours after the induction of experimental sepsis in rats (Barichello et al, 2006).
It should be noted that the available evidence on sepsis-associated lipid peroxidation in the brain is almost exclusively based on TBARS measurements. Although the measurement of TBARS works well when applied to well-defined membrane systems in vitro, its application to body fluids and tissue extracts is problematic (Moore and Roberts, 1998). TBARS assays are relatively nonspecific, since aldehydes other than malondialdehyde are present in body fluids, many of which form chromogens that may yield false-positive readings. Furthermore, the TBARS assays may in themselves produce TBARS, due to the decomposition of lipid peroxides. Consequently, these indirect findings must be interpreted with caution. Electron paramagnetic resonance spectroscopy is the only technique that exists for the direct molecular detection and subsequent characterization of free radicals in vivo (Holley and Cheeseman, 1993). This technique has not yet been applied to investigate neuro-oxidative-nitrosative stress in sepsis.
We propose that free radical-mediated lipid peroxidation causes structural membrane damage in the cerebrovasculature and brain parenchyma from the very early stages of sepsis.
Inflammation
In sepsis, the widespread structural membrane damage throughout the cerebrovascular endothelium and surrounding brain parenchyma is accompanied by a local inflammatory response with increased intrathecal levels of cytokines (Sacoccio et al, 1998). Tumor necrosis factor (TNF)-α expression increases, initially in the circumventricular organs and subsequently in glial cells throughout the brain parenchyma (Gatti and Bartfai, 1993; Pitossi et al, 1997; Nadeau and Rivest, 2000; Semmler et al, 2008); this may facilitate the glial expression of several other cytokines ‘downstream' of TNF-α, such as interleukin (IL)-1β and IL-6 (Pitossi et al, 1997; Meyer et al, 1997; Satta et al, 1998; Semmler et al, 2008).
No studies have yet succeeded in disassociating oxidative-nitrosative stress from inflammation in the brain during systemic inflammation. Nevertheless, the biological half-lives of ROS/RNS are relatively short when compared with those of cytokines; the half-lives of ROS/RNS are as low as 10−9 seconds for •OH (Sies, 1993), whereas the reported half-lives of TNF-α, IL-1β, and IL-6, range from 6 to 20 minutes (Tracey and Cerami, 1993). This may favor free radicals as inducers, rather than mere propagators of inflammation. Furthermore, free radical-mediated blood-brain barrier disruption may allow noxious metabolites to enter the brain parenchyma and thus compound inflammation.
We propose that the release of free radicals and free radical-mediated structural membrane damage is an upstream signal that induces focal inflammation in the brain in sepsis.
NO depletion
In the brain, the gaseous signaling molecule NO serves a number of cellular functions, including neurotransmission, regulation of blood-vessel tone, and immunity (Pacher et al, 2007). NO, an endogenous vasodilator, is produced from arginine by the members of the NO synthase (NOS) family of proteins, that is neuronal NOS (nNOS, type I), inducible NOS (iNOS, type II), and endothelial NOS (eNOS, type III) (Pacher et al, 2007). Whereas nNOS and eNOS are constitutively expressed and require the formation of Ca2+-calmodulin complexes for their activation, iNOS exerts its activity in a Ca2+-independent manner.
Any free radical, for instance O2•−, lipid-derived alkoxyl free radical (LO•) or •OH may react with NO to yield ONOO− in a diffusion-controlled manner (Nauser and Koppenol, 2002):
Hence, free radicals may deplete NO through the formation of ONOO−, thus decreasing the vascular bioavailability of NO. Data from clinical studies support that the bioavailability of NO is decreased in sepsis (Doerschug et al, 2007; Davis et al, 2009), which, combined with endothelial damage, may contribute to the inhomogenity of microvascular perfusion that was recently demonstrated in septic sheep (Taccone et al, 2010). This may furthermore impair neurovascular coupling (Rosengarten et al, 2009) and dynamic cerebral blood flow autoregulation (Pfister et al, 2008; Steiner et al, 2009), and thus prompt focal ischemia/hypoxia and blood–brain barrier damage upon acute surges in blood pressure. Changes in the levels of various vasoactive peptides that are involved in the regulation of cerebrovascular resistance (Pittet et al, 1991; Linscheid et al, 2004; Berg et al, 2009), as well as a reduced deformability of red blood cells due to oxidative modifications, which complicates their passage through the cerebral microvessels (Papadopoulos et al, 2000), may contribute to focal cerebral hypoxia. Since hypoxia compounds mitochondrial free radical formation (Guzy and Schumacker, 2006), this forms the basis for a vicious cycle, in which the free radical-mediated depletion of NO is maintained. Accordingly, we recently reported evidence of a similar vicious cycle during acute inspiratory hypoxia in healthy humans (Bailey et al, 2009).
In the healthy state, iNOS activation is low, but this enzyme may be induced in glial cells and increase NO production in response to a variety of noxious stimuli (Pacher et al, 2007), such as TNF-α and IL-1β (Wong et al, 1996; Alexander et al, 2008) and, possibly, decreased ambient NO levels (Galijasevic et al, 2003). A decreased NO bioavailability may therefore act in concert with focal inflammation to enhance iNOS expression. Thus, in experimentally septic rats, iNOS messenger RNA levels increase in vascular, glial, and neuronal structures throughout the brain (Wong et al, 1996; Jacobs et al, 1997; Harada et al, 1999), and levels of the NO metabolites, nitrate (NO3−) and nitrite (NO2−), both of which reduce to evolve NO, have been found to be elevated in the brain parenchyma and cerebrospinal fluid (Wong et al, 1996; Abd El-Gawad and Khalifa, 2001). Moreover, one study has provided in vivo evidence for increased intrathecal NO formation in septic rats using electron paramagnetic resonance combined with an NO spin-trapping technique with an iron and N,N-diethyldithiocarbamate complex (Suzuki et al, 1998).
Although it may seem counterintuitive, an enhanced iNOS-dependent NO production may coincide with a decreased bioavailability of NO, since NO is rapidly depleted when free radicals are present (equation (4); Table 1). Therefore, iNOS is likely to enhance oxidative-nitrosative stress through the formation of ONOO−. ONOO− is much more reactive than NO (Table 1), causes tyrosine nitration and cysteine oxidation in various proteins (Radi et al, 1991), and decomposes to highly toxic free radicals, such as NO2• and •OH (Buettner, 1993; Bartberger et al, 2002). Cerebrospinal fluid levels of 3-nitrotyrosine have recently been found to be elevated in children with sepsis (Hamed et al, 2009); 3-nitrotyrosine, which is formed when a tyrosyl radical combines with NO2• during tyrosine nitration (Pacher et al, 2007), is relatively stable, and can thus be considered a surrogate molecular ‘footprint' of nitrosative stress.
We propose that free radical-mediated NO depletion decreases the cerebrovascular bioavailability of NO in sepsis, a reaction which is further fuelled by focal hypoxia and the upregulation of iNOS; the resultant generation of ONOO− augments oxidative-nitrosative stress by decomposing to other highly reactive free radicals.
Mitochondrial dysfunction
The presence of an enhanced NO- and free radical-driven ONOO− production within the brain may prove critical to neuronal function in sepsis. NO donors, which yield NO and subsequently ONOO−, induce a rapid decrease in oxygen consumption by inhibiting complex I and IV of the mitochondrial electron transport chain when added to cell cultures and intact mitochondria (Clementi et al, 1998; Brookes et al, 1999). NO reversibly inhibits mitochondrial respiration by competing with oxygen for the binding site at complex I, whereas ONOO− attenuates electron transport both at complex I (Cassina and Radi, 1996; Clementi et al, 1998), and to a lesser extent at complex IV (Brookes et al, 1999) through irreversible oxidative modifications. This may explain the presence of impaired complex I and complex IV activities which have been demonstrated in experimentally septic rats (Chuang et al, 2002; Comim et al, 2008; d'Avila et al, 2008).
O2•− Formation
Impaired complex I and complex IV activities should theoretically cause a marked increase in the leakage of O2•− from the electron transport chain (Riobo et al, 2001). Direct evidence demonstrating an increased mitochondrial O2•− production is, however, limited. It has been reported that the O2•− formation increases in the brain stem tissue of experimentally septic rats, as determined by luminometry after incubation of the tissue in a lucigenin-containing HEPES buffer (Chuang et al, 2003). Likewise, the O2•− content, measured by the reduction of nitroblau-tetrazolium, has been found to increase in the brain capillaries of experimentally septic rats (Ninkovic et al, 2006). However, no extracellular O2•− could be detected in the brains of experimentally septic pigs by use of the same method for O2•− measurements (Deutschman et al, 1990), most likely because the negative charge of O2•− principally restricts it to the intracellular compartment.
Mitochondrial Uncoupling
Efficient oxidative metabolism depends on the coupling of electron transport and oxidative phosphorylation through a proton gradient across the inner mitochondrial membrane. O2•− and various lipid peroxides are able to activate uncoupling proteins, which increase proton permeability across the inner mitochondrial membrane (Crompton, 1999; Echtay et al, 2002). This allows protons to pass into the mitochondrial matrix to join electrons in the form of oxide ions (O22−) to form water in an exothermic reaction and without the generation of ATP from ADP. Uncoupling of oxidative phosphorylation was recently demonstrated in the brain mitochondria of septic mice (d'Avila et al, 2008). Although mitochondrial uncoupling may diminish the generation of ROS by neutralizing electrons that leak from the electron transport chain, it may also cause cytopathic hypoxia, which signifies that mitochondria are unable to use oxygen, regardless of whether it is available or not (Fink, 2001). Additionally, ONOO− may introduce single-strand breaks in DNA, thus triggering the activation of various repair processes, including the nuclear NAD+-consuming enzyme poly(ADP-ribose) polymerase, which increases the cellular metabolic requirements (Zhang et al, 1994). Combined with a state of cytopathic hypoxia, this may prompt neuronal bioenergetic failure. Accordingly, the global cerebral metabolic rate of oxygen has been found to be reduced in patients with severe sepsis (Maekawa et al, 1991). However, sedation remains a potential source of error in such clinical studies. In contrast, the cerebral metabolic rate of oxygen has been found to be largely unaffected in healthy humans subjected to experimental sepsis by means of lipopolysaccharide infusion (Møller et al, 2002; Berg et al, 2010). Since, however, these healthy volunteers did not exhibit any changes in the level of consciousness, the relevance of such observations for critically ill septic patients showing signs of encephalopathy is unclear. As of now, regional cerebral oxidative metabolism has not been assessed in humans, either in experimental or clinical sepsis.
We propose that oxidative-nitrosative stress causes a global cerebral dysfunction in sepsis by prompting neuronal bioenergetic failure, and, consequently, impairing cerebral oxidative metabolism.
Apoptosis
Apart from bioenergetic failure, free radicals may induce mitochondria-dependent apoptosis, by impairing membrane integrity and function in mitochondria and the endoplasmatic reticulum (Polster and Fiskum, 2004). This increases intracellular Ca2+ (Satrustegui and Richter, 1984), which induces permeability transition pores in neuronal mitochondria (Crompton, 1999). Cytochrome c, which transports electrons from complex III to IV, is consequently translocated from the inner mitochondrial membrane to the cytoplasm (Brustovetsky et al, 2002). This translocation is considered a key step in the initiation of mitochondria-dependent apoptosis, since cytochrome c, once present in the cytoplasm, comprises an upstream signal in the apoptotic cascade (Polster and Fiskum, 2004). The presence of mitochondria-dependent neuronal apoptosis in sepsis is supported by studies showing increased free intracellular Ca2+ (Zhan et al, 1996) and enhanced cytoplasmic cytochrome c immunoreactivity (Messaris et al, 2004) in experimentally septic animals. Accordingly, apoptotic neurons with degenerating mitochondria have been observed in several brain regions of experimentally septic animals and deceased septic patients, including the cerebral cortex and hippocampus (Papadopoulos et al, 1999; Vereker et al, 2000; Semmler et al, 2005; Barichello et al, 2006; Orihuela et al, 2006; Kafa et al, 2010), the cerebellum (Messaris et al, 2004; Barichello et al, 2006), and some autonomic centers in the brain stem (Sharshar et al, 2003, 2004).
Intrinsic and Extrinsic Factors
The underlying mechanisms that render some brain regions more susceptible to apoptosis than others are largely unknown, but likely involve both intrinsic and extrinsic factors. For example, the hippocampus has been found to be particularly vulnerable to apoptosis when compared with other brain regions in experimentally septic rats (Semmler et al, 2005); even so, the regional cerebral metabolic rate of oxygen in the hippocampus is not remarkably high (Yamaguchi et al, 1986), nor does it contain large amounts of iron (Haacke et al, 2005). Rather, other intrinsic properties, such as its relatively high content of docosahexaenoic acid (McNamara and Carlson, 2006), as well as an insufficient antioxidant capacity (Barichello et al, 2006), are likely to render this region ‘at risk' of free radical-induced apoptosis.
Extrinsic factors, such as cerebral ischemia and/or hemorrhage may prime a given region to apoptosis and render it ‘at risk' by compounding free radical-mediated damage. Based on a multiwavelength near-infrared spectroscopy technique, a hypoxia-dependent reduction of the mitochondrial complex IV has been reported in experimentally septic rats (Schaefer and Biber, 1993). Furthermore, multiple ischemic zones and hemorrhages are frequent findings in autopsy studies of patients dying from septic shock (Sharshar et al, 2003, 2004). These findings may for instance be explained by microvascular failure with an impaired cerebral blood flow autoregulation, thus causing alternating regional hypo- and hyperperfusion in response to fluctuations in the cerebral perfusion pressure. Another mechanism that may be involved is ROS/RNS-mediated contraction of capillary pericytes, which has previously been found to compromise microvascular perfusion in experimental stroke (Yemisci et al, 2009).
Whereas a cerebral hemorrhage may prime a brain region to oxidative-nitrosative stress by increasing the extracellular availability of Fe2+, ischemia may increase the leakage of electrons from the mitochondrial complex III (Guzy and Schumacker, 2006), thus leading to increased free radical formation. This ‘hypoxia priming' could explain the presence of apparently randomly scattered groups of degenerating neurons in the cortex of experimentally septic pigs (Papadopoulos et al, 1999). However, the relationship between histological signs of ischemia and apoptosis are not clearcut in the autopsy studies of deceased septic patients, and markers of oxidative-nitrosative damage have not been assessed (Sharshar et al, 2003, 2004).
Neuronal apoptosis was found to be associated with an enhanced endothelial iNOS expression in deceased septic patients (Sharshar et al, 2003, 2004), and in experimentally septic rats, this was found to be particularly pronounced in the hippocampus (Semmler et al, 2005). Furthermore, neuronal apoptosis in this area was markedly reduced upon iNOS inhibition (Semmler et al, 2005). This implies that the degree of iNOS upregulation may reflect the severity of oxidative-nitrosative stress upon various intrinsic and extrinsic factors in a given brain region, and further supports the notion that iNOS is involved as a critical propagator of oxidative-nitrosative stress.
Cognitive Decline
Longitudinal studies of cognitive function in sepsis survivors and experimental studies in rodents may shed some light on the clinical impact of neuronal apoptosis. Elderly sepsis survivors were recently reported to exhibit a pronounced cognitive decline when compared with nonseptic patients at 8-year follow-up (Iwashyna et al, 2010). In another study, a global intellectual decline was found to be present in patients who had recovered from severe sepsis complicated by adult respiratory distress syndrome (Hopkins et al, 1999). In this cohort, most of the cognitive deficits were spontaneously reversed 1 year after discharge (Hopkins et al, 1999); however, some impairment of the hippocampus-dependent processes tended to persist in the majority of patients (Hopkins et al, 1999, 2004) and did not cease at 2-year follow-up (Hopkins et al, 2005).
Data from animal studies also indicate that hippocampus-dependent learning and memory is permanently attenuated after a septic episode (Barichello et al, 2005, 2007; Semmler et al, 2007; Weberpals et al, 2009). This is likely to involve apoptosis of neuronal progenitor cells within the dentate gyrus. These cells are critical to memory processing and formation (Aimone et al, 2006) and rapidly undergo apoptosis upon H2O2-induced oxidative stress in vitro (Braun et al, 2002).
In rats surviving sepsis, permanent cognitive deficits were alleviated by the combined antioxidant treatment with N-acetylcysteine and desferrioxamine (Barichello et al, 2007). Thus, learning and memory performance, as evaluated by inhibitory avoidance and open field habituation tests, was impaired to a lesser extent in treated animals after recovery from sepsis (Barichello et al, 2007). Moreover, N-acetylcysteine and desferrioxamine decreased the hippocampal content of protein carbonyls, indicators of protein oxidation (Barichello et al, 2007). Likewise, iNOS gene deficiency appears to protect experimentally septic mice from permanent cognitive deficits (Weberpals et al, 2009), again supporting the role of iNOS as a critical propagator of oxidative-nitrosative stress. To validate the concept of free radical generation being responsible for regional neuronal apoptosis and impaired cognitive function, further studies are required of the time course, magnitude, and reversibility of either process alone, as well as their interrelation, in particular in patients with (rather than in experimental models of) sepsis.
We propose that free radicals cause apoptosis in selected brain regions and that this contributes to the permanent cognitive deficits that are commonly encountered in sepsis survivors.
Complementary mechanisms
Brain dysfunction in sepsis may involve a number of alternative mechanisms, which have been reviewed extensively elsewhere (Papadopoulos et al, 2000; Ebersoldt et al, 2007; Iacobone et al, 2009), and will only briefly be outlined here. These mechanisms and the six principal components of the oxidative-nitrosative stress cascade proposed above are not mutually exclusive, and may to different extents interact to affect brain function in sepsis.
Components of the innate immune system, such as proinflammatory cytokines and complement may affect blood–brain barrier function directly. TNF-α, IL-1β, and IL-6 attenuate blood–brain barrier integrity in vitro, assessed by electrical resistance and electron microscopy of rat cerebrovascular endothelial cells (Duchini et al, 1996; de Vries et al, 1996), and septic mice devoid of the TNF receptor 1 develop less cerebral edema compared with control mice (Alexander et al, 2008). By use of electron microscopy, it was similarly found that administration of an antibody to TNF-α protected blood-brain barrier integrity (Tsao et al, 2001). Likewise, treatment of septic rats with a neutralizing antibody to the complement anaphylatoxin C5a reduced breaching of the blood-brain barrier (Flierl et al, 2009).
Alterations in neurotransmitter signaling may cause brain dysfunction. Accordingly, various experimental studies suggest that sepsis is associated with abnormal cholinergic, β-adrenergic, γ-aminobutyric, serotoninergic, and glutamatergic signaling (Winder et al, 1988; Shimizu et al, 1999; Kadoi and Saito, 1996; Kadoi et al, 1996; Semmler et al, 2007; Toklu et al, 2009). Furthermore, the combination of an increased peripheral protein breakdown, and the concomitant increased hepatic synthesis of acute-phase reactants may affect central noradrenergic signaling. From the very early stages of sepsis, this causes a decrease in the plasma ratio between branched-chain and aromatic amino acids, which compete for transport through the same saturable carrier across the blood-brain barrier (Basler et al, 2002; Berg et al, 2010). This may increase the intracerebral levels of aromatic amino acids, thereby facilitating the generation of ‘false' neurotransmitters, such as β-phenylethylamine and octopamine, both of which inhibit noradrenergic pathways (Freund et al, 1978, 1979).
Conclusion
Oxidative-nitrosative stress may contribute to brain dysfunction in sepsis, both in terms of SAE and permanent cognitive deficits. As of now, direct evidence for this hypothesis is, however, lacking in humans. Although brain dysfunction is frequently the initial symptom of sepsis, it is not an isolated phenomenon. As the clinical condition deteriorates and multiorgan failure evolves, brain function may decline further. Prompt and specific antibiotic therapy to eradicate the causative pathogen as well as supportive therapy to maintain and restore organ function in the septic patient are the most critical measures to improve mortality and morbidity. However, there is currently no specific therapy for the treatment of brain dysfunction in sepsis. Therefore, the presence of neuro-oxidative-nitrosative stress is an attractive concept warranting further consideration. Future studies should include interventional experimental and clinical trials incorporating targeted delivery of nootropic ‘radical scavengers' to the blood-brain barrier and brain parenchyma. Researchers would need to consider the physiochemical suitability of proposed neurotherapeutic agents since effective delivery is a complex outcome of the agent's lipophilicity, ionic charge, molecular mass, relative brain tissue/plasma binding affinities, and ability to activate endogenous transporters (Lo et al, 2001). Recent pharmacogenomic advances have led to the rapid development of novel strategies that promote effective delivery to the injured or susceptible brain (Scherrmann, 2002; Barber et al, 2009). Future incorporation of these ‘hit compounds' to the central nervous system will clearly facilitate a more comprehensive examination of the role that free radicals and associated reactants have in the pathophysiology of brain dysfunction in sepsis.
The authors declare no conflict of interest.
Footnotes
Author contributions
RMGB drafted the manuscript. KM and DMB made critical revisions. All authors read and approved the final manuscript.
CIM is supported by a grant from the Danish National Research Foundation (#02-512-55). This study was further supported by the Danish Council for Independent Research-Medical Sciences, the Commission of the European Communities (Grant Agreement No. 223576-MYOAGE). CIM is part of the UNIK Project: Food, Fitness & Pharma for Health and Disease, supported by the Danish Ministry of Science, Technology, and Innovation. CIM is a member of DD2-the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, Grant No. 09-067009 and 09-075724).
References
- Abd El-Gawad HM, Khalifa AE. Quercetin, coenzyme Q10, and L-canavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res. 2001;43:257–263. doi: 10.1006/phrs.2000.0781. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52:447–456. doi: 10.1016/j.neuint.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babincova M, Bacova Z, Machova E, Kogan G. Antioxidant properties of carboxymethyl glucan: comparative analysis. J Med Food. 2002;5:79–83. doi: 10.1089/109662002760178159. [DOI] [PubMed] [Google Scholar]
- Bailey DM. Supplemental ascorbate and exercise-induced IL-6 metabolism: focus on Fenton chemistry and redox-regulation of vascular homeostasis. Eur J Appl Physiol. 2005;94:487–489. doi: 10.1007/s00421-005-1346-z. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Morris-Stiff G, McCord JM, Lewis MH. Has free radical release across the brain after carotid endarterectomy traditionally been underestimated? Significance of reperfusion hemodynamics. Stroke. 2007;38:1946–1948. doi: 10.1161/STROKEAHA.106.475376. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Raman S, McEneny J, Young IS, Parham KL, Hullin DA, Davies B, McKeeman G, McCord JM, Lewis MH. Vitamin C prophylaxis promotes oxidative lipid damage during surgical ischemia-reperfusion. Free Radic Biol Med. 2006;40:591–600. doi: 10.1016/j.freeradbiomed.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Taudorf S, Berg RMG, Lundby C, McEneny J, Young IS, Evans KA, James PE, Shore A, Hullin DA, McCord JM, Pedersen BK, Møller K. Increased cerebral output of free radicals during hypoxia: implications for acute mountain sickness. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1283–R1292. doi: 10.1152/ajpregu.00366.2009. [DOI] [PubMed] [Google Scholar]
- Barber SC, Higginbottom A, Mead RJ, Barber S, Shaw PJ. An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free Radic Biol Med. 2009;46:1127–1138. doi: 10.1016/j.freeradbiomed.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barichello T, Fortunato JJ, Vitali AM, Feier G, Reinke A, Moreira JC, Quevedo J, Dal Pizzol F. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med. 2006;34:886–889. doi: 10.1097/01.CCM.0000201880.50116.12. [DOI] [PubMed] [Google Scholar]
- Barichello T, Machado RA, Constantino L, Valvassori SS, Reus GZ, Martins MR, Petronilho F, Ritter C, Quevedo J, Dal Pizzol F. Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Crit Care Med. 2007;35:2186–2190. doi: 10.1097/01.ccm.0000281452.60683.96. [DOI] [PubMed] [Google Scholar]
- Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal Pizzol F. Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med. 2005;33:221–223. doi: 10.1097/01.ccm.0000150741.12906.bd. [DOI] [PubMed] [Google Scholar]
- Bartberger MD, Liu W, Ford E, Miranda KM, Switzer C, Fukuto JM, Farmer PJ, Wink DA, Houk KN. The reduction potential of nitric oxide (NO) and its importance to NO biochemistry. Proc Natl Acad Sci USA. 2002;99:10958–10963. doi: 10.1073/pnas.162095599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler T, Meier-Hellmann A, Bredle D, Reinhart K. Amino acid imbalance early in septic encephalopathy. Intensive Care Med. 2002;28:293–298. doi: 10.1007/s00134-002-1217-6. [DOI] [PubMed] [Google Scholar]
- Berg RMG, Strauss GI, Tofteng F, Qvist T, Edvinsson L, Fahrenkrug J, Qvist J, Fonsmark L, Skinhøj P, Møller K. Circulating levels of vasoactive peptides in patients with acute bacterial meningitis. Intensive Care Med. 2009;35:1604–1608. doi: 10.1007/s00134-009-1515-3. [DOI] [PubMed] [Google Scholar]
- Berg RMG, Taudorf S, Bailey DM, Lundby C, Larsen FS, Pedersen BK, Møller K. Cerebral net exchange of large neutral amino acids after lipopolysaccharide infusion in healthy humans. Crit Care. 2010;14:R16. doi: 10.1186/cc8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl D, Halle A, Freyer D, Dagand E, Braun JS, Bechmann I, Schröder NW, Weber JR. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J Clin Invest. 2005;115:1607–1615. doi: 10.1172/JCI23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton CF, Young GB, Zochodne DW. The neurological complications of sepsis. Ann Neurol. 1993;33:94–100. doi: 10.1002/ana.410330115. [DOI] [PubMed] [Google Scholar]
- Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med. 1996;24:163–172. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res. 2009;77:26–34. doi: 10.1016/j.mvr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, Weber JR. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J Clin Invest. 2002;109:19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Bolanos JP, Heales SJ. The assumption that nitric oxide inhibits mitochondrial ATP synthesis is correct. FEBS Lett. 1999;446:261–263. doi: 10.1016/s0014-5793(99)00217-3. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- Chen K, Suh J, Carr AC, Morrow JD, Zeind J, Frei B. Vitamin C suppresses oxidative lipid damage in vivo, even in the presence of iron overload. Am J Physiol Endocrinol Metab. 2000;279:E1406–E1412. doi: 10.1152/ajpendo.2000.279.6.E1406. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Chan JY, Chang AY, Sikorska M, Borowy-Borowski H, Liou CW, Chan SH. Neuroprotective effects of coenzyme Q10 at rostral ventrolateral medulla against fatality during experimental endotoxemia in the rat. Shock. 2003;19:427–432. doi: 10.1097/01.shk.0000048900.46342.37. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Tsai JL, Chang AY, Chan JY, Liou CW, Chan SH. Dysfunction of the mitochondrial respiratory chain in the rostral ventrolateral medulla during experimental endotoxemia in the rat. J Biomed Sci. 2002;9:542–548. doi: 10.1159/000064727. [DOI] [PubMed] [Google Scholar]
- Clawson CC, Harman JF, Vernier RL. Electron microscopy of the effect of gram-negative endotoxin on the blood-brain barrier. J Comp Neurol. 1966;127:183–198. doi: 10.1002/cne.901270204. [DOI] [PubMed] [Google Scholar]
- Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comim CM, Rezin GT, Scaini G, Di Pietro PB, Cardoso MR, Petronilho FC, Ritter C, Streck EL, Quevedo J, Dal Pizzol F. Mitochondrial respiratory chain and creatine kinase activities in rat brain after sepsis induced by cecal ligation and perforation. Mitochondrion. 2008;8:313–318. doi: 10.1016/j.mito.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- d'Avila JC, Santiago AP, Amancio RT, Galina A, Oliveira MF, Bozza FA. Sepsis induces brain mitochondrial dysfunction. Crit Care Med. 2008;36:1925–1932. doi: 10.1097/CCM.0b013e3181760c4b. [DOI] [PubMed] [Google Scholar]
- Davis JS, Yeo TW, Thomas JH, McMillan M, Darcy CJ, McNeil YR, Cheng AC, Celermajer DS, Stephens DP, Anstey NM. Sepsis-associated microvascular dysfunction measured by peripheral arterial tonometry: an observational study. Crit Care. 2009;13:R155. doi: 10.1186/cc8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschman CS, Kirsch JR, Breslow MJ, Miller CF, Traystman RJ. Failure of endotoxic shock to elicit superoxide anion production in pig brain. Circ Shock. 1990;31:149–158. [PubMed] [Google Scholar]
- Doerschug KC, Delsing AS, Schmidt GA, Haynes WG. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol. 2007;293:H1065–H1071. doi: 10.1152/ajpheart.01237.2006. [DOI] [PubMed] [Google Scholar]
- Duchini A, Govindarajan S, Santucci M, Zampi G, Hofman FM. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996;44:474–482. [PubMed] [Google Scholar]
- du Moulin GC, Paterson D, Hedley-Whyte J, Broitman SA. E. coli peritonitis and bacteremia cause increased blood-brain barrier permeability. Brain Res. 1985;340:261–268. doi: 10.1016/0006-8993(85)90922-9. [DOI] [PubMed] [Google Scholar]
- Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33:941–950. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Eckman PL, King WM, Brunson JG. Studies on the blood brain barrier. I. Effects produced by a single injection of gramnegative endotoxin on the permeability of the cerebral vessels. Am J Pathol. 1958;34:631–643. [PMC free article] [PubMed] [Google Scholar]
- Esen F, Erdem T, Aktan D, Orhan M, Kaya M, Eraksoy H, Cakar N, Telci L. Effect of magnesium sulfate administration on blood-brain barrier in a rat model of intraperitoneal sepsis: a randomized controlled experimental study. Crit Care. 2005;9:R18–R23. doi: 10.1186/cc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin. 2001;17:219–237. doi: 10.1016/s0749-0704(05)70161-5. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Stahel PF, Rittirsch D, Huber-Lang M, Niederbichler AD, Hoesel LM, Touban BM, Morgan SJ, Smith WR, Ward PA, Ipaktchi K. Inhibition of complement C5a prevents breakdown of the blood-brain barrier and pituitary dysfunction in experimental sepsis. Crit Care. 2009;13:R12. doi: 10.1186/cc7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund H, Atamian S, Holroyde J, Fischer JE. Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg. 1979;190:571–576. doi: 10.1097/00000658-197911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund HR, Ryan JA, Jr, Fischer JE. Amino acid derangements in patients with sepsis: treatment with branched chain amino acid rich infusions. Ann Surg. 1978;188:423–430. doi: 10.1097/00000658-197809000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci USA. 2003;100:14766–14771. doi: 10.1073/pnas.2435008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti S, Bartfai T. Induction of tumor necrosis factor-alpha mRNA in the brain after peripheral endotoxin treatment: comparison with interleukin-1 family and interleukin-6. Brain Res. 1993;624:291–294. doi: 10.1016/0006-8993(93)90090-a. [DOI] [PubMed] [Google Scholar]
- Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, Ely EW. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraca A, Aslanowicz-Antkowiak K. Prophylaxis with alpha-lipoic acid against lipopolysaccharide-induced brain injury in rats. Arch Immunol Ther Exp (Warsz) 2009;57:141–146. doi: 10.1007/s00005-009-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now. J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hamed SA, Hamed EA, Abdella MM. Septic encephalopathy: relationship to serum and cerebrospinal fluid levels of adhesion molecules, lipid peroxides and S-100B protein. Neuropediatrics. 2009;40:66–72. doi: 10.1055/s-0029-1231054. [DOI] [PubMed] [Google Scholar]
- Harada S, Imaki T, Chikada N, Naruse M, Demura H. Distinct distribution and time-course changes in neuronal nitric oxide synthase and inducible NOS in the paraventricular nucleus following lipopolysaccharide injection. Brain Res. 1999;821:322–332. doi: 10.1016/s0006-8993(99)01124-5. [DOI] [PubMed] [Google Scholar]
- Holley AE, Cheeseman KH. Measuring free radical reactions in vivo. Br Med Bull. 1993;49:494–505. doi: 10.1093/oxfordjournals.bmb.a072626. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Chan KJ, Orme JF., Jr Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004;10:1005–1017. doi: 10.1017/s135561770410711x. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med. 2009;37 (10 Suppl:S331–S336. doi: 10.1097/CCM.0b013e3181b6ed58. [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RA, Satta MA, Dahia PL, Chew SL, Grossman AB. Induction of nitric oxide synthase and interleukin-1beta, but not heme oxygenase, messenger RNA in rat brain following peripheral administration of endotoxin. Brain Res Mol Brain Res. 1997;49:238–246. doi: 10.1016/s0169-328x(97)00150-2. [DOI] [PubMed] [Google Scholar]
- Kadoi Y, Saito S. An alteration in the gamma-aminobutyric acid receptor system in experimentally induced septic shock in rats. Crit Care Med. 1996;24:298–305. doi: 10.1097/00003246-199602000-00020. [DOI] [PubMed] [Google Scholar]
- Kadoi Y, Saito S, Kunimoto F, Imai T, Fujita T. Impairment of the brain beta-adrenergic system during experimental endotoxemia. J Surg Res. 1996;61:496–502. doi: 10.1006/jsre.1996.0153. [DOI] [PubMed] [Google Scholar]
- Kafa IM, Uysal M, Bakirci S, Ayberk Kurt M. Sepsis induces apoptotic cell death in different regions of the brain in a rat model of sepsis. Acta Neurobiol Exp (Wars) 2010;70:246–260. doi: 10.55782/ane-2010-1796. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Muller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32:1715–1721. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- Lo EH, Singhal AB, Torchilin VP, Abbott NJ. Drug delivery to damaged brain. Brain Res Brain Res Rev. 2001;38:140–148. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Fujii Y, Sadamitsu D, Yokota K, Soejima Y, Ishikawa T, Miyauchi Y, Takeshita H. Cerebral circulation and metabolism in patients with septic encephalopathy. Am J Emerg Med. 1991;9:139–143. doi: 10.1016/0735-6757(91)90175-j. [DOI] [PubMed] [Google Scholar]
- Martins PS, Kallas EG, Neto MC, Dalboni MA, Blecher S, Salomao R. Upregulation of reactive oxygen species generation and phagocytosis, and increased apoptosis in human neutrophils during severe sepsis and septic shock. Shock. 2003;20:208–212. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Messaris E, Memos N, Chatzigianni E, Konstadoulakis MM, Menenakos E, Katsaragakis S, Voumvourakis C, Androulakis G. Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med. 2004;32:1764–1770. doi: 10.1097/01.ccm.0000135744.30137.b4. [DOI] [PubMed] [Google Scholar]
- Meyer TA, Wang JJ, Tiao GM, Ogle CK, Fischer JE, Hasselgren PO. Sepsis and endotoxaemia in mice stimulate the expression of interleukin-I and interleukin-6 in the central nervous system. Clin Sci (Lond) 1997;92:519–525. doi: 10.1042/cs0920519. [DOI] [PubMed] [Google Scholar]
- Moore K, Roberts LJ. Measurement of lipid peroxidation. Free Radic Res. 1998;28:659–671. doi: 10.3109/10715769809065821. [DOI] [PubMed] [Google Scholar]
- Møller K, Strauss GI, Qvist J, Fonsmark L, Knudsen GM, Larsen FS, Krabbe KS, Skinhøj P, Pedersen BK. Cerebral blood flow and oxidative metabolism during human endotoxemia. J Cereb Blood Flow Metab. 2002;22:1262–1270. doi: 10.1097/01.WCB.0000037999.34930.CA. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauser T, Koppenol WH. The rate constant of the reaction of superoxide with nitrogen monoxide: approaching the diffusion limit. J Phys Chem. 2002;106:4084–4086. [Google Scholar]
- Ninkovic M, Malicevic I, Jelenkovic A, Jovanovic DM, Dukic M, Vasiljevic I. Oxidative stress in the rats brain capillaries in sepsis--the influence of 7-nitroindazole. Acta Physiol Hung. 2006;93:315–323. doi: 10.1556/APhysiol.93.2006.4.7. [DOI] [PubMed] [Google Scholar]
- Orihuela CJ, Fillon S, Smith-Sielicki SH, El Kashmi KC, Gao G, Soulis K, Patil A, Murray PJ, Tuomanen EI. Cell wall-mediated neuronal damage in early sepsis. Infect Immun. 2006;74:3783–3789. doi: 10.1128/IAI.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med. 2000;28:3019–3024. doi: 10.1097/00003246-200008000-00057. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Lamb FJ, Moss RF, Davies DC, Tighe D, Bennett ED. Faecal peritonitis causes oedema and neuronal injury in pig cerebral cortex. Clin Sci (Lond) 1999;96:461–466. [PubMed] [Google Scholar]
- Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Ruegg S, Strebel SP, Marsch SC, Pargger H, Steiner LA. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitossi F, del Rey A, Kabiersch A, Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res. 1997;48:287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Morel DR, Hemsen A, Gunning K, Lacroix JS, Suter PM, Lundberg JM. Elevated plasma endothelin-1 concentrations are associated with the severity of illness in patients with sepsis. Ann Surg. 1991;213:261–264. doi: 10.1097/00000658-199103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- Riobo NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, Poderoso JJ. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem J. 2001;359:139–145. doi: 10.1042/0264-6021:3590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten B, Wolff S, Klatt S, Schermuly RT. Effects of inducible nitric oxide synthase inhibition or norepinephrine on the neurovascular coupling in an endotoxic rat shock model. Crit Care. 2009;13:R139. doi: 10.1186/cc8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacoccio C, Dornand J, Barbanel G. Differential regulation of brain and plasma TNFalpha produced after endotoxin shock. Neuroreport. 1998;9:309–313. doi: 10.1097/00001756-199801260-00024. [DOI] [PubMed] [Google Scholar]
- Satrustegui J, Richter C. The role of hydroperoxides as calcium release agents in rat brain mitochondria. Arch Biochem Biophys. 1984;233:736–740. doi: 10.1016/0003-9861(84)90501-0. [DOI] [PubMed] [Google Scholar]
- Satta MA, Jacobs RA, Kaltsas GA, Grossman AB. Endotoxin induces interleukin-1beta and nitric oxide synthase mRNA in rat hypothalamus and pituitary. Neuroendocrinology. 1998;67:109–116. doi: 10.1159/000054305. [DOI] [PubMed] [Google Scholar]
- Schaefer CF, Biber B. Effects of endotoxemia on the redox level of brain cytochrome a,a3 in rats. Circ Shock. 1993;40:1–8. [PubMed] [Google Scholar]
- Scherrmann JM. Drug delivery to brain via the blood-brain barrier. Vascul Pharmacol. 2002;38:349–354. doi: 10.1016/s1537-1891(02)00202-1. [DOI] [PubMed] [Google Scholar]
- Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, Heneka MT. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Semmler A, Hermann S, Mormann F, Weberpals M, Paxian SA, Okulla T, Schafers M, Kummer MP, Klockgether T, Heneka MT. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Sener G, Toklu H, Ercan F, Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. Int Immunopharmacol. 2005a;5:1387–1396. doi: 10.1016/j.intimp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Sener G, Toklu H, Kapucu C, Ercan F, Erkanli G, Kacmaz A, Tilki M, Yegen BC. Melatonin protects against oxidative organ injury in a rat model of sepsis. Surg Today. 2005b;35:52–59. doi: 10.1007/s00595-004-2879-1. [DOI] [PubMed] [Google Scholar]
- Sharma MK, Buettner GR. Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radic Biol Med. 1993;14:649–653. doi: 10.1016/0891-5849(93)90146-l. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Annane D, de la Grandmaison GL, Brouland JP, Hopkinson NS, Francoise G. The neuropathology of septic shock. Brain Pathol. 2004;14:21–33. doi: 10.1111/j.1750-3639.2004.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharshar T, Gray F, Lorin dlG, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael JC, Gajdos P, Annane D. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362:1799–1805. doi: 10.1016/s0140-6736(03)14899-4. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Adachi N, Liu K, Lei B, Nagaro T, Arai T. Sepsis facilitates brain serotonin activity and impairs learning ability in rats. Brain Res. 1999;830:94–100. doi: 10.1016/s0006-8993(99)01396-7. [DOI] [PubMed] [Google Scholar]
- Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, Hinshaw LB. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18:801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- Stanbury DM. Reduction potentials involving inorganic free radicals in aqueous solution. Adv Inorg Chem. 1989;33:69–138. [Google Scholar]
- Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10:122–128. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Fujii S, Numagami Y, Tominaga T, Yoshimoto T, Yoshimura T. In vivo nitric oxide detection in the septic rat brain by electron paramagnetic resonance. Free Radic Res. 1998;28:293–299. doi: 10.3109/10715769809069281. [DOI] [PubMed] [Google Scholar]
- Taccone FS, Su F, Pierrakos C, He X, James S, Dewitte O, Vincent JL, De Backer D. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14:R140. doi: 10.1186/cc9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Shimada Y, Okada T, Amano M, Sakai T, Yoshiya I. Lipid peroxidation in experimental septic rats. Crit Care Med. 1986;14:719–723. doi: 10.1097/00003246-198608000-00010. [DOI] [PubMed] [Google Scholar]
- Takezawa J, Taenaka N, Nishijima MK, Hirata T, Okada T, Shimada Y, Yoshiya I. Amino acids and thiobarbituric acid reactive substances in cerebrospinal fluid and plasma of patients with septic encephalopathy. Crit Care Med. 1983;11:876–879. doi: 10.1097/00003246-198311000-00007. [DOI] [PubMed] [Google Scholar]
- Toklu HZ, Uysal MK, Kabasakal L, Sirvanci S, Ercan F, Kaya M. The effects of riluzole on neurological, brain biochemical, and histological changes in early and late term of sepsis in rats. J Surg Res. 2009;152:238–248. doi: 10.1016/j.jss.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- Tsao N, Hsu HP, Wu CM, Liu CC, Lei HY. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J Med Microbiol. 2001;50:812–821. doi: 10.1099/0022-1317-50-9-812. [DOI] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- Voigt K, Kontush A, Stuerenburg HJ, Muench-Harrach D, Hansen HC, Kunze K. Decreased plasma and cerebrospinal fluid ascorbate levels in patients with septic encephalopathy. Free Radic Res. 2002;36:735–739. doi: 10.1080/10715760290032557. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, Berger M, Schafers M, Heneka MT. NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci. 2009;29:14177–14184. doi: 10.1523/JNEUROSCI.3238-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder TR, Minuk GY, Sargeant EJ, Seland TP. gamma-aminobutyric acid (GABA) and sepsis-related encephalopathy. Can J Neurol Sci. 1988;15:23–25. doi: 10.1017/s0317167100027128. [DOI] [PubMed] [Google Scholar]
- Wong ML, Rettori V, al Shekhlee A, Bongiorno PB, Canteros G, McCann SM, Gold PW, Licinio J. Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nat Med. 1996;2:581–584. doi: 10.1038/nm0596-581. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke. 1986;17:1220–1228. doi: 10.1161/01.str.17.6.1220. [DOI] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA. The encephalopathy associated with septic illness. Clin Invest Med. 1990;13:297–304. [PubMed] [Google Scholar]
- Zhan RZ, Fujiwara N, Shimoji K. Regionally different elevation of intracellular free calcium in hippocampus of septic rat brain. Shock. 1996;6:293–297. doi: 10.1097/00024382-199610000-00012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]