Abstract
Despite an undisputed association between vasospasm and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage (SAH), there is debate if this association implies causality. It has been suggested that cerebral infarction is a better outcome measure than vasospasm in clinical trials and observational studies. To further investigate the relationship between infarction and outcome, we performed a systematic review and meta-analysis of all randomized, double-blind, placebo-controlled trials that studied the efficacy of pharmaceutical preventive strategies in SAH patients, and had both cerebral infarction and clinical outcome as outcome events. Effect sizes were expressed in (pooled) risk ratio (RR) estimates with corresponding 95% confidence intervals (CIs). Sensitivity analyses were performed for studies with a low risk of bias and for those who reported outcome at 3 months after SAH. Twenty-four studies including 8,552 patients were included. Pharmaceutical treatments decreased the incidence of both cerebral infarction (RR: 0.83; 95% CI: 0.74 to 0.93) and of poor functional outcome (RR: 0.92; 95% CI: 0.86 to 0.98). The sensitivity analyses did not change the results essentially. These data suggest that the previously observed association between cerebral infarction and functional outcome implies causality, and that cerebral infarction is a better outcome measure than vasospasm in clinical trials and observational studies.
Keywords: cerebral infarction, delayed cerebral ischemia, meta-analysis, outcome, subarachnoid hemorrhage, systematic review
Introduction
Patients with aneurysmal subarachnoid hemorrhage (SAH) are at high risk of developing delayed cerebral ischemia (DCI). Delayed cerebral ischemia can progress to cerebral infarction, resulting in severe disability or death in 50% of patients. The pathogenesis of DCI remains incompletely understood. Despite the undisputed association between arterial narrowing (‘vasospasm') and DCI, there is debate if this association implies causality (Millikan, 1975).
Many randomized controlled trials have been performed over the last decades aiming to find a treatment that effectively prevents DCI. Although several drugs were identified that successfully prevent vasospasm, no effects on clinical outcome were observed (Macdonald et al, 2008; Etminan et al, 2011). It has been proposed that cerebral infarction and functional outcome, and not vasospasm, should be the main outcome measures in clinical trials and observational studies (Vergouwen et al, 2010). The aim of the present study was to investigate if a lower incidence of cerebral infarction after SAH correlates with better functional outcomes.
Materials and methods
For this systematic review, the Cochrane Collaboration format was used (Higgins and Green, 2008).
Selection Criteria
Types of studies
All randomized, double-blind, placebo-controlled trials that studied the efficacy of pharmaceutical strategies aiming to prevent cerebral infarction and poor outcome in SAH patients were included, regardless of type and dosage of drug and follow-up duration.
Types of outcome measures
Only trials that included both cerebral infarction and clinical outcome as outcome events were included.
Definition of outcome measures
Cerebral infarction was defined as ‘the presence of cerebral infarction on computed tomography (CT) or magnetic resonance scan of the brain within 6 weeks after SAH, or on the latest CT or magnetic resonance scan made before death within 6 weeks, or proven at autopsy, not present on the CT or magnetic resonance scan between 24 and 48 hours after early aneurysm occlusion, and not attributable to other causes such as surgical clipping or endovascular treatment. Hypodensities on CT imaging resulting from ventricular catheter or intraparenchymal hematoma should not be regarded as cerebral infarctions from DCI' (Vergouwen et al, 2010). Poor clinical outcome was defined as severe disability, vegetative state or death, as defined by the investigators of the individual studies, either measured with the modified Rankin scale or (extended) Glasgow outcome scale. If the investigators provided data on the individual categories of the used outcome scales, we considered a modified Rankin scale of 3 to 6 or a Glasgow outcome scale of 1 to 3 as poor clinical outcome. In those studies where an inverted Glasgow outcome scale was used, poor clinical outcome was readjusted to the original scale. For all included studies, we used data from the last blinded outcome measurement. If the study only provided a figure or the percentage of patients with an outcome event, the actual numbers were calculated from the figure or percentages.
Search strategy for identification of studies
Two of the authors (NE and MDIV) conducted a systematic search of the literature using the Pubmed database (http://www.ncbi.nlm.nih.gov/pubmed) up to February 2011 (week 8) for the variable ‘SAH.' The search was limited to ‘clinical trials,' ‘controlled clinical trials,' and ‘human studies.' Abstracts of the resulting studies were reviewed independently and invalid studies were excluded in a three-step manner. In the fourth and final step, only double-blinded, placebo-controlled, randomized studies were screened for valid end points, that is cerebral infarction and functional outcome. Further, additional studies in previous meta-analyses that investigated the efficacy of pharmaceutical interventions to prevent cerebral infarction after SAH were searched.
Statistics
Data were processed in Review manager 5.0.25 as supplied by the Cochrane Collaboration. Effect sizes were expressed in (pooled) risk ratio (RR) estimates. Statistical uncertainty was expressed in 95% confidence intervals (CIs). Pooled data were interpreted to be heterogeneous in case the probability value of the χ2 test was ⩽0.10. If no heterogeneity could be demonstrated, we used a fixed-effects model. Otherwise, a random-effects model was used. Two authors (MDIV and NE) independently assessed risk of bias by reviewing methodological quality of the included studies for allocation concealment and blinding. Funnel plots, including Begg's and Egger's statistical tests, were generated in Stata (version 9, College Station, TX, USA) to investigate potential bias. Sensitivity analyses were performed for studies with a low risk of bias (both on the items ‘allocation concealment' and ‘blinding'), and for studies that reported functional outcome at 3 months after SAH.
Results
The initial search yielded 780 articles, of which ultimately 756 were excluded. The remaining 24 trials were included in the analysis, including 8,552 patients (Macdonald et al, 2008; Asano et al, 1996; Chou et al, 2008; Gomis et al, 2010; Haley et al, 1993, 1997; Hop et al, 2000; Kassell et al, 1996; Lanzino et al, 1999; Lanzino and Kassell, 1999; Ohman and Heiskanen, 1988; Petruk et al, 1988; Pickard et al, 1989; Saito et al, 1998; Shaw et al, 2000; Shibuya et al, 1992; Siironen et al, 2003; Juvela et al, 2005; Springborg et al, 2007; Suzuki et al, 1989; Tseng et al, 2009; van den Bergh et al, 2005, 2006; Westermaier et al, 2010; Wong et al, 2010, 2011). A total of 4,818 patients were randomized to pharmaceutical treatment, and 3,734 patients to placebo. Characteristics of the included studies are listed in Table 1.
Table 1. Characteristics of included studies.
| Study | Drug studied | Imaging modality | Definition of cerebral infarction | Definition of poor outcome | Adequate allocation concealment | Adequate blinding |
|---|---|---|---|---|---|---|
| Asano et al (1996) | AVS | CT | All patients were required to undergo repeat CT scans on completion of treatment (∼day 14) and at ∼day 30, in addition to those routinely obtained on admission, immediately after surgery, or on exacerbation of neurologic deficits. The findings of each CT scan and the presumed cause of the postoperative appearance of LDAs were documented according to the surgeon's judgment | GOS 1–3 at 3 months | Yes | Yes |
| Chou et al (2008) | Simvastatin | CT or MRI | The development of a new lesion consistent with infarction on CT or MRI in the vascular territory of angiographic or TCD vasospasm (timing of imaging unclear) | mRS 3–6 on discharge | Unclear | Unclear |
| Gomis et al (2010) | Methyl-prednisolone | CT | A new ischemic area following vasospasm was defined as any new low-density zone on CT scan not explained by other causes of focal cerebral ischemia between 4 and 10 days after SAH onset | GOS 1–3 at 12 months | Unclear | Yes |
| Haley et al (1993) | Nicardipine | CT | Infarction on a CT scan obtained at 3 months | GOS 1–3 at 3 months | Yes | Yes |
| Haley et al (1997) | Tirilazad | CT | A follow-up CT scan of the head was obtained at 3 months and sent to the Central Registry for blinded interpretation and measurement of infarct volume using planimetric techniques | GOS 1–3 at 3 months | Unclear | Yes |
| Hop et al (2000) | Aspirin | CT | New hypodense lesion consistent with clinical signs of delayed cerebral ischemia | mRS 3–6 at 4 months | Yes | Yes |
| Kassell et al (1996) | Tirilazad | CT | A follow-up CT scan of the head was obtained at 3 months and sent to the Central Registry for blinded interpretation and measurement of infarct volume using planimetric techniques | GOS 1–3 at 3 months | Unclear | Yes |
| Lanzino et al (1999) | Tirilazad | CT | Unclear | GOS 1–3 at 3 months | Unclear | Yes |
| Lanzino and Kassell (1999) | Tirilazad | CT | Unclear | GOS 1–3 at 3 months | Unclear | Yes |
| Macdonald et al (2008) | Clazosentan | CT | New cerebral infarct due to vasospasm within 6 weeks of SAH | GOS 1–3 at 12 weeks | Yes | Yes |
| Ohman and Heiskanen (1988) | Nimodipine | CT | Unclear | GOS 1–3 at 3 months | Unclear | Unclear |
| Petruk et al (1988) | Nimodipine | CT | Hypodense areas consistent with infarction were categorized at 3 months as being (1) in the same area as a previous ICH; (2) secondary to vasospasm if they occurred in an area where there had not been a previous ICH; or (3) indeterminate, if no previous CT scan was available or if they were not easily placed into one of the previous two categories | GOS 1–3 at 3 months | Yes | Yes |
| Pickard et al (1989) | Nimodipine | CT | The diagnosis of infarction was classified as definitive or probable according to whether confirmatory evidence was available from CT or at necropsy | GOS 1–3 at least 3 months after entry to the trial | Yes | Yes |
| Saito et al (1998) | Ebselen | CT | LDAs on CT scans obtained around day 14 or day 30 after SAH. LDAs that appeared within 2 days of surgery or within 24 hours of onset and were considered to be caused by surgery, and temporary clipping or intracerebral hematoma were excluded from assessment | GOS 1–3 at 3 months | Unclear | Unclear |
| Shaw et al (2000) | TAK-044 | CT or postmortem studies | Development of a cerebral infarct visualized on a CT scan or at autopsy during the 3 months following onset of SAH | GOS 1–3 at 3 months | Yes | Yes |
| Shibuya et al (1992) | Fasudil | CT | Larger LDAs (due to vasospasm) between two CT scans (obtained at 1 or 2 weeks after the SAH, and at 1 month) | GOS 1–3 at 1 month | Yes | Yes |
| Siironen et al (2003) | Enoxaparin | CT | A hypodense lesion, caused by delayed cerebral ischemia (with or without symptoms and after excluding other causes) and appearing later than on the 1st postoperative CT scan | GOS 1–3 at 3 months | Unclear | Yes |
| Springborg et al (2007) | Erythropoietin | CT or MRI | CT or MRI scans performed around day 14 after the hemorrhage were evaluated for signs of vasospastic infarction | GOS 1–3 at 6 months | Yes | Yes |
| Suzuki et al (1989) | OKY-046 | CT | Abnormal low densities (LD) with 48 hours postoperatively and after 21 days were classified into five grades: –=no LD; +=small definite lacunar infarction; ++=moderate infarction larger than 2 cm but smaller than the territory of one vessel; +++=multiple small-to-moderate infarctions; ++++=large infarction over the entire MCA territory or extended territories of several vessels. All grades were included in the analysis | Functional ADL was evaluated at 1 and 3 months after aneurysmal rupture by of nine grades (0=normal; 1=almost normal; 2=slight neurologic deficit with usable hand finger; 3=unaided daily life; 4=assisted daily life; 5=can leave bad with assistance; 6=confined to bed; 7=vegetative state; 8=deceased at 3 months. Grades 4–8 were considered poor functional outcome | Unclear | Unclear |
| Tseng et al (2009) | Erythropoietin | CT | Unclear | mRS 4–6, GOS 1–3 or NIHSS >20 at 6 months | Yes | Yes |
| Van den Bergh et al (2005) | Magnesium sulfate | CT | Any new hypodensity on brain CT regardless of its cause | mRS 4–6 at 3 months | Yes | Yes |
| Van den Bergh et al (2006) | Aspirin | CT | Any new hypodensity on brain CT, regardless of its cause | mRS 4–6 at 3 months | Yes | Yes |
| Westermaier et al (2010) | Magnesium sulfate | CT | Parenchymal hypodensity appearing between day 3 and the end of the observation period | GOS 1–3 at 6 months | Unclear | Yes |
| Wong et al (2010) | Magnesium sulfate | Unclear | A new cerebral infarction within 3 weeks that was not related to posttreatment (coiling or clipping) complications, the ventricular catheter track, a rebleed, or hydrocephalus | GOSE 1–4 at 6 months | Yes | Yes |
ADL, activity in daily living; AVS, (±)-N,N'-propylenedinicotinamide (nicaraven); CT, computed tomography; GOS, Glasgow outcome score; GOSE, extended Glasgow outcome score; ICH, intracerebral hemorrhage; LDAs, low-density areas; MCA, middle cerebral artery; MRI, magnetic resonance imaging; mRS, modified Rankin scale; NIHSS, National Institute of Health Stroke Scale; SAH, subarachnoid hemorrhage; TCD, transcranial Doppler.
Analyses
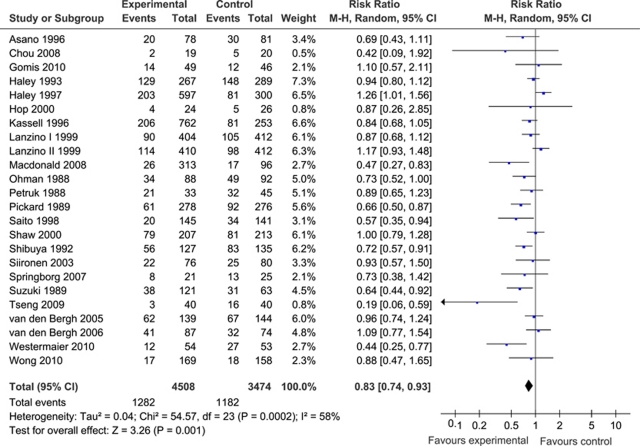
For the analysis of cerebral infarction, data from 7,982 patients were available (4,508 patients randomized to pharmaceutical treatment and 3,474 to placebo). The overall number of patients with cerebral infarction was 1,282 in the group of patients randomized to pharmaceutical treatment and 1,182 in the placebo group. Our meta-analysis demonstrated a significant and favorable effect of treatment compared with placebo on cerebral infarction (pooled RR: 0.83; 95% CI: 0.74 to 0.93). Heterogeneity between the trials was high (I2=58% and P=0.0002), which justified the use of a random-effects meta-analysis model (Figure 1).
Figure 1.
(Pooled) risk ratio (RR) estimates for patients on pharmaceutical treatment to have cerebral infarction. CI, confidence interval.
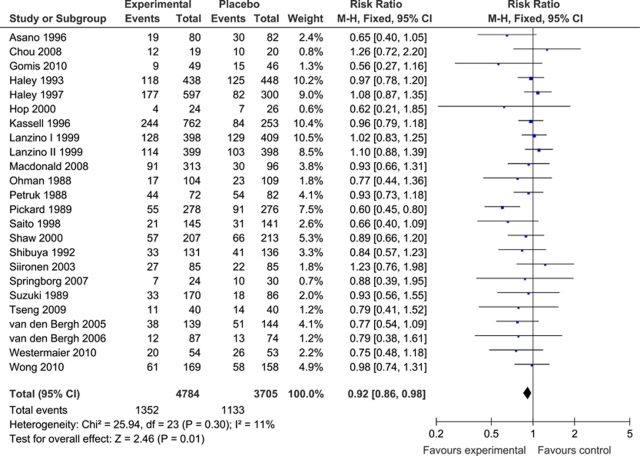
For clinical outcome, most studies used the Glasgow outcome scale (Macdonald et al, 2008; Asano et al, 1996; Gomis et al, 2010; Haley et al, 1993, 1997; Kassell et al, 1996; Lanzino et al, 1999; Lanzino and Kassell, 1999; Ohman and Heiskanen, 1988; Petruk et al, 1988; Pickard et al, 1989; Saito et al, 1998; Shaw et al, 2000; Shibuya et al, 1992; Siironen et al, 2003; Springborg et al, 2007; Westermaier et al, 2010; Wong et al, 2010) or modified Rankin scale (Chou et al, 2008; Hop et al, 2000; Tseng et al, 2009; van den Bergh et al, 2005, 2006) (Table 1). One study used a functional activity in daily living scale (Suzuki et al, 1989). For the meta-analysis of poor clinical outcome, data from 8,489 patients were available (4,784 patients randomized to pharmaceutical treatment, 3,705 to placebo). The number of patients with poor outcome was 1,352 in the pharmaceutically treated group and 1,133 in the placebo group. Meta-analysis showed a beneficial effect of treatment compared with placebo on poor clinical outcome (pooled RR: 0.92; 95% CI: 0.86 to 0.98). Heterogeneity between the trials was not significant (I2=11% and P=0.30), which justified the use of a fixed-effects model (Figure 2).
Figure 2.
(Pooled) risk ratio (RR) estimates for patients on pharmaceutical treatment to have poor clinical outcome. CI, confidence interval.
Risk of Bias Assessments
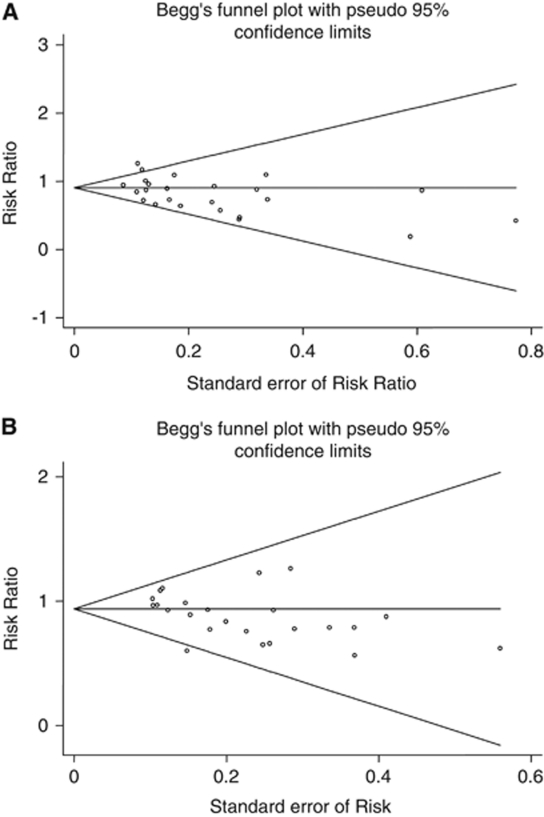
For allocation concealment, risk of bias was low in 13 (Macdonald et al, 2008; Asano et al, 1996; Haley et al, 1993; Hop et al, 2000; Petruk et al, 1988; Pickard et al, 1989; Shaw et al, 2000; Shibuya et al, 1992; Springborg et al, 2007; Tseng et al, 2009; van den Bergh et al, 2005, 2006; Wong et al, 2010) and unclear in 11 studies (Chou et al, 2008; Gomis et al, 2010; Haley et al, 1997; Kassell et al, 1996; Lanzino et al, 1999; Lanzino and Kassell, 1999; Ohman and Heiskanen, 1988; Saito et al, 1998; Siironen et al, 2003; Suzuki et al, 1989; Westermaier et al, 2010) (Table 1). For blinding, risk of bias was low in 20 studies (Macdonald et al, 2008; Asano et al, 1996; Gomis et al, 2010; Haley et al, 1993, 1997; Hop et al, 2000; Kassell et al, 1996; Lanzino et al, 1999; Lanzino and Kassell, 1999; Petruk et al, 1988; Pickard et al, 1989; Shaw et al, 2000; Shibuya et al, 1992; Siironen et al, 2003; Springborg et al, 2007; Tseng et al, 2009; van den Bergh et al, 2005, 2006; Westermaier et al, 2010; Wong et al, 2010), and unclear in four studies (Chou et al, 2008; Ohman and Heiskanen, 1988; Saito et al, 1998; Suzuki et al, 1989). High risk of bias was not observed in any of the studies. One of the authors of this systematic review acknowledges potential risk of bias, because he is an author of one of the studies included in this systematic review (Macdonald et al, 2008). Funnel plots are shown in Figures 3A and 3B. For the cerebral infarction outcome measure, the Egger's test supports the evidence of significant level of bias (P=0.037) in the pooled publications. However, Begg's test (P=0.172) and the close to symmetry orientation of the funnel plot (Figure 3A) suggested otherwise. For the poor clinical outcome measure, both the Egger's test (P=0.037) and Begg's test (P=0.038) with the close to asymmetry orientation of the funnel plot (Figure 3B) support the evidence of significant level of bias.
Figure 3.
Funnel plot for outcome measure cerebral infarction (A) and functional outcome (B). The name funnel plot arises from the fact that precision of the estimated intervention effect increases as the size of the study increases. Effect estimates from small studies will therefore scatter more widely at the y-axis, with the spread narrowing among larger studies. In the absence of bias, the plot should approximately resemble a symmetrical funnel. If there is bias, from for example publication bias or studies with low methodological quality, this will lead to an asymmetrical appearance of the funnel plot (Higgins and Green, 2008).
Sensitivity Analyses
In the sensitivity analysis that only included studies with a low risk of bias, as assessed on the items ‘allocation concealment' and ‘blinding,' 13 studies were included with 3,831 patients (Macdonald et al, 2008; Asano et al, 1996; Haley et al, 1993; Hop et al, 2000; Petruk et al, 1988; Pickard et al, 1989; Shaw et al, 2000; Shibuya et al, 1992; Springborg et al, 2007; Tseng et al, 2009; van den Bergh et al, 2005, 2006; Wong et al, 2010). The pooled RR for cerebral infarction was 0.70 (95% CI: 0.56 to 0.88) and for poor outcome 0.78 (95% CI: 0.67 to 0.90) (figures not shown). Heterogeneity between the trials was significant in the cerebral infarction analysis (I2=45% and P=0.04), but not in the poor outcome analysis (I2=0% and P=0.62). In the sensitivity analysis that only included studies with an outcome assessment 3 months after SAH, 16 studies were included with 7,530 patients (Macdonald et al, 2008; Asano et al, 1996; Haley et al, 1993, 1997; Kassell et al, 1996; Lanzino et al, 1999; Lanzino and Kassell, 1999; Ohman and Heiskanen, 1988; Petruk et al, 1988; Pickard et al, 1989; Saito et al, 1998; Shaw et al, 2000; Siironen et al, 2003; Suzuki et al, 1989; van den Bergh et al, 2005, 2006). The pooled RR for cerebral infarction was 0.87 (95% CI: 0.78 to 0.98) and for poor outcome 0.93 (95% CI: 0.86 to 1.00) (figures not shown). Again, heterogeneity between the trials was high in the cerebral infarction analysis (I2=59% and P=0.002), but not in the poor outcome analysis (I2=28% and P=0.14).
Discussion
This systematic review including 8,552 patients shows that a lower incidence of cerebral infarction after SAH correlates with a lower incidence of poor functional outcome. The sensitivity analysis that only included studies with a low risk of bias confirmed these findings. The sensitivity analysis that reported on functional outcome 3 months after SAH showed similar findings, though the poor outcome analysis had CIs that included 1.00.
Previous studies found strong associations between vasospasm, DCI, cerebral infarction, and poor functional outcome (Fisher et al, 1977; Vergouwen et al, 2011; Rabinstein et al, 2004; Fergusen and Macdonald 2007). However, associations do not always represent causal relationships. Especially for vasospasm, it is debated if it causes DCI and poor functional outcome (Millikan, 1975; Vergouwen et al, 2008). A recent systematic review including 4,235 patients with SAH showed that pharmaceutical interventions decreased vasospasm after SAH, but not poor functional outcome (Etminan et al, 2011). This dissociation between vasospasm and clinical outcome could result from methodological problems, sample size, insensitivity of clinical outcome measures, or from mechanisms other than vasospasm that also contribute to poor outcome. Current understanding is that the pathogenesis of DCI is multifactorial. Microthromboembolism, cortical spreading ischemia, delayed effects of acute SAH-induced brain injury, and impaired cerebral autoregulation have been suggested to have a role in clinical outcome (Vergouwen et al, 2008; Dreier et al, 2009; Yundt et al, 1998). Since cerebral infarction is the ultimate outcome of DCI, it has been suggested that cerebral infarction is a better outcome measure in observational studies and clinical trials than vasospasm (Vergouwen et al, 2010). The findings of the present study support this suggestion. Obviously, the main outcome measure in such studies should be functional outcome (Vergouwen et al, 2010).
This study has some limitations. Significant heterogeneity was observed in the cerebral infarction meta-analyses. This might result from the many different drugs used in the various studies, which all have different pharmacological properties. The studies that were included did not use a uniform definition of cerebral infarction, which may introduce some variability. To account for the observed heterogeneity, we used a random-effects model instead of a fixed-effects model. In addition, the meta-analysis shows an association between cerebral infarction and poor outcome. As with the relation between angiographic vasospasm and DCI, it is still conceivable that this relation is not causal, although this seems unlikely since it would require one to accept that dead brain is not somehow detrimental to outcome. Furthermore, for the outcome measure ‘functional outcome,' statistical tests showed evidence of bias. However, our sensitivity analysis that only included studies with low risk of bias confirmed our finding that lower rates of infarction are correlated with better functional outcomes. Finally, in many of the studies included in the meta-analysis, diagnosis of cerebral infarction was based on CT scans only. Since the sensitivity of CT to detect cerebral infarction is low, the result of the meta-analysis might significantly underestimate the presence of infarction (Dreier et al, 2002). Future studies should investigate the relationship between cerebral infarction on magnetic resonance imaging and functional outcome after SAH.
In conclusion, pharmaceutical treatments decreased both cerebral infarction and poor functional outcome after SAH. These data suggest that the previously observed association between cerebral infarction and functional outcome implies causality. Considering our prior meta-analysis studying the relationship between angiographic vasospasm and outcome that showed no relationship (Etminan et al, 2011), the present results suggest that cerebral infarction may be a better outcome measure than vasospasm in clinical trials and observational studies.
Acknowledgments
MDIV is financially supported by a grant from the Netherlands Thrombosis Foundation, The Netherlands (2010-4). Drs N Etminan and RL Macdonald received research support from The Physicians Services Incorporated Foundation.
Dr RL Macdonald is a consultant for Actelion Pharmaceuticals and Chief Scientific Officer of Edge Therapeutics, Inc.
References
- Asano T, Takakura K, Sano K, Kikuchi H, Nagai H, Saito I, Tamura A, Ochiai C, Sasaki T. Effects of a hydroxyl radical scavenger on delayed ischemic neurological deficits following aneurysmal subarachnoid hemorrhage: results of a multicenter, placebo-controlled double-blind trial. J Neurosurg. 1996;84:792–803. doi: 10.3171/jns.1996.84.5.0792. [DOI] [PubMed] [Google Scholar]
- Chou SH, Smith EE, Badjatia N, Nogueira RG, Sims JR, Ogilvy CS, Rordorf GA, Ayata C. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:2891–2893. doi: 10.1161/STROKEAHA.107.505875. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ, COSBID study group Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Sakowitz OW, Harder A, Zimmer C, Dirnagl U, Valdueza JM, Unterberg AW. Focal laminar cortical MR signal abnormalities after subarachnoid hemorrhage. Ann Neurol. 2002;52:825–829. doi: 10.1002/ana.10383. [DOI] [PubMed] [Google Scholar]
- Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL.2011Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia and clinical outcome in patients with aneurysmal subarachnoid hemorrhage—a systematic review and meta-analysis J Cereb Blood Flow MetabEpub ahead of print 2 February 2011: PMID: 21285966 [DOI] [PMC free article] [PubMed]
- Fergusen S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:658–667. doi: 10.1227/01.NEU.0000255396.23280.31. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Roberson GH, Ojemann RG. Cerebral vasospasm with ruptured saccular aneurysm--the clinical manifestations. Neurosurgery. 1977;1:245–248. doi: 10.1227/00006123-197711000-00004. [DOI] [PubMed] [Google Scholar]
- Gomis P, Graftieaux JP, Sercombe R, Hettler D, Scherpereel B, Rousseaux P. Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2010;112:681–688. doi: 10.3171/2009.4.JNS081377. [DOI] [PubMed] [Google Scholar]
- Haley EC, Jr, Kassell NF, Torner JC. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1993;78:537–547. doi: 10.3171/jns.1993.78.4.0537. [DOI] [PubMed] [Google Scholar]
- Haley EC, Jr, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86:467–474. doi: 10.3171/jns.1997.86.3.0467. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S.(eds) (2008Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane CollaborationAvailable from http://www.cochrane-handbook.org
- Hop JW, Rinkel GJ, Algra A, Berkelbach van der Sprenkel JW, van Gijn J. Randomized pilot trial of postoperative aspirin in subarachnoid hemorrhage. Neurology. 2000;54:872–878. doi: 10.1212/wnl.54.4.872. [DOI] [PubMed] [Google Scholar]
- Juvela S, Siironen J, Varis J, Poussa K, Porras M. Risk factors for ischemic lesions following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005;102:194–201. doi: 10.3171/jns.2005.102.2.0194. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Haley ECJ, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF, Dorsch NW, Pasqualin A, Brandt L, Schmiedek P, Truskowski LL, Alves WM. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part I. A cooperative study in Europe, Australia, New Zealand, and South Africa. J Neurosurg. 1999;90:1011–1017. doi: 10.3171/jns.1999.90.6.1011. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–1024. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, CONSCIOUS-1 Investigators Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- Millikan CH. Cerebral vasospasm and ruptured intracranial aneurysm. Arch Neurol. 1975;32:433–449. doi: 10.1001/archneur.1975.00490490037003. [DOI] [PubMed] [Google Scholar]
- Ohman J, Heiskanen O. Effect of nimodipine on the outcome of patients after aneurysmal subarachnoid hemorrhage and surgery. J Neurosurg. 1988;69:683–686. doi: 10.3171/jns.1988.69.5.0683. [DOI] [PubMed] [Google Scholar]
- Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, Disney LB, Khan MI, Grace M, Holness RO, Karwon MS, Ford RM, Cameron GS, Tucker WS, Purves GB, Miller JDR, Hunter KM, Richard MT, Durity FA, Chan R, Clein LJ, Maroun FB, Godon A. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg. 1988;68:505–517. doi: 10.3171/jns.1988.68.4.0505. [DOI] [PubMed] [Google Scholar]
- Pickard JD, Murray GD, Illingworth R, Shaw MD, Teasdale GM, Foy PM, Humphrey PR, Lang DA, Nelson R, Richards P. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989;298:636–642. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–1866. doi: 10.1161/01.STR.0000133132.76983.8e. [DOI] [PubMed] [Google Scholar]
- Saito I, Asano T, Sano K, Takakura K, Abe H, Yoshimoto T, Kikuchi H, Ohta T, Ishibashi S. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1998;42:269–277. doi: 10.1097/00006123-199802000-00038. [DOI] [PubMed] [Google Scholar]
- Shaw MD, Vermeulen M, Murray GD, Pickard JD, Bell BA, Teasdale GM. Efficacy and safety of the endothelin, receptor antagonist TAK-044 in treating subarachnoid hemorrhage: a report by the Steering Committee on behalf of the UK/Netherlands/Eire TAK-044 Subarachnoid Haemorrhage Study Group. J Neurosurg. 2000;93:992–997. doi: 10.3171/jns.2000.93.6.0992. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, Nagata I, Kikuchi H, Takemae T, Hidaka H, Nakashima M. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–577. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- Siironen J, Juvela S, Varis J, Porras M, Poussa K, Ilveskero S, Hernesniemi J, Lassila R. No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled clinical trial. J Neurosurg. 2003;99:953–959. doi: 10.3171/jns.2003.99.6.0953. [DOI] [PubMed] [Google Scholar]
- Springborg JB, Moller C, Gideon P, Jorgensen OS, Juhler M, Olsen NV. Erythropoietin in patients with aneurysmal subarachnoid haemorrhage: a double blind randomised clinical trial. Acta Neurochir (Wien) 2007;149:1089–1101. doi: 10.1007/s00701-007-1284-z. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Sano K, Handa H, Asano T, Tamura A, Yonekawa Y, Ono H, Tachibana N, Hanaoka K. Clinical study of OKY-046, a thromboxane synthetase inhibitor, in prevention of cerebral vasospasms and delayed cerebral ischaemic symptoms after subarachnoid haemorrhage due to aneurysmal rupture: a randomized double-blind study. Neurol Res. 1989;11:79–88. doi: 10.1080/01616412.1989.11739867. [DOI] [PubMed] [Google Scholar]
- Tseng MY, Hutchinson PJ, Richards HK, Czosnyka M, Pickard JD, Erber WN, Brown S, Kirkpatrick PJ. Acute systemic erythropoietin therapy to reduce delayed ischemic deficits following aneurysmal subarachnoid hemorrhage: a phase II randomized, double-blind, placebo-controlled trial. Clinical article. J Neurosurg. 2009;111:171–180. doi: 10.3171/2009.3.JNS081332. [DOI] [PubMed] [Google Scholar]
- van den Bergh WM, Algra A, van Kooten F, Dirven CM, van Gijn J, Vermeulen M, Rinkel GJ, MASH Study Group Magnesium sulfate in aneurysmal subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2005;36:1011–1015. doi: 10.1161/01.STR.0000160801.96998.57. [DOI] [PubMed] [Google Scholar]
- van den Bergh WM, Algra A, Dorhout Mees SM, van Kooten F, Dirven CM, van Gijn J, Vermeulen M, Rinkel GJ. Randomized controlled trial of acetylsalicylic acid in aneurysmal subarachnoid hemorrhage: the MASH Study. Stroke. 2006;37:2326–2330. doi: 10.1161/01.STR.0000236841.16055.0f. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–929. doi: 10.1161/STROKEAHA.110.597914. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies. Proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- Westermaier T, Stetter C, Vince GH, Pham M, Tejon JP, Eriskat J, Kunze E, Matthies C, Ernestus RI, Solymosi L, Roosen K. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage: a randomized, placebo-controlled, clinical study. Crit Care Med. 2010;38:1284–1290. doi: 10.1097/CCM.0b013e3181d9da1e. [DOI] [PubMed] [Google Scholar]
- Wong GK, Boet R, Poon WS, Chan MT, Gin T, Ng SC, Zee BC. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage: an updated systemic review and meta-analysis. Crit Care. 2011;15:R52. doi: 10.1186/cc10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, Zee BC. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke. 2010;41:921–926. doi: 10.1161/STROKEAHA.109.571125. [DOI] [PubMed] [Google Scholar]
- Yundt KD, Grubb RL, Jr, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998;18:419–424. doi: 10.1097/00004647-199804000-00010. [DOI] [PubMed] [Google Scholar]