Abstract
Reinstatement of perilesional activation and connectivity may underlie functional recovery after stroke. To measure activation responsiveness in perilesional cortex in relation to white matter integrity, we performed functional functional magnetic resonance imaging during stimulation of the contralesional cortex, together with diffusion tensor imaging, 3 and 28 days after stroke in rats. Despite disturbed sensorimotor function and abnormal callosal appearance at day 3, activation amplitudes were preserved in the perilesional sensorimotor cortex, although time-to-peak was significantly delayed. This indicates that in spite of dysfunction, perilesional cortical tissue can be activated subacutely after stroke, while delay of the hemodynamic activation response suggests impaired neurovascular coupling.
Keywords: brain connectivity, direct cortical stimulation, MRI, plasticity, rats, stroke
Introduction
There is increasing evidence for the adult brain's capacity to reorganize after stroke. Structural and functional remodeling of intact neuronal tissue nearby or remote from an ischemic infarct may significantly alter signaling within bilateral neuronal networks, which may contribute to compensation or partial restoration of acutely disturbed functions. Task- or stimulus-induced functional magnetic resonance imaging (fMRI) studies have shown that subacute loss of activation responses in the ipsilesional, structurally intact, sensorimotor cortex in patients and rats with sensorimotor dysfunction after unilateral stroke, can recover at chronic stages in parallel with improvement of sensorimotor function (Calautti and Baron, 2003; Dijkhuizen et al, 2003; Weber et al, 2008). More recently, resting-state fMRI (rs-fMRI) studies in stroke patients and animal models have revealed a strong correlation between baseline interhemispheric functional connectivity and behavioral outcome (Carter et al, 2010; van Meer et al, 2010). However, it remains unknown to what extent perilesional activation responsiveness and direct transcallosal neuronal signal transmission between the two hemispheres are affected at different stages after stroke.
The aim of this study was to determine the degree of activation of the perilesional sensorimotor cortex in response to direct stimulation of the contralesional primary motor cortex (M1) at a subacute and chronic stage after unilateral stroke in rats. We hypothesized that contralesional cortical stimulation elicits perilesional activation responses that are disturbed subacutely after stroke, but recover chronically, dependent on the integrity of the connecting corpus callosum.
Materials and methods
All animal procedures were approved by the Animal Experiments Committee of the University Medical Center Utrecht and Utrecht University, and experiments were performed in accordance with the guidelines of the European Communities Council Directive.
Rat Stroke Model and Behavioral Testing
Twenty-one male Sprague Dawley rats, weighing 250 to 330 g, were included in the study. Twelve rats underwent transient focal cerebral ischemia by 90 minutes occlusion of the right middle cerebral artery with an intraluminal filament (Longa et al, 1989). Procedures for anesthesia, physiological monitoring, and surgery were performed as previously described (van Meer et al, 2010). Nine rats served as controls.
Sensorimotor function after stroke was measured using the sensorimotor performance score, which is based on six different motor, sensory, and tactile tests that provide an overall score on a scale of 0 to −20 points, with −20 as maximum deficit score (van Meer et al, 2010).
Direct Intracortical Stimulation and Magnetic Resonance Imaging
For placement of electrodes, animals were anesthetized by mechanical ventilation with 2.0% isoflurane in air/O2 (2:1). After exposure of the skull, two holes were drilled above the left (contralesional) M1, at 2.5 mm lateral from midline, and 0.5 and 3.0 mm anterior from bregma, respectively. Home-built bipolar insulated gold (18 karats) electrodes with 200 μm diameter exposed tips, which cause minimal susceptibility artifacts on MR images, were positioned in cortical layer III at a depth of 1.7 mm.
Magnetic resonance imaging was performed on a Varian 4.7 T MR system with the same experimental setup and physiological monitoring as previously described (van Meer et al, 2010). T2-weighted MRI (repetition time/echo time=3,600/15 milliseconds; echo train length=12; 19 1 mm coronal slices; field-of-view=32 × 32 mm2; acquisition matrix=256 × 128) was performed to determine the extent of the ischemic lesion. Diffusion tensor imaging was performed with a four-shot spin-echo echo planar imaging sequence (repetition time/echo time=3,500/26 milliseconds; 25 0.5 mm axial slices; field-of-view=32 × 32 mm2; acquisition matrix=64 × 64; diffusion weighting in six directions with b=1,250 s/mm2; two images with b=0; number of averages=4).
Before fMRI measurements, end-tidal isoflurane was reduced to 1% for 15 minutes (we have previously demonstrated that rats remain anesthetized at 1% isoflurane for 20 to 30 minutes, if this is preceded by 1 to 2 hours anesthesia at 1.5% to 2% isoflurane (Wang et al, 2011)). To prevent direct intracortical stimulation (DICS)-induced motion of the contralateral forelimb, a bolus of pancuronium bromide was intravenously administered (0.67 mg/kg), followed by continuous infusion (0.67 mg/kg per hour). Blood oxygenation level-dependent (BOLD) fMRI was performed with a gradient-echo echo planar imaging sequence (echo time/repetition time=19/1,000 milliseconds; flip angle=50° 13 1.5 mm coronal slices; field-of-view=32 × 32 mm2; acquisition matrix=64 × 64), during a DICS paradigm (Austin et al, 2003), involving five blocks of 3 seconds periods of stimulation (1.5 mA with 0.3 milliseconds duration at 300 Hz in 50 milliseconds trains, repeated five times per second) with a home-built constant current stimulator with 60 seconds rest intervals.
After MRI, rats were euthanized, and brains were extracted and sectioned. Coronal brain slices (2 mm thickness) were stained with triphenyltetrazolium chloride to evaluate potential electrolytic damage at the site of stimulation.
Data Processing and Analysis
Images were registered to a rat brain atlas (Paxinos and Watson, 2005; van Meer et al, 2010). Fractional anisotropy (FA) maps were derived from diffusion tensor imaging data (Basser and Pierpaoli, 1996). Fractional anisotropy was measured in a manually segmented region-of-interest (ROI) encompassing the corpus callosum between the bilateral motor cortices over multiple slices.
A support vector machines-based classification algorithm (Vapnik, 1995) was used to identify voxels that were part of the ischemic lesion, based on T2 and spatial coordinates (http://cran.r-project.org/web/packages/kernlab).
Statistical activation maps were calculated for each rat using fMRI expert analysis tool (FEAT) (http://www.fmrib.ox.ac.uk/fsl). Preprocessing of the fMR images included rigid-body motion correction (MCFLIRT), temporal high-pass filtering (63 seconds cutoff), and spatial smoothing with a Gaussian kernel (full width at half maximum=0.5 mm). A γ-convoluted block design was used to fit the BOLD response to the five stimulation blocks. Z-statistic images were cluster-corrected (P<0.05) and thresholded at a Z value of 1.64 (P<0.05). Higher-level FEAT analysis using a mixed-effects model was applied to statistically compare activation patterns between groups and to calculate mean activation maps that were significant at a group level (P<0.05) (Woolrich et al, 2004). The group-significant Z values were cluster-corrected (P<0.05) and projected on a T2-weighted anatomical rat brain template.
Ipsilesional and contralesional sensorimotor cortical ROIs were defined according to a rat brain atlas (Paxinos and Watson, 2005) (see Figure 1, top panel). Cortical voxels inside the lesion area and near the cortical stimulation site were excluded from ROI analysis (so that perilesional ROIs only included voxels with normal T2 values). For each ROI, BOLD time courses were averaged over the five stimulation blocks. The stimulation response was fitted with a γ-variate function, from which we calculated the amplitude (MAX) (percent signal change) and time-to-peak (TTP) (seconds) of the profile. One-way analysis of variance with post hoc Bonferroni testing was used to statistically compare ROI size, MAX, TTP, and FA between groups. Unpaired Student's t-testing was performed to compare sensorimotor performance scores between time points. P<0.05 was considered significant. Data are reported as mean±s.d., unless otherwise mentioned.
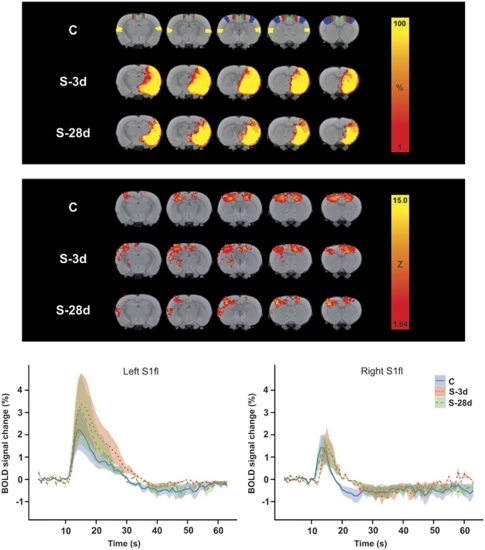
Figure 1.
(Top panel) Color-coded local incidence of T2-based lesion (percent of group size) in control rats (C; n=6) and rats at 3(S-3d; n=6) or 28 days after stroke (S-28d; n=4), overlaid on consecutive coronal rat brain slices from a T2-weighted template. The bilateral regions-of-interest (ROIs) S2 (secondary somatosensory cortex) (yellow), S1fl (forelimb region of the primary somatosensory cortex) (blue), M1 (primary cortex) (red), and M2 (secondary motor cortex) (green) are depicted on the template of group C. (Middle panel) Mean maps of significant blood oxygenation level-dependent (BOLD) activation in response to direct intracortical stimulation (DICS) of the left (contralesional) M1 in rats from groups C, S-3d, and S-28d. Color-coded maps display Z values, overlaid on consecutive coronal rat brain slices from a T2-weighted template. (Bottom panel) Averaged BOLD signal time courses (lines)±s.e.m. (shadings) in left (contralesional) (left graph) and right (ipsilesional) (right graph) S1fl in response to DICS of left (contralesional) M1, for groups C (solid blue lines), S-3d (dotted red lines), and S-28d (dashed green lines).
Experimental Groups
Five animals were excluded from further analysis because of DICS-induced cortical spreading depolarization, which was detected as prolonged BOLD signal increase spreading over the entire cortex, followed by incomplete hemodynamic responses to subsequent DICS. Remaining animals were divided in three experimental groups: a control group, C (n=6); a subacute stroke group at 3 days after stroke, S-3d (n=6); and a chronic stroke group at 28 days after stroke, S-28d (n=4).
Results
All rats displayed subcortical and cortical tissue damage, including the secondary somatosensory cortex (S2) and parts of the primary somatosensory cortex (S1), as characterized by T2 prolongation (see T2-based lesion incidence maps in Figure 1, top panel). Total lesion volumes were 380±44 mm3 and 220±53 mm3 in groups S-3d and S-28d, respectively. None of the animals showed electrolytic tissue damage at the stimulation site on triphenyltetrazolium chloride-stained sections. Sensorimotor performance score at day 3 was significantly lower than at day 28 (−6.2±3.1 versus −1.8±1.7, respectively).
Figure 1 (middle panel) shows mean maps of significant BOLD activation in response to DICS of the left (contralesional) M1 for the C, S-3d, and S-28d groups. Significant activation responses were observed in primary and secondary sensorimotor cortical areas ipsilateral and contralateral to the stimulation site. Some stroke animals exhibited more pronounced activation of contralesional S2, as displayed on the mean BOLD activation maps; however, higher-level FEAT analysis revealed no significant differences in activation patterns between groups.
The average time courses of BOLD signal changes in the left (contralesional) and right (ipsilesional) forelimb region of S1 (S1fl) for all groups are also shown in Figure 1 (bottom panel). In accordance with Austin et al (2003), DICS resulted in a positive BOLD response, that peaked at about 5 seconds after stimulation, followed by an undershoot before returning to baseline. There were no significant differences in MAX and TTP of the average BOLD response in left (contralesional) sensorimotor cortical ROIs, i.e., around the stimulation site, between groups (Table 1). Also, in the opposite (ipsilesional) cortex, MAX was not significantly different between groups. However, a significant delay in TTP was evident in all ipsilesional sensorimotor cortical ROIs in S-3d animals (P<0.05 versus C group). Time-to-peak was not significantly prolonged in ipsilesional ROIs after 28 days.
Table 1. ROI size (mm3), and MAX (% of baseline) and TTP (seconds) of the BOLD response in left (contralesional (cl)) and right (ipsilesional (il)) sensorimotor cortical areas, following DICS in left (contralesional) M1 for groups C, S-3d, and S-28d.
| C | S-3d | S-28d | |
|---|---|---|---|
| S2 (left (cl)) | |||
| Size (mm3) | 11.6±3.4 | 10.7±2.2 | 11.0±1.6 |
| MAX (%) | 1.2±1.4 | 2.2±1.1 | 2.2±0.6 |
| TTP (seconds) | 3.3±0.7 | 4.1±0.9 | 3.8±0.4 |
| S1fl (left (cl)) | |||
| Size (mm3) | 13.1±1.4 | 12.1±2.7 | 10.7±6.4 |
| MAX (%) | 2.3±1.1 | 3.4±2.0 | 3.1±1.5 |
| TTP (seconds) | 5.0±1.5 | 6.4±2.2 | 5.9±1.5 |
| M2 (left (cl)) | |||
| Size (mm3) | 12.0±6.6 | 9.5±5.4 | 8.5±4.8 |
| MAX (%) | 3.6±2.2 | 2.3±1.1 | 4.6±2.7 |
| TTP (seconds) | 5.4±0.9 | 6.4±2.6 | 8.0±4.9 |
| M2 (right (il)) | |||
| Size (mm3) | 18.4±4.5 | 16.7±7.2 | 10.9±4.5 |
| MAX (%) | 2.0±1.8 | 2.6±1.1 | 3.7±1.1 |
| TTP (seconds) | 4.1±0.6 | 6.6±0.3a | 5.2±1.4 |
| M1 (right (il)) | |||
| Size (mm3) | 19.9±3.4 | 15.0±5.0 | 13.8±4.9 |
| MAX (%) | 1.5±1.1 | 2.3±1.4 | 2.5±1.1 |
| TTP (seconds) | 4.2±0.4 | 6.6±0.6a | 5.3±1.5 |
| S1fl (right (il)) | |||
| Size (mm3) | 13.0±3.3 | 3.2±4.5a | 8.0±3.3 |
| MAX (%) | 1.7±1.8 | 1.6±0.4 | 1.6±0.7 |
| TTP (seconds) | 3.0±0.7 | 5.1±0.7a | 4.3±1.3 |
BOLD, blood oxygenation level-dependent; DICS, direct intracortical stimulation; M1, primary motor cortex; M2, secondary motor cortex; ROI, region-of-interest; S1fl, forelimb region of the primary somatosensory cortex; S2, secondary somatosensory cortex; TTP, time-to-peak.
Regions around the cortical stimulation site (left M1) and inside the lesion territory (right, ipsilesional S2) were excluded from ROI analysis.
P<0.05 versus C.
Fractional anisotropy in the corpus callosum was significantly lowered in the S-3d group (FA=0.49±0.04; ROI size: 8.8±2.1 mm3; P<0.05), but not in the S-28d groups (FA=0.53±0.04; ROI size: 9.4±0.7 mm3), as compared with the C group (FA=0.58±0.00; ROI size: 7.5±2.1 mm3).
Discussion
Our study shows that activation responses in the perilesional sensorimotor cortex could still be evoked by DICS in the opposite M1 at a subacute time point after experimental stroke, despite ischemic damage in large part of the subcortical and cortical sensorimotor network and significant white matter abnormality in the corpus callosum. This indicates that cortical neurons in the lesion borderzone were still receptive to stimuli through intact transcallosal signal transmission. In contrast, activation of the perilesional sensorimotor cortex in response to electrical stimulation of the affected forelimb has been shown to be largely absent at this stage (Dijkhuizen et al, 2003; Weber et al, 2008), which may be related to dysfunctioning of ascending (and descending) pathways. Moreover, we recently detected loss of synchronization of baseline fluctuations in low-frequency BOLD signals between contralesional and perilesional sensorimotor cortices at 3 days after stroke in rats, indicative of reduced functional connectivity (van Meer et al, 2010). Nevertheless, these studies have also demonstrated that the forelimb stimulation-induced activation and resting-state interhemispheric functional connectivity of the perilesional sensorimotor cortex can recover at chronic stages, which correlated with improvement of sensorimotor function (Dijkhuizen et al, 2003; Weber et al, 2008; van Meer et al, 2010). The current data point out that neurons in perilesional cortical tissue can still respond to stimuli subacutely after stroke, in spite of presumed disturbance of normal function. Furthermore, reduced FA, potentially caused by (partial) axonal degeneration or edema (Shereen et al, 2010), in callosal white matter may not necessarily be accompanied by loss of transcallosal neuronal signal transmission. Apparently, critical connections remain intact and functional, and the preserved activatibility may be crucial for successful reinstatement of perilesional cortical functioning at later stages.
Although the DICS-induced BOLD response was largely preserved, we detected a significant delay in TTP in perilesional sensorimotor cortical regions subacutely after stroke. This delay of around 2 seconds, which is about 500 times larger than the time for transcallosal transmission of electrical signals in rat brain (Hoffmeyer et al, 2007), most probably reflects locally compromised neurovascular coupling. Previous fMRI studies in stroke patients have reported increased TTP of BOLD responses in both perilesional and contralesional sensorimotor cortices during motor and language tasks (Pineiro et al, 2002; Altamura et al, 2009). The slower hemodynamic responses in poststroke brain have been explained by reduced cerebral autoregulation, which may be due to altered endothelial function (Altamura et al, 2009). Evidently, such disturbances in cerebrovascular hemodynamics can occur outside the infarct zone and may not necessarily be associated with neuronal dysfunction.
In conclusion, although neuronal function in ischemic borderzone regions has been shown to be impaired subacutely after stroke, our study demonstrates that perilesional cortical tissue can still respond to contralesional stimulation, even when the integrity of the corpus callosum is affected. This preserved activation responsiveness may be critical for subsequent functional plasticity and could therefore inform on the capacity of perilesional tissue to recover or reorganize after stroke.
Acknowledgments
The authors thank André van Dieren for providing the bipolar gold electrodes, and Steven Mocking, Annette van der Toorn, Gerard van Vliet, and René Zwartbol for their technical assistance.
The authors declare no conflict of interest.
Footnotes
This study was supported by the Alexandre Suerman program of the University Medical Center Utrecht, Utrecht University's High Potential program, and the Netherlands Organization for Scientific Research (NWO).
References
- Altamura C, Reinhard M, Vry MS, Kaller CP, Hamzei F, Vernieri F, Rossini PM, Hetzel A, Weiller C, Saur D. The longitudinal changes of BOLD response and cerebral hemodynamics from acute to subacute stroke. A fMRI and TCD study. BMC Neurosci. 2009;10:151. doi: 10.1186/1471-2202-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin VC, Blamire AM, Grieve SM, O'Neill MJ, Styles P, Matthews PM, Sibson NR. Differences in the BOLD fMRI response to direct and indirect cortical stimulation in the rat. Magn Reson Med. 2003;49:838–847. doi: 10.1002/mrm.10428. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J Cereb Blood Flow Metab. 2007;27:575–587. doi: 10.1038/sj.jcbfm.9600372. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2005The Rat Brain in Stereotaxic Coordinates5th ed.Burlington, MA: Elsevier Academic Press [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke. 2002;33:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- Shereen A, Nemkul N, Yang D, Adhami F, Dunn RS, Hazen ML, Nakafuku M, Ning G, Lindquist DM, Kuan CY. Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia-ischemia-induced thrombotic stroke. J Cereb Blood Flow Metab. 2010;31:1155–1169. doi: 10.1038/jcbfm.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnik VN. The Nature of Statistical Learning Theory. New York, NY: Springer-Verlag; 1995. [Google Scholar]
- Wang K, van Meer MP, van der Marel K, van der Toorn A, Xu L, Liu Y, Viergever MA, Jiang T, Dijkhuizen RM. Temporal scaling properties and spatial synchronization of spontaneous blood oxygenation level-dependent (BOLD) signal fluctuations in rat sensorimotor network at different levels of isoflurane anesthesia. NMR Biomed. 2011;24:61–67. doi: 10.1002/nbm.1556. [DOI] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Justicia C, Wiedermann D, Strecker C, Sprenger C, Hoehn M. Early prediction of functional recovery after experimental stroke: functional magnetic resonance imaging, electrophysiology, and behavioral testing in rats. J Neurosci. 2008;28:1022–1029. doi: 10.1523/JNEUROSCI.4147-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]