Abstract
Defects of the mitochondrial respiratory chain are associated with a diverse spectrum of clinical phenotypes, and may be caused by mutations in either the nuclear or the mitochondrial genome (mitochondrial DNA (mtDNA)). Isolated complex I deficiency is the most common enzyme defect in mitochondrial disorders, particularly in children in whom family history is often consistent with sporadic or autosomal recessive inheritance, implicating a nuclear genetic cause. In contrast, although a number of recurrent, pathogenic mtDNA mutations have been described, historically, these have been perceived as rare causes of paediatric complex I deficiency. We reviewed the clinical and genetic findings in a large cohort of 109 paediatric patients with isolated complex I deficiency from 101 families. Pathogenic mtDNA mutations were found in 29 of 101 probands (29%), 21 in MTND subunit genes and 8 in mtDNA tRNA genes. Nuclear gene defects were inferred in 38 of 101 (38%) probands based on cell hybrid studies, mtDNA sequencing or mutation analysis (nuclear gene mutations were identified in 22 probands). Leigh or Leigh-like disease was the most common clinical presentation in both mtDNA and nuclear genetic defects. The median age at onset was higher in mtDNA patients (12 months) than in patients with a nuclear gene defect (3 months). However, considerable overlap existed, with onset varying from 0 to >60 months in both groups. Our findings confirm that pathogenic mtDNA mutations are a significant cause of complex I deficiency in children. In the absence of parental consanguinity, we recommend whole mitochondrial genome sequencing as a key approach to elucidate the underlying molecular genetic abnormality.
Keywords: respiratory chain, complex I, mitochondrial DNA, mutation, genetic counselling
INTRODUCTION
Isolated complex I deficiency is the most frequently observed mitochondrial respiratory chain disorder, and is associated with a wide range of clinical presentations, including marked and often fatal lactic acidosis, cardiomyopathy, leukoencephalopathy, pure myopathy and hepatopathy with tubulopathy.1, 2, 3 Complex I deficiency is one of the most common mitochondrial disorders of childhood; onset is usually early and death often occurs within the first year of life.4, 5, 6, 7
Defining the molecular basis of complex I deficiency is a difficult task, not least because of the dual genetic control governing the correct assembly and function of complex I, but also as the largest complex in the mitochondrial respiratory chain, it is also the most intricate. Complex I is composed of 45 subunits,8 7 of which are encoded by mitochondrial DNA (mtDNA), and the remaining 38 are nuclear encoded. Moreover, a number of proteins are involved in the correct processing and assembly of complex I, the total number of which as yet remains unknown.9
To date, pathogenic mutations in structural complex I proteins, leading to complex I deficiency and associated with a disease phenotype, have been identified in 21 nuclear genes: 11 of the 36 autosomal genes encoding complex I subunits (namely NDUFS1, NDUFS2, NDUFS3, NDUFS4, NDUFS6, NDUFS7, NDUFS8, NDUFV1, NDUFV2, NDUFA2 and NDUFA11), 1 of the 2 chromosome X genes encoding complex I subunits (NDUFA1) and 9 autosomal genes encoding proteins involved in complex I assembly, namely NDUFAF1 (CIA30), NDUFAF2 (B17.2L), NDUFAF3, NDUFAF4, C8orf38, C20orf7, NUBPL, FOXRED1 and ACAD9.10, 11, 12, 13, 14, 15, 16 Mutations in nuclear genes involved in mtDNA maintenance, in particular POLG, which encodes the catalytic subunit of the mitochondrial polymerase γ, may also cause isolated complex I deficiency in patients with Alpers' syndrome.10 Equally, mutations have been identified in all seven of the mtDNA genes encoding subunits of complex I,17 and also in mtDNA tRNA (mt-tRNA) genes, particularly mitochondrial tRNALeu(UUR).2
The importance of mtDNA mutations as a potential molecular aetiology for complex I deficiency was therefore not in doubt, but thus far their prevalence remained unclear. Indeed, it was previously believed that complex I deficiency due to mtDNA mutations accounted for only 5–10% of paediatric complex I deficiency cases.18, 19 Recent screening of mitochondrial complex I subunit genes has shown mutations in mtDNA accounting for a much higher number of cases,20, 21, 22, 23, 24, 25, 26, 27 and therefore, its screening is an essential step in the genetic diagnosis of complex I deficiency. Previous studies assessing the prevalence of nuclear gene mutations in complex I-deficient paediatric cases have found the prevalence of these to also be high. Three studies have reported mutations in nuclear genes encoding subunits, assembly factors or related proteins in 20–25% of children with complex I deficiency.15, 28, 29
In this study, we report a detailed analysis of a large cohort of 109 patients, representing all patients diagnosed with childhood-onset complex I deficiency at a large national referral centre. We present the identification of the underlying molecular basis of the enzyme defects in a number of these patients, and have used these data to estimate the prevalence of such mutations in these disorders and to present data surveying the overall clinical findings in these patients.
PATIENTS AND METHODS
Patient cohort
The cohort of 109 patients (from 101 families) represents all patients diagnosed in Melbourne between 1992 and 2007, with definite childhood-onset mitochondrial disease and an enzyme diagnosis of complex I deficiency. The Melbourne laboratory is referred almost all children from Australia and New Zealand who are biopsied for investigation of mitochondrial disease. Before biopsy, children had been referred to a geneticist or paediatrician specialising in inborn errors of metabolism. Testing for metabolic or genetic conditions with phenotypes similar to mitochondrial disease would typically have been performed (eg, organic acids, acylcarnitines, chromosome analysis), but the extent of this testing varied. Histological and enzyme histochemical analyses were routinely performed on muscle biopsies, but usually produced normal or equivocal results, as reported previously for patients with complex I deficiency.2 The criteria used to establish the diagnosis of complex I deficiency were those described by Kirby et al,2 namely: (1) percentage complex I activity relative to citrate synthase (CS) or complex II was <25% of the control mean in one or more tissues or (2) percentage complex I ratios were 25–40% plus one of (a) similar activity in multiple tissues, cell lines or siblings, (b) supportive histology or electron microscopy (ie, presence of abnormal mitochondria in at least one tissue), and (c) supportive functional tests in fibroblasts, low ratio of ATP synthesis with glutamate and malate (complex I-linked substrate) compared with the rate with succinate and rotenone (complex II-linked substrate) or inability to grow in a galactose/azide medium. Complex I deficiency was considered isolated if other respiratory chain complexes had CS ratios that were not clearly deficient (ie, >25%) and had residual activities at least two-fold higher than complex I. In addition to the enzyme data, to make the diagnosis of complex I deficiency as stringent as possible, all patients required supportive clinical and other evidences to establish a definite diagnosis of a respiratory chain defect to be included in the study.30 Kaplan–Meier cumulative probability survival analyses (for calculating the proportion of unaffected cases at any time point) were performed using GraphPad Prism, version 4 (GraphPad Software, La Jolla, CA, USA).
Biochemical analyses
Respiratory chain enzymes and CS were measured in tissue homogenates and fibroblasts and Epstein–Barr virus-transformed lymphoblast mitochondrial fractions as described previously.2, 31
Molecular genetic studies
Total DNA was extracted from tissues, cultured cells and blood by standard procedures. Samples were screened for four common mtDNA mutations, namely m.3243A>G causing mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS), m.8344A>G causing myoclonic epilepsy and ragged-red fibres, m.8993T>G and m.8993T>C associated with neurogenic weakness, ataxia and retinitis pigmentosa and maternally inherited Leigh syndrome phenotypes by PCR restriction fragment-length polymorphism (RFLP) analysis,2 and the entire mitochondrial genome was sequenced as described previously.32 For some patient cell lines, the presence of a mtDNA mutation was excluded by enzyme analysis of either cybrid fusions22 or heterokaryons formed by fusing patient cells with ρ0 cells.33
Fibroblast cDNAs obtained from patients in 32 of the 101 families were screened for mutations in the NDUFS1, NDUFS2, NDUFS4, NDUFS7, NDUFS8 and NDUFV1 genes by denaturing HPLC, as described elsewhere.34 Next-generation sequencing was also used to screen patients from 94 families for mutations in 103 genes. These included the 45 subunits, the 15 mt-tRNA genes flanking the complex I subunit genes, known assembly factors and genes that shared an evolutionary relationship with complex I. The approach involved a discovery screen that sequenced PCR-amplified targets in pools of DNA obtained from 20 patients, with each pool analysed in a single lane of an Illumina Genome Analyzer (Illumina, San Diego, CA, USA). Sequence variants were prioritised by bioinformatic analyses and assigned to individual patients by Sequenom genotyping (Sequenom, San Diego, CA, USA), followed by experimental studies to determine pathogenicity. The inclusion of 43 patients with known pathogenic mutations allowed us to determine a detection sensitivity of 78% for causal nuclear DNA variants and 28% for causal mtDNA variants; the latter figure was low because of highly variable amplification of mtDNA from different patients in the whole-genome amplification step before pooling.15
RESULTS
mtDNA mutations
A total of 30 patients (from 29 families) were found to harbour a pathogenic mutation in mtDNA (29% of probands), either in MTND subunit genes (21 probands from 22 families) or in MTTL1 or MTTW tRNA genes (8 probands) (Table 1). Each of the mtDNA mutations has been reported to be pathogenic in previous studies that provided evidence to support pathogenicity such as sequence conservation, the presence of lower levels of heteroplasmy in healthy maternal relatives and either cybrid analysis or single-fibre PCR used to confirm an association between heteroplasmic mutant load and mitochondrial dysfunction. In all, 15 of the 30 mtDNA patients presented in infancy or early childhood with Leigh syndrome, including a pair of siblings with Leigh disease caused by the m.14459G>A mutation. The next most common group (7 of 29 probands) comprised children presenting typically in teenage years with MELAS. One patient with a known pathogenic MTTW mutation presented and died on the first day of life, whereas two others with MTND3 mutations died in the first months of life with lethal infantile mitochondrial disease (LIMD). Two patients with MTTL1 mutations had a pure mitochondrial myopathy.
Table 1. Mitochondrial DNA mutations in complex I deficiency.
| Gene and mutation | Clinical presentation and family history | % Mutant load (tissue) | Maternal % mutant load (tissue) | Publications in which patient was described previously |
|---|---|---|---|---|
| MTND1 | ||||
| m.3697G>A (p.G131S) | MELASa | 80 (muscle) | 3 (blood) | Kirby et al33 |
| m.3946G>A (p.E214K) | MELASb | 60 (muscle) | n.d. (urine) | Kirby et al33 |
| m.3949T>C (p.Y215H) | MELASb | 93 (muscle) | n.d. (blood) | Kirby et al33 |
| MTND3 | ||||
| m.10158T>C (p.S34P) | LSb | 91 (muscle) | n.d. (blood) | McFarland et al22 |
| LSb | 97 (muscle) | n.d. (blood) | McFarland et al22 | |
| LIMDa | >98 (muscle) | 7 (fibroblasts) | McFarland et al22 | |
| m.10191T>C (p.S45P) | LIMDa | >98 (liver) | n.d. (blood) | McFarland et al22 |
| m.10197G>A (p.A47T) | MEb | ∼100 (muscle) | Kirby et al34 | |
| MEa | ∼90 (blood) | Calvo et al15 | ||
| MTND5 | ||||
| m.12706T>C (p.F124 L) | LSa | 65 (muscle) | ||
| m.13094T>C (p.V253A) | LSa | ∼60 (muscle) | Calvo et al15 | |
| m.13513G>A (p.D393N) | LSa | 31 (muscle) | 4 (urine) | Kirby et al7 (3 of 4 patients) |
| LSa | 44 (fibroblasts) | |||
| LSa | ∼30 (blood) | |||
| LSb | 26 (muscle) | 2 (blood) | ||
| MTND6 | ||||
| m.14487T>C (p.M63V) | LSa | >98 (muscle) | ||
| LSb | >98 (fibroblasts) | 20 (urine) | ||
| MEc | >98 (muscle) | 24 (blood) | ||
| m.14453G>A (p.A74V) | LSa | 41 (fibroblasts) | n.d. (blood) | |
| m.14459G>A (p.A72V) | LSa | 95 (muscle) | n.d. (blood) | Kirby et al46 |
| LSb,d | 97 (fibroblasts) | Kirby et al46 | ||
| LSb,d | 97 (muscle) | Kirby et al46 | ||
| MTTL1 | ||||
| m.3242A>G | MEc | >98 (muscle) | 72 (blood) | Kirby et al2 |
| m.3243A>G | MELASc | ∼60 (muscle) | n.d. (fibroblasts) | Kirby et al2 |
| MELASc | ∼60 (muscle) | ∼5% (blood) | Kirby et al2 | |
| MELASc | ∼70 (muscle) | |||
| m.3250A>G | MMb | 81 (muscle) | 43 (blood) | Ogle et al47 |
| m.3271C>T | MELASa | >98% (muscle) | ∼10% (blood) | Kirby et al2 |
| m.3303C>T | MMa | 97 (muscle) | 58 (blood) | Bruno et al48 |
| MTTW | ||||
| m.5567T>C | LIMDa | >90 (muscle) | Calvo et al15 | |
Abbreviations: LIMD, lethal infantile mitochondrial disease; LS, Leigh syndrome; ME, mitochondrial encephalomyopathy; MELAS, mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes; MM, mitochondrial myopathy; n.d., not detected.
No family history suggestive of mtDNA disease.
Family history possibly suggestive of mtDNA disease.
Family history strongly suggestive of mtDNA disease.
Affected siblings.
Four patients had other mitochondrial encephalomyopathies. The first patient had an m.3242G>A mutation and presented at 2.5 years of age with failure to thrive, hypotonia, ataxia and renal tubular acidosis. The second patient had an m.14487T>C mutation. He presented at 12.5 years of age with deteriorating vision and pale optic discs and subsequently developed migraines, deterioration in school performance, bilateral hearing loss and obsessive compulsive disorder. The other two patients presented at 18 months and 3 years of age, respectively. They had progressive dystonia and dysarthria and were apparently unrelated, but both had the m.10197G>A mutation and were of Tongan ancestry. One had a de novo balanced chromosomal translocation, which we initially believed may contribute to the disease phenotype. However, her symptoms are similar to other reported m.10197G>A patients;23, 35 hence, it seems likely that her symptoms are mostly or entirely explained by the mtDNA mutation. We described finding the m.10197G>A mutation in this patient previously but misinterpreted it as a benign variant, which appeared to be present in Polynesian controls.34 The latter finding was erroneous because of an RFLP assay that failed to distinguish this variant from an m.10238T>C polymorphism, which was also cut by the HphI restriction enzyme.
Maternal DNA samples were available for 19 of the 29 probands (Table 1). Mutant mtDNA was detected in 11 mothers, at mutant loads substantially below that found in their affected child. Eight mothers had no mtDNA mutation detected, which may imply that the mutations were de novo events but could also be due to loss of the mutation from the tested tissue, which was usually blood. Only 5 of the 29 families with mtDNA disease had a family history that strongly implied maternal inheritance, for example, a maternal aunt, uncle or cousin having symptoms consistent with disease. These 5 families represented 4 of the 8 with MTT (mt-tRNA) gene mutations and only 1 of the 21 with subunit mutations. Nine families had a family history that may have been consistent with or suggestive of mitochondrial disease, for example, maternal relatives with epilepsy, learning difficulties, migraine, diabetes or SIDS. The remaining 15 family histories, representing half of this group, lacked any suspicion of maternal inheritance.
Nuclear gene mutations
Nuclear gene mutations were identified in 28 patients (from 22 families) by sequencing of candidate genes (22% of probands; Table 2). Pathogenicity of sequence variants was assessed by approaches including analysis of sequence conservation, use of predictive algorithms such as PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), segregation in families and absence in controls plus protein or RNA studies, as described in individual references listed in Table 2. Overall, 13 patients (from 11 families) had 2 autosomal recessive mutations in complex I subunit genes. In all, 11 patients from 7 families had 2 autosomal recessive mutations in genes encoding complex I assembly factors. In addition to these obvious complex I candidate genes, four patients classified as having isolated complex I deficiency had mutations in genes involved in the broader aspects of mitochondrial biogenesis. Two probands with Alpers' syndrome and complex I deficiency in the liver had two pathogenic mutations in the POLG gene, encoding the mtDNA γ-polymerase. A patient with hepatoencephalopathy and liver and muscle complex I deficiency had a homozygous mutation in the C10orf2 (formerly PEO1) gene, encoding the Twinkle helicase. All three patients with POLG or C10orf2 mutations had hepatic mtDNA depletion. A patient with Barth's syndrome (but lacking cyclic neutropaenia) had a pathogenic mutation in the TAZ gene.36
Table 2. Nuclear gene mutations in complex I deficiency.
| Gene and mutation(s) | Clinical presentation and family history | Reference |
|---|---|---|
| Complex I subunits | ||
| NDUFV1 p.K111E/p.R386H, p.P252R/p.R386H, homozygous p.E377K | 2 LS, 1 LIMDa | Calvo et al15 |
| NDUFS2 p.R221X/p.R333Q, p.M292T/p.L261WfsX19 | 1 LS, 1 ME | Calvo et al15 |
| NDUFS4 homozygous p.T74IfsX17, p.K154NfsX34/p.S34IfsX4 (in 2 siblings), p.S34IfsX4/c.351-2A>G | 4 LS | Calvo et al15; Lazarou et al49 |
| NDUFS6 homozygous p.V63EfsX9 (in 2 siblings), homozygous deletion: chr5:g.1,865,477_1,869,744del | 3 LIMDb | Kirby et al34 |
| NDUFS8 homozygous p.G154S | 1 ME | Calvo et al15 |
| Other complex I genes | ||
| NDUFAF1 p.T207P/p.K253R | 1 ME | Dunning et al50 |
| NDUFAF2 homozygous p.I35SfsX17 (in 2 siblings), homozygous p.W74X | 3 LSb | Calvo et al15 |
| C8orf38 homozygous p.Q99R | 2 LSc | Pagliarini et al13 |
| C20orf7 homozygous p.L229P | 3 LIMDd | Sugiana et al14 |
| FOXRED1 p.Q232X/p.N430S | 1 LS | Calvo et al15 |
| NUBPL p.G56R+p.D273QfsX31/chr14:g.[(30,932,976_30,953,766)_ (31,193,278_31,194,846)del]+ [(31,211,800_31,212,780)_ (31,345,080_31,350,225)dup] | 1 ME | Calvo et al15 |
| Other genes | ||
| POLG p.W748S/p.G848S, p.A467T/p.R852C | 2 Alpers' syndrome | Hakonen et al51 |
| C10orf2 homozygous p.R400C | 1 MEa | |
| TAZ hemizygous p.R123X | 1 Barth's syndrome | McKenzie et al36 |
Abbreviations: LIMD, lethal infantile mitochondrial disease; LS, Leigh syndrome; ME, mitochondrial encephalomyopathy.
For each gene, the number of patients with each clinical presentation is listed. Patients were singleton cases from non-consanguineous families and had two pathogenic mutations, unless stated otherwise.
One child from a consanguineous family.
Two siblings from a non-consanguineous family and one child from a consanguineous family.
Two siblings from a consanguineous family.
Three siblings from a consanguineous family.
A further 17 patients from 16 families were classified as having nuclear gene mutations on the basis of having either (1) no mtDNA mutations identified by sequencing of the entire mtDNA genome in DNA prepared from a tissue with demonstrated complex I deficiency (15 patients from 14 families) or (2) cell-fusion studies demonstrating nuclear inheritance of the enzyme defect (2 patients from 2 families).
Undefined inheritance
The mode of inheritance in the remaining 34 patients (from 34 families) remains unclear (34% of probands). This is a large group of patients, and as such, presents a challenge for the subsequent genetic counselling of complex I deficiency in these families.
Clinical and biochemical features
Leigh or Leigh-like syndrome accounts for the majority of phenotypes associated with paediatric complex I deficiency, and the proportion of affected cases is similar whether associated with an mtDNA or with a nuclear gene mutation (Table 3). Of the remaining phenotypes associated with complex I deficiency, there are a number of differences in proportions caused by mtDNA and nuclear DNA defects. For example, LIMD was about threefold more common in patients with nuclear gene defects than in those with mtDNA defects. No cases of MELAS were attributable to a nuclear gene mutation in our series, although occasional MELAS patients have previously been shown to have POLG mutations.37
Table 3. Clinical and biochemical features of complex I patients.
| mtDNA | Nuclear genea | Undefined | |
|---|---|---|---|
| Sex (M:F) | 16:14 | 28:16 | 21:13 |
| Clinical presentation | |||
| LIMD | 3 | 12 | 8 |
| Leigh syndrome | 14 | 18 | 3 |
| MELAS | 6 | 0 | 0 |
| Other mito encephalopathies | 5 | 12 | 14 |
| Myopathy | 2 | 2 | 1 |
| Cardiomyopathy | 0 | 1 | 8 |
| Tissue-specificityb | |||
| Systemic | 18 | 29 | 4 |
| Tissue specific | 3 | 12 | 21 |
| Not determined | 9 | 4 | 9 |
Abbreviations: LIMD, lethal infantile mitochondrial disease; MELAS, mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes.
Three individuals in the nuclear gene group were identified by prenatal diagnosis and pregnancies were terminated. The sex of one individual was not known and clinical presentation for each of the three was assumed to be the same as for their affected sibling.
An enzyme defect was regarded as systemic if it was shown to be expressed in a cultured cell line and was deficient in all tissues assayed. It was regarded as tissue specific if at least one tissue or cell line had normal activity. Samples in which a cultured cell line was not assayed were classed as not determined.
Patients with mutations in genes encoding complex I subunits or assembly factors usually have an isolated complex I defect. However, we along with others have found several such patients in whom complex III activity was borderline low or deficient.38 Similarly, many patients with mtDNA depletion or mutations in mt-tRNA genes have combined defects affecting complexes I, III and IV, but a substantial number may have enzyme profiles indistinguishable from an isolated complex I defect. The difficulty in predicting the class of genes affected based on the profile of enzyme activities is demonstrated by Table 4, which summarises the median enzyme activities for each group of patients in this study in which mutations were identified.
Table 4. Enzyme activities in patients with identified mutations.
| Complex I | Complex II | Complex III | Complex IV | |
|---|---|---|---|---|
| Subunit/assembly factor | ||||
| Muscle (32) | 16 | 136 | 81 | 86 |
| Liver (14) | 23 | 87 | 72 | 119 |
| Fibroblast (38) | 24 | 129 | 76 | 92 |
| mtDNA tRNA (MTTL1) | ||||
| Muscle (7) | 16 | 130 | 91 | 77 |
| mtDNA tRNA (MTTW) | ||||
| Liver (1) | 31 | 146 | 172 | 318 |
| Cardiolipin metabolism (TAZ) | ||||
| Muscle (1) | 14 | 144 | n.d. | 47 |
| mtDNA depletion | ||||
| Liver (3) | 4 | 69 | 47 | 61 |
Abbreviation: n.d., not determined.
Enzyme activities are expressed as % of control mean value relative to citrate synthase. Data are the median values for each group with the number of patient samples per group shown in parentheses.
Ages at onset and survival
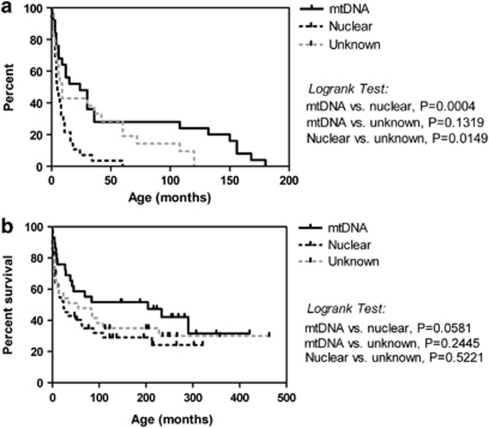
Patients with mtDNA mutations had a higher median age at onset (12 months) than did those with nuclear gene defects or undefined inheritance (both 3 months, Figure 1a). However, ages at onset varied over a wide range, with 20–40% of the patients in each group having onset in the first month of life. Kaplan–Meier survival analysis suggested a trend toward longer survival in the group of patients with mtDNA mutations compared with those with nuclear gene defects, although this did not reach significance (Figure 1b). Survival of patients with undefined inheritance was not significantly different from either of the other two groups. Individuals in all three patient group had survivals varying from <1 month to >25 years, again emphasising the variable severity within each group.
Figure 1.
Age at onset and survival in complex I patients. (a) Kaplan–Meier graph showing age at onset as the percentage of patients remaining unaffected with increasing age for patients with mtDNA mutations (solid line, median 12.0 months, n=30), nuclear gene mutations (dashed black line, median 3.0 months, n=41) and unknown inheritance (dashed grey line, median 3.0 months, n=33). (b) Kaplan–Meier survival analysis for patients with mtDNA mutations (solid line, n=30), nuclear gene mutations (dashed black line, n=42) and unknown inheritance (dashed grey line, n=34).
DISCUSSION
The identification of the underlying molecular basis of complex I deficiency in paediatric patients is of great importance, not least because of the implications this has in the genetic counselling of these disorders. The identification or exclusion of mtDNA as the underlying cause is important as mtDNA is inherited solely through the maternal lineage.
This cohort is, to our knowledge, the largest cohort in which the prevalence of mtDNA mutations in complex I deficiency has been assessed. The molecular defect was found to be of mitochondrial origin in 29% of probands investigated, including one mutation that has been previously erroneously reported by us to be a polymorphism (m.10197G>A)34 and two mutations (m.13094T>C and m.5576T>C) that had each been reported in only one other previous patient.26 Other recent studies have found a prevalence of mtDNA mutations in 20–27% of complex I deficiencies, with all mutations reported to be in MTND genes only.20, 21, 29, 39 Although this seems to be where the majority of mutations leading to complex I deficiency are located, we found various mutations in MTT genes, confirming that these mutations are not confined to the subunit genes and that it may be more appropriate to screen the whole mitochondrial genome in cases of complex I deficiency. Indeed, the prevalence of 29% mtDNA mutations that we found is still likely an underestimation, as only 15 of the 51 patients without a pathogenic mutation identified had full mtDNA genome sequencing.
After a correct biochemical diagnosis of complex I deficiency, we believe the most appropriate and effective way to confirm/exclude the involvement of mtDNA as the underlying cause is to sequence the mtDNA genome to look for changes that may be associated with the biochemical defect observed. At present, sequencing remains the method of choice, as this enables the identification of both heteroplasmic and homoplasmic variants in a single assay. Other methods, for example, dHPLC, would potentially miss homoplasmic variants.
Some associations have been suggested between the genotype and the phenotype of mitochondrial gene mutations, for example, MTND5 and MTND1 mutations are often associated with a MELAS phenotype,33 and MTND5 is postulated as a ‘hotspot' for disease-causing mutations.40 A number of common ‘recurrent' mtDNA mutations have also been identified in multiple unrelated pedigrees by different diagnostic centres. However, preferential screening of mtDNA genes or of recurrent mutations based on phenotype, postulated ‘hotspots' or common mutations will fail to diagnose many patients as genotype–phenotype correlations are variable and the majority of mtDNA mutations have still only been reported once.17 This issue is even more of a problem in the case of complex I nuclear gene mutations as there are even fewer of these mutations that have been found in more than one family. Furthermore, not all of the nuclear genes involved in the correct functioning of complex I are known, and given that the number and size of these genes far exceeds those of their mitochondrial counterparts, this means that deciding which nuclear genes to investigate in complex I-deficient patients remains a great diagnostic challenge.
Owing to the clinical heterogeneity of complex I disorders, patients often do not present with the traditional hallmarks of mitochondrial disease.41 This reinforces the observation that biochemical and metabolic analyses are usually the most appropriate methods to diagnose complex I deficiency in paediatric patients. Although essential, these studies are only indicative of the underlying genetic abnormality and cannot alone settle between an mtDNA or nuclear DNA origin. Clear evidence of maternal transmission can help in indicating that a mutation in mtDNA is likely, but a lack of maternal transmission cannot rule out mtDNA involvement, as many of these mutations appear to arise sporadically.20, 22, 42 We observed some differences in the clinical phenotypes and age at onset of symptoms between mtDNA mutations and nuclear DNA mutations, and although in many cases these may be subtle, these differences may provide important clues for focusing genetic testing in mitochondrial disease.
Owing to the supercomplex formation of the respiratory chain,43 patients with mutations in complex I subunit genes (either mtDNA or nuclear), can present with combined complex I and III deficiency.38 In contrast, patients with mutations in genes expected to affect multiple complexes of the respiratory chain (eg, mt-tRNA, POLG, C10orf2, TAZ, etc.) can appear to have isolated complex I defects. Some degree of variability in correlating enzyme profiles with mutated genes should thus be expected. As mtDNA depletion disorders can give enzyme profiles suggesting an isolated complex defect, analysis of the mtDNA:nDNA ratio in a relevant tissue is well worth considering in patients with complex I deficiency. Similarly, findings such as 3-methylglutaconic aciduria and cardiomyopathy should prompt sequencing of the TAZ gene, even in patients with an apparently isolated complex I defect. Where possible, a biochemical diagnosis, clinical presentation and tissue involvement/histology should all be considered in guiding mutation analysis, rather than relying solely on an enzyme diagnosis.
The use of the correct tissue for assessing complex I activity is also essential. Many complex I deficiencies expressed in the muscle are not expressed in fibroblasts.44 Of 87 complex I patients in this study for whom multiple tissues were studied, the enzyme defect was tissue specific in 36 (41%). In most of these patients, the defect was not expressed in fibroblasts, but in 13 patients, complex I was normal in the skeletal muscle but deficient in the liver or heart. If only fibroblasts had been used to assess the complex I function in this cohort, we would have failed to identify three patients with MTT mutations and all four patients with mutations in genes involved in mitochondrial biogenesis (POLG, C10orf2 and TAZ).
Analysis of nuclear DNA in our cohort revealed 22% of probands with an identified mutation, 18% in complex I subunit or assembly genes and 4% in genes involved in mitochondrial biogenesis. A further 16% are assumed to have a nuclear DNA mutation based on normal mitochondrial genome sequencing or cell-fusion assays.
As there is no clear genotype–phenotype correlation associated with mutations in either mtDNA or nuclear DNA leading to complex I deficiency, a correct biochemical diagnosis is fundamental. If 25–30% of complex I deficiencies are caused by mutations in mtDNA, and 20–25% by identified genes in nDNA, this still means that in nearly half of the cases, the genetic basis of complex I deficiency is undefined.14 We suggested previously that of all genes that could potentially cause complex I deficiency, no one gene is responsible for any more than 10% of all cases.34 As mtDNA mutations account for >25% of cases, coupled with the relative ease of sequencing the entire genome, mtDNA should typically be the first target for mutation analysis in most cases of paediatric complex I deficiency. Provided the DNA was prepared from a tissue with demonstrated complex I deficiency, then the lack of any detectable mtDNA mutation essentially excludes maternal inheritance, which is of great benefit in genetic counselling of the family. Sequencing the large range of nuclear-encoded complex I subunits plus the known and putative assembly factors remains a substantial challenge using conventional sequencing technologies. This problem may soon be alleviated by the application of next-generation sequencing of large numbers of candidate genes,45 although significant challenges remain before such methods can achieve high sensitivity in routine genetic diagnostic studies.15
Acknowledgments
We thank the large number of physicians who referred patients for investigative studies, Dr Henrik Dahl for advice and Drs Vamsi Mootha and Sarah Calvo for identification of three of the mtDNA mutations described herein, as part of a High Throughput sequencing study of complex I deficiency. We also thank Drs Lisa Worgan, Edwin Kirk, Peter Taylor and Michael Buckley for the dHPLC analyses that identified subunit mutations in five patients. This work was supported by grants and a Principal Research Fellowship from the Australian National Health and Medical Research Council (DRT), the Muscular Dystrophy Association, USA (DRT), the Medical Research Council, UK (RM) and the Wellcome Trust, UK (DMT and RWT). Diagnostic studies in the Newcastle laboratory are supported by the UK National Commissioning Group ‘Rare Mitochondrial Disorders of Adults and Children' service (http://www.mitochondrialncg.nhs.uk). This study was performed under the ethical guidelines issued by each of our Institutions for clinical studies.
The authors declare no conflict of interest.
References
- Robinson BH. Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. Biochim Biophys Acta. 1998;1364:271–286. doi: 10.1016/s0005-2728(98)00033-4. [DOI] [PubMed] [Google Scholar]
- Kirby DM, Crawford M, Cleary MA, Dahl HHM, Dennett X, Thorburn DR. Respiratory chain complex I deficiency. An underdiagnosed energy generation disorder. Neurology. 1999;52:1255–1264. doi: 10.1212/wnl.52.6.1255. [DOI] [PubMed] [Google Scholar]
- Loeffen JL, Smeitink JA, Trijbels JM, et al. Isolated complex I deficiency in children: clinical, biochemical and genetic aspects. Hum Mutat. 2000;15:123–134. doi: 10.1002/(SICI)1098-1004(200002)15:2<123::AID-HUMU1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Moreadith RW, Batshaw ML, Ohnishi T, et al. Deficiency of the iron-sulfur clusters of mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase (complex I) in an infant with congenital lactic acidosis. J Clin Invest. 1984;74:685–697. doi: 10.1172/JCI111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH, McKay N, Goodyer P, Lancaster G. Defective intramitochondrial NADH oxidation in skin fibroblasts from an infant with fatal neonatal lacticacidemia. Am J Hum Genet. 1985;37:938–946. [PMC free article] [PubMed] [Google Scholar]
- Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987;80:71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby DM, Boneh A, Chow CW, et al. Low mutant load of mitochondrial DNA G13513A mutation can cause Leigh disease. Ann Neurol. 2003;54:473–478. doi: 10.1002/ana.10687. [DOI] [PubMed] [Google Scholar]
- Carroll J, Fearnley IM, Skehel JM, Shannon RJ, Hirst J, Walker JE. Bovine complex I is a complex of 45 different subunits. J Biol Chem. 2006;281:32724–32727. doi: 10.1074/jbc.M607135200. [DOI] [PubMed] [Google Scholar]
- Lazarou M, Smith SM, Thorburn DR, Ryan MT, McKenzie M. Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J. 2009;276:6701–6713. doi: 10.1111/j.1742-4658.2009.07384.x. [DOI] [PubMed] [Google Scholar]
- Kirby DM, Thorburn DR. Approaches to finding the molecular basis of mitochondrial oxidative phosphorylation disorders. Twin Res Hum Genet. 2008;11:395–411. doi: 10.1375/twin.11.4.395. [DOI] [PubMed] [Google Scholar]
- Hoefs SJ, Dieteren CE, Distelmaier F, et al. NDUFA2 complex I mutation leads to Leigh disease. Am J Hum Genet. 2008;82:1306–1315. doi: 10.1016/j.ajhg.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada A, Vogel RO, Hoefs SJ, et al. Mutations in NDUFAF3 (C3ORF60), encoding an NDUFAF4 (C6ORF66)-interacting complex I assembly protein, cause fatal neonatal mitochondrial disease. Am J Hum Genet. 2009;84:718–727. doi: 10.1016/j.ajhg.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiana C, Pagliarini DJ, McKenzie M, et al. Mutation of c20orf7 disrupts complex I assembly and causes lethal neonatal mitochondrial disease. Am J Hum Genet. 2008;83:468–478. doi: 10.1016/j.ajhg.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo SE, Tucker EJ, Compton AG, et al. High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat Genet. 2010;42:851–858. doi: 10.1038/ng.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouws J, Nijtmans L, Houten SM, et al. Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I. Cell Metab. 2010;12:283–294. doi: 10.1016/j.cmet.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lott MT, Procaccio V, et al. An enhanced MITOMAP with a global mtDNA mutational phylogeny. Nucleic Acids Res. 2007;35:D823–D828. doi: 10.1093/nar/gkl927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeitink J, Sengers R, Trijbels F, van den Heuvel L. Human NADH: ubiquinone oxidoreductase. J Bioenerg Biomembr. 2001;33:259–266. doi: 10.1023/a:1010743321800. [DOI] [PubMed] [Google Scholar]
- Triepels RH, van den Heuvel LP, Trijbels JM, Smeitink JA. Respiratory chain complex I deficiency. Am J Med Genet. 2001;106:37–45. doi: 10.1002/ajmg.1397. [DOI] [PubMed] [Google Scholar]
- Lebon S, Chol M, Benit P, et al. Recurrent de novo mitochondrial DNA mutations in respiratory chain deficiency. J Med Genet. 2003;40:896–899. doi: 10.1136/jmg.40.12.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M, Invernizzi F, Alberio S, et al. Clinical and molecular findings in children with complex I deficiency. Biochim Biophys Acta. 2004;1659:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- McFarland R, Kirby DM, Fowler KJ, et al. de novo mutations in the mitochondrial ND3 gene as a cause of infantile mitochondrial encephalopathy and complex I deficiency. Ann Neurol. 2004;55:58–64. doi: 10.1002/ana.10787. [DOI] [PubMed] [Google Scholar]
- Sarzi E, Brown MD, Lebon S, et al. A novel recurrent mitochondrial DNA mutation in ND3 gene is associated with isolated complex I deficiency causing Leigh syndrome and dystonia. Am J Med Genet A. 2007;143:33–41. doi: 10.1002/ajmg.a.31565. [DOI] [PubMed] [Google Scholar]
- Shanske S, Coku J, Lu J, et al. The G13513A mutation in the ND5 gene of mitochondrial DNA as a common cause of MELAS or Leigh syndrome: evidence from 12 cases. Arch Neurol. 2008;65:368–372. doi: 10.1001/archneurol.2007.67. [DOI] [PubMed] [Google Scholar]
- Moslemi AR, Darin N, Tulinius M, Wiklund LM, Holme E, Oldfors A. Progressive encephalopathy and complex I deficiency associated with mutations in MTND1. Neuropediatrics. 2008;39:24–28. doi: 10.1055/s-2008-1076739. [DOI] [PubMed] [Google Scholar]
- Valente L, Piga D, Lamantea E, et al. Identification of novel mutations in five patients with mitochondrial encephalomyopathy. Biochim Biophys Acta. 2009;1787:491–501. doi: 10.1016/j.bbabio.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Naess K, Freyer C, Bruhn H, et al. MtDNA mutations are a common cause of severe disease phenotypes in children with Leigh syndrome. Biochim Biophys Acta. 2009;1787:484–490. doi: 10.1016/j.bbabio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Ugalde C, Janssen RJ, van den Heuvel LP, Smeitink JA, Nijtmans LG. Differences in assembly or stability of complex I and other mitochondrial OXPHOS complexes in inherited complex I deficiency. Hum Mol Genet. 2004;13:659–667. doi: 10.1093/hmg/ddh071. [DOI] [PubMed] [Google Scholar]
- Rotig A, Lebon S, Zinovieva E, et al. Molecular diagnostics of mitochondrial disorders. Biochim Biophys Acta. 2004;1659:129–135. doi: 10.1016/j.bbabio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59:1406–1411. doi: 10.1212/01.wnl.0000033795.17156.00. [DOI] [PubMed] [Google Scholar]
- Rahman S, Blok RB, Dahl HHM, et al. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol. 1996;39:343–351. doi: 10.1002/ana.410390311. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Taylor GA, Durham SE, Turnbull DM. The determination of complete human mitochondrial DNA sequences in single cells: implications for the study of somatic mitochondrial DNA point mutations. Nucleic Acids Res. 2001;29:E74–E744. doi: 10.1093/nar/29.15.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby DM, McFarland R, Ohtake A, et al. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J Med Genet. 2004;41:784–789. doi: 10.1136/jmg.2004.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby DM, Salemi R, Sugiana C, et al. NDUFS6 mutations are a novel cause of lethal neonatal mitochondrial complex I deficiency. J Clin Invest. 2004;114:837–845. doi: 10.1172/JCI20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae JH, Lee JS, Kim KJ, et al. A novel ND3 mitochondrial DNA mutation in three Korean children with basal ganglia lesions and complex I deficiency. Pediatr Res. 2007;61:622–624. doi: 10.1203/pdr.0b013e3180459f2d. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J Mol Biol. 2006;361:462–469. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Deschauer M, Tennant S, Rokicka A, et al. MELAS associated with mutations in the POLG1 gene. Neurology. 2007;68:1741–1742. doi: 10.1212/01.wnl.0000261929.92478.3e. [DOI] [PubMed] [Google Scholar]
- Budde SM, van den Heuvel LP, Janssen AJ, et al. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochem Biophys Res Commun. 2000;275:63–68. doi: 10.1006/bbrc.2000.3257. [DOI] [PubMed] [Google Scholar]
- Esteitie N, Hinttala R, Wibom R, et al. Secondary metabolic effects in complex I deficiency. Ann Neurol. 2005;58:544–552. doi: 10.1002/ana.20570. [DOI] [PubMed] [Google Scholar]
- Blok MJ, Spruijt L, de Coo IF, Schoonderwoerd K, Hendrickx A, Smeets HJ. Mutations in the ND5 subunit of complex I of the mitochondrial DNA are a frequent cause of oxidative phosphorylation disease. J Med Genet. 2007;44:e74. doi: 10.1136/jmg.2006.045716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinopoulos A, Smeitink J, ter Laak H. Unusual features of mitochondrial degeneration in skeletal muscle of patients with nuclear complex I mutation. Acta Neuropathol. 2005;110:199–202. doi: 10.1007/s00401-005-1036-2. [DOI] [PubMed] [Google Scholar]
- Thorburn DR. Mitochondrial disorders: prevalence, myths and advances. J Inherit Metab Dis. 2004;27:349–362. doi: 10.1023/B:BOLI.0000031098.41409.55. [DOI] [PubMed] [Google Scholar]
- Schagger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem. 2004;279:36349–36353. doi: 10.1074/jbc.M404033200. [DOI] [PubMed] [Google Scholar]
- Ruitenbeek W, Wendel U, Hamel BC, Trijbels JM. Genetic counselling and prenatal diagnosis in disorders of the mitochondrial energy metabolism. J Inherit Metab Dis. 1996;19:581–587. doi: 10.1007/BF01799118. [DOI] [PubMed] [Google Scholar]
- Vasta V, Ng SB, Turner EH, Shendure J, Hahn SH. Next generation sequence analysis for mitochondrial disorders. Genome Med. 2009;1:100. doi: 10.1186/gm100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby DM, Kahler SG, Freckmann ML, Reddihough D, Thorburn DR. Leigh disease caused by the mitochondrial DNA G14459A mutation in two unrelated families. Ann Neurol. 2000;48:102–104. [PubMed] [Google Scholar]
- Ogle RF, Christodoulou J, Fagan E, et al. Mitochondrial myopathy with tRNALeu(UUR) mutation and complex I deficiency responsive to riboflavin. J Pediatr. 1997;130:138–145. doi: 10.1016/s0022-3476(97)70323-8. [DOI] [PubMed] [Google Scholar]
- Bruno C, Kirby DM, Koga Y, et al. The mitochondrial DNA C3303T mutation can cause cardiomyopathy and/or skeletal myopathy. J Pediatr. 1999;135:197–202. doi: 10.1016/s0022-3476(99)70022-3. [DOI] [PubMed] [Google Scholar]
- Lazarou M, McKenzie M, Ohtake A, Thorburn DR, Ryan MT. Analysis of the assembly profiles for mitochondrial- and nuclear-DNA-encoded subunits into complex I. Mol Cell Biol. 2007;27:4228–4237. doi: 10.1128/MCB.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning CJ, McKenzie M, Sugiana C, et al. Human CIA30 is involved in the early assembly of mitochondrial complex I and mutations in its gene cause disease. EMBO J. 2007;26:3227–3237. doi: 10.1038/sj.emboj.7601748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakonen A, Davidzon G, Salemi R, et al. Abundance of the POLG disease mutations in Europe, Australia, New Zealand, and the United States explained by single ancient European founders. Eur J Hum Gen. 2007;15:779–783. doi: 10.1038/sj.ejhg.5201831. [DOI] [PubMed] [Google Scholar]