Abstract
Local cutaneous heating produces vasodilation that is largely nitric oxide (NO) dependent. We showed that angiotensin II (ANG II) attenuates this by an ANG II receptor, type 1 (AT1R)-dependent mechanism that is reversible with the antioxidant ascorbate, indicating oxidative stress. Reactive oxygen species (ROS) produced by ANG II employ NADPH and xanthine oxidase pathways. To determine whether these mechanisms pertain to skin, we measured cutaneous local heating with 10 μM ANG II, using apocynin to inhibit NADPH oxidase and allopurinol to inhibit xanthine oxidase. We also inhibited superoxide with tempol, and H2O2 with ebselen. We heated the skin of the calf in 8 healthy volunteers (24.5–29.9 yr old) to 42°C and measured local blood flow to assess the percentage of maximum cutaneous vascular conductance. We remeasured while perfusing allopurinol, apocynin, ebselen, and tempol through individual microdialysis catheters. This was then repeated with ANG II combined with antioxidant drugs. tempol and apocynin alone had no effect on the heat response. Allopurinol enhanced the entire response (125% of heat alone), while ebselen suppressed the heat plateau (76% of heat alone). ANG II alone caused significant attenuation of the entire heat response (52%). When added to ANG II, Allopurinol partially reversed the ANG II attenuation. Heat with ebselen and ANG II were similar to heat and ANG II; ebselen only partially reversed the ANG II attenuation. Apocynin and tempol each partially reversed the attenuation caused by ANG II. This suggests that ROS, produced by ANG II via NADPH and xanthine oxidase pathways, modulates the response of skin to the application of heat, and thus contributes to the control of local cutaneous blood flow.
Keywords: cutaneous heat response, nitric oxide, reactive oxygen species, microdialysis
we have previously described the role of ANG II in the modulation of cutaneous blood flow using the vasodilation response of nonglabrous skin to local heating (39–41). This response has three distinct phases of cutaneous blood flow: an initial peak, a nadir, and an increase to a plateau which has been shown to be NO dependent (16, 24). When its effect on local skin blood flow was evaluated, ANG II exhibited vasoconstrictive actions which are thought to be primarily mediated by ANG II receptor, type 1 (AT1R) activation of oxidases (4, 10, 11, 16, 19). These reductions in cutaneous blood flow can be reversed by the ANG II receptor antagonist losartan (39–41) and are improved by intradermal administration of Ang-(1–7) and the antioxidant ascorbate (39, 41). ANG II binds to the AT1 receptor at NADPH oxidase, which is thought to increase superoxide, H2O2 (4, 52) and peroxynitrite (28), thus increasing the level of local oxidative stress. In contrast, ascorbate, acting as an antioxidant, has been shown to reverse age-dependent decreases in cutaneous blood flow due to heat (14). Reactive oxygen species (ROS) are therefore likely involved in the regulation of skin blood flow and may modulate the response of skin blood flow to NO.
Our hypothesis, based on the effects of ANG II, is that the cutaneous response to local heat is mediated by products of oxidative stress. Therefore, to evaluate the role that ROS products play in the regulation of skin blood flow, we investigated the response to local heat as measured by laser-Doppler flowmetry in normal, healthy control subjects. Since some vasoactive actions of ANG II are mediated by AT1R-dependent activation of NADPH oxidase and xanthine oxidase causing increased ROS (7, 11, 19), we used allopurinol and apocynin, respectively, to inhibit these enzymes. These ROS include superoxide and H2O2 which exert important vasoactive and sympathetic effects (44, 46, 52); thus we reduced local cutaneous levels of superoxide (SO) with tempol and H2O2 with ebselen.
METHODS
Subjects.
In the first series of experiments, we studied the effects of NADPH oxidase inhibition, xanthine oxidase inhibition, superoxide reduction, and H2O2 reduction on the cutaneous heat response in eight healthy volunteer subjects aged 24.5–29.5 yr, median age 27.15 yr (5 men and 3 women). In a second series of experiments, we studied the effects of ANG II on the heat response in the presence of NADPH oxidase inhibition, xanthine oxidase inhibition, superoxide reduction, and H2O2 reduction in the same study population. Subjects with a history of orthostatic intolerance were specifically excluded. Only subjects free from cutaneous, systemic, and cardiovascular diseases were eligible. Subjects were not taking any medications and refrained from alcohol and caffeinated beverages for at least 24 h before the study. There were no smokers or trained competitive athletes. Informed consent was obtained, and the Committee for the Protection of Human Subjects (Institutional Review Board) of New York Medical College approved all protocols. Female subjects were enrolled without regard to the phase of their menstrual cycle except that none were menstruating during testing procedures.
Instrumentation.
All testing was conducted in a temperature-controlled room (25°C) at least 2 h after a light breakfast. Skin temperature was continuously monitored by the laser-Doppler flow (LDF) probes used to make the skin blood flow measurements. Measurements were made in the left calf. Since all experiments were performed with the subject supine, the leg was at the level of the heart throughout all procedures. Subjects were instrumented in the dermal space of the lateral aspect of the left calf after hair was gently removed from the insertion site. Each site was cooled with ice before catheter insertion to reduce discomfort. Each probe (MD-2000 Linear Microdialysis Probes; Bioanalytical Systems, West Lafayette, IN) has a 10-mm microdialysis membrane section that is placed in the intradermal space using a 25-gauge needle as an introducer. Catheters were randomly designated. The molecular mass cutoff is nominally 30,000 Da. Following placement, all catheters were initially perfused with Ringer's solution at 2 μl/min. An integrating LDF probe (Probe 413; Perimed) containing seven individual probe tips (each contains a separate transmitting and receiving fiber) was then placed directly over each microdialysis catheter to measure skin blood flow, designated as LDF. LDF was thereafter recorded until values were similar to those measured over the same area before catheter insertion. The return of LDF to approximately preinsertion values indicated recovery from the trauma of the catheter emplacement and usually occurred by 60–90 min (1, 22). When necessary, longer times were allowed until preinsertion LDF was reached. Baseline untreated LDF was then recorded during local heating and 30 min post-heat recovery.
Local heating.
Once baseline LDF values were obtained, the areas under each laser were gradually heated at 1°C/10 s to 42°C which was maintained for at least 30 min until a plateau was reached. The area underneath the heating unit is 3 cm2. Heat was turned off to allow for recovery to baseline LDF.
Blood pressure measurements.
Blood pressure was measured by finger plethysmography (Finometer, Arnhem, Netherlands), intermittently recalibrated against oscillometry. Mean arterial pressure was obtained by averaging the signal over 5 min and compared with oscillometry [using the formula mean arterial pressure = (systolic arterial pressure + 2 × diastolic arterial pressure)/3], as previously described (39). Finometer and oscillometric blood pressure were in agreement.
General protocol.
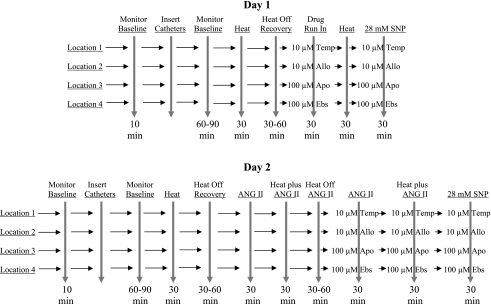
The experiments were performed on 2 separate days on the same subjects. The order of the experimental days was randomized. During each day, four microdialysis catheters were placed to infuse drugs locally into the intradermal space of the calf. Before the microdialysis catheter insertion, LDF was measured over each of the four insertion sites to estimate baseline flows for later use in determining when the area had recovered from the trauma of catheter insertion. Laser probes were removed, and the four microdialysis catheters were inserted. After recovery, LDF was measured while perfusing the catheters with lactated Ringer's solution, and values were recorded for 10 min. Following this, LDF was recorded during local heating at each site with continued perfusion of lactated Ringer solution. A recovery from heat period followed, requiring 30–60 min. After recovery, catheters were perfused with drugs dissolved in lactated Ringer's solution, and local heating was repeated. The sequence of events for both experimental days is shown in Fig. 1.
Fig. 1.
Sequence of events for experimental maneuvers. SNP, sodium nitroprusside; Apo, apocynin; Allo, allopurinol; Ebs, ebselen; Temp, tempol.
On 1 day, the effects on the heating response of ROS inhibition using tempol, a superoxide dismutase mimetic (10 μM), xanthine oxidase inhibition using allopurinol (10 μM), NADPH oxidase inhibition using apocynin (100 μM) and H2O2 inhibition plus 15-lipoxygenase/cyclooxygenase inhibition using ebselen (100 μM) was evaluated by perfusing each of these drugs through individual microdialysis catheters dissolved in lactated Ringer's solution. After a 30-min run-in period, local heating and heating recovery were recorded at all sites. Following this, maximum blood flow and conductance were elicited by perfusing 28 mM sodium nitroprusside through each of the four microdialysis catheters.
The concentrations of tempol, allopurinol, apocynin, and ebselen we initially used in preliminary trials were based on studies in animals (26, 27, 53) and humans (23). We then performed preliminary experiments to determine appropriate concentrations for these inhibitors. In all cases, the concentrations used were the minimum dose required to elicit a maximal response. In addition, preliminary determinations confirmed that none of the inhibitors used influenced the response of skin blood flow to SNP; thus they did not alter maximal cutaneous vascular conductance (CVC).
On another day, we repeated a similar experimental design that evaluated the effects of ANG II on the same group of subjects. Four microdialysis catheters were used. Baseline LDF, catheter insertion with Ringer's perfusion, and initial local heating at each site were performed as in the first series of experiments while the catheter was perfused with Ringer's solution. Following this, ANG II (10 μM) was perfused through each catheter for 30 min while taking baseline measurements and throughout the heating period. Subjects were then allowed to recover from the heat response. The response to heat was then reevaluated while 10 μM ANG II plus 10 μM tempol was perfused through the first catheter, 10 μM ANG II plus 10 μM allopurinol was perfused through the second catheter, 10 μM ANG II plus 100 μM apocynin was perfused through the third catheter, and 10 μM ANG II plus 100 μM ebselen was perfused through the fourth catheter. Following this, maximum blood flow and conductance were elicited by perfusing 28 mM sodium nitroprusside through each of the four microdialysis catheters.
Use of heat-reheat assessment.
In these experiments, we employed a heat-reheat protocol where we measured the cutaneous response to locally applied heat up to three times, while allowing for the skin blood flow to return to “baseline” in between, as we have employed in previous studies (40, 41). The utility of performing heat-and-reheat comparisons has been reported in detail elsewhere (40, 41) and shows that the variability between the plateau phase of the local heating response during sequential heat-reheat in each catheter is significantly less than that between local heating plateaus of different catheters placed in the same subject. This is in contrast to another study that showed repeated heating of the skin can affect its responses (6).
Data and statistical analysis.
Laser-Doppler skin blood flows were measured in arbitrary perfusion units (pfu). Continuous LDF data were collected at a sampling rate of 200 Hz during experiments. Data from the lasers were multiplexed and interfaced to a personal computer through a analog-to-digital converter (DI-720; Dataq, Milwaukee, WI) using custom data acquisition software. LDF data were converted to units of CVC by dividing by the mean arterial blood pressure.
CVC measurements were then converted to a percentage of maximum CVC (%CVCmax) by dividing CVC by the CVCmax achieved after the administration of 28 mM sodium nitroprusside at the end of experiments. This fraction was converted to a percentile by multiplication by 100. Conductance data are therefore displayed as %CVCmax. Changes in baseline LDF before and after drugs were compared by two-way ANOVA. Results are shown and reported as means ± SE. Other comparisons were made by repeated-measures ANOVA, with a Bonferroni post hoc test to look at differences in the local heating response between pre- and post-drug infusion using the particular microdialysis catheter as the within factor. Results were calculated using Statistical Package for the Social Sciences software version 11.0. The value for significance was P < 0.05.
RESULTS
The effects of anti-oxidants on LDF.
These results are shown in Table 1. While none of these drugs altered the baseline (nonheated) skin blood flow significantly, the presence of apocynin (P = 0.10) and allopurinol (P = 0.07) increased %CVC above the average flow measured in the absence of drug. Intradermal infusion of allopurinol produced a significantly augmented response to local heat resulting in an increased LDF of the first thermal peak, nadir, and final heat plateau, compared with that measured using lactated Ringer's alone (P < 0.05). On the other hand, the infusion of ebselen caused a significant reduction of the final heat plateau compared with lactated Ringer's given alone (P < 0.05).
Table 1.
Magnitudes of heat responses
| Ringer's (n = 8) | Drug (n = 8) | |
|---|---|---|
| Baseline | ||
| Apocynin | 10.31 ± 2.04 | 16.36 ± 5.25 |
| Allopurinol | 11.50 ± 2.29 | 17.91 ± 4.60 |
| Ebselen | 12.73 ± 2.45 | 10.07 ± 2.22 |
| Tempol | 9.40 ± 1.49 | 9.29 ± 1.88 |
| First thermal peak | ||
| Apocynin | 49.75 ± 4.18 | 49.29 ± 8.33 |
| Allopurinol | 42.38 ± 4.49 | 63.77 ± 3.70* |
| Ebselen | 50.24 ± 2.78 | 45.78 ± 3.16 |
| Tempol | 54.72 ± 6.67 | 59.92 ± 3.87 |
| Nadir | ||
| Apocynin | 29.80 ± 4.57 | 31.84 ± 8.37 |
| Allopurinol | 23.23 ± 3.78 | 42.60 ± 1.89‡ |
| Ebselen | 33.36 ± 2.26 | 29.87 ± 2.88 |
| Tempol | 33.48 ± 7.40 | 40.47 ± 4.38 |
| Plateau | ||
| Apocynin | 74.07 ± 6.01 | 58.25 ± 10.01 |
| Allopurinol | 73.46 ± 2.49 | 84.54 ± 3.08‡ |
| Ebselen | 80.08 ± 2.70 | 60.89 ± 4.68† |
| Tempol | 78.06 ± 5.42 | 76.67 ± 4.87 |
Values are means ± SE and are shown as percentage of maximal cutaneous vascular conductance (%CVCmax).
P < 0.01, significantly different from value with Ringer alone, comparing %CVCmax.
P < 0.05, significantly different from value with Ringer alone, comparing %CVCmax.
P < 0.002, significantly different from value with Ringer alone, comparing %CVCmax.
The effects of ANG II on LDF.
As shown in Table 2, 10 μM ANG II caused significant reductions in all aspects of the cutaneous response to the application of local heat compared with Ringer's alone (P < 0.05). This inhibition was evident over the entire time course measured (not shown) and resulted in a significant reduction of baseline, first heat peak, nadir, and heat plateau.
Table 2.
Effect of 10 μM ANG II on the cutaneous response to local heat
| Heat + Ringer's | Heat + ANG II | P Value Comparing Heat to ANG II | |
|---|---|---|---|
| Baseline | 11.91 ± 1.14 | 8.31 ± 0.73 | P < 0.0001 |
| First heat peak | 56.18 ± 1.83 | 13.19 ± 2.53 | P < 0.0001 |
| Nadir | 35.26 ± 2.07 | 22.70 ± 1.68 | P < 0.0001 |
| Plateau | 87.65 ± 2.45 | 45.58 ± 3.07 | P < 0.0001 |
Values are means ± SE and are shown as %CVCmax.
The effects of ANG-II + antioxidants on LDF.
To evaluate whether the cutaneous effects of ANG II are mediated by products of oxidative stress, we measured the response to local heat, first alone, then during infusions of 10 μM ANG II, and finally during infusions of 10 μM ANG II plus either apocynin, allopurinol, ebselen or tempol.
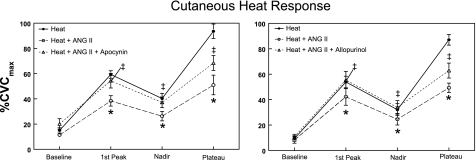
The effects of NADPH oxidase and xanthine oxidase inhibition, using apocynin and allopurinol, respectively, on the heat response are shown in Fig. 2. This figure depicts %CVC for baseline, the first heat peak, nadir, and heat plateau elicited by the application of local heat. Heat alone elicits this characteristic multiphasic response measured during the 30 min of local skin heating. Infusion of 10 μM ANG II alone caused a significant suppression of skin blood flow during the entire course of the heat response. Apocynin and allopurinol reversed the suppression of the heat response by ANG II in the first peak and nadir phases and caused a significant increase in the plateau blood flow above that caused by ANG II alone. The plateau value for ANG II plus either allopurinol or apocynin was midway between and significantly different from heat alone, or heat plus ANG II.
Fig. 2.
The response of skin to local heating alone and after administration of ANG II, or ANG II plus apocynin (left) or ANG II plus allopurinol (right). Data are expressed as percentage of maximum cutaneous vascular conductance (%CVCmax) and are averaged over all subjects. In this figure averaged data ± SE are shown at baseline, first thermal peak, nadir, and heat plateau. *Significantly different from heat alone, P < 0.05. ‡Significantly different from heat + ANG II, P < 0.05.
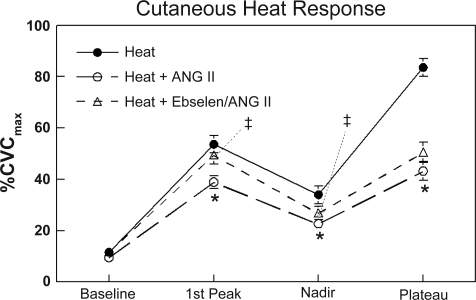
The effects of ANG II, with and without ebselen scavenging H2O2 and hydroxyperoxides, are shown in Fig. 3. In contrast to that seen with apocynin and allopurinol, the heat response with ebselen plus ANG II was intermediate to and significantly different from heat alone and heat with ANG II, when comparing the first heat peak and nadir. The heat plateau values however were the same as heat plus ANG II and significantly less than with heat alone.
Fig. 3.
The response of skin to local heating alone and after administration of ANG II, or ANG II plus ebselen. Data are expressed as %CVCmax and are averaged over all subjects. In this figure averaged data ± SE are shown at baseline, first thermal peak, nadir, and heat plateau. *Significantly different from heat alone, P < 0.05. ‡Significantly different from heat + ANG II, P < 0.05.
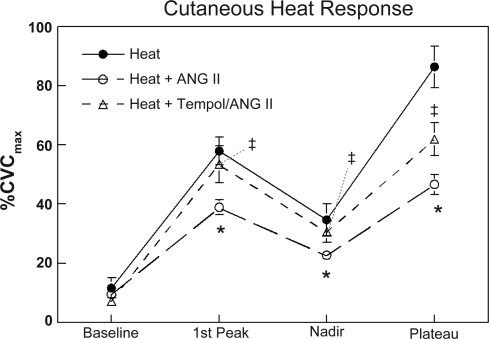
Last, to evaluate the effect of scavenging superoxide and decrease the formation of hydroxyl radicals, we performed similar determinations as described above but instead perfused the microdialysis catheters with tempol, an SOD mimetic agent. As shown in Fig. 4, the response to local heat with tempol plus ANG II was the same as heat alone for baseline, the first heat peak, and nadir, and therefore improved compared with that measured with ANG II alone. In a fashion similar to that seen with apocynin and allopurinol, the heat plateau values for tempol plus ANG II were intermediate between heat alone and heat plus ANG II.
Fig. 4.
The response of skin to local heating alone and after administration of ANG II, or ANG II plus Tempol. Data are expressed as %CVCmax, and are averaged over all subjects. In this figure averaged data ± SE are shown at baseline, first thermal peak, nadir, and heat plateau. *Significantly different from heat alone, P < 0.05. ‡Significantly different from heat + ANG II, P < 0.05.
DISCUSSION
In previous studies of the cutaneous response to local heat, we have shown that ANG II exhibits vasoconstrictive effects (39–41) mediated through the actions of the AT1R through activation of local oxidases. The primary effect of ANG II is thought to occur through direct activation of NADPH oxidase (4, 10–12) and secondarily through increased ROS via xanthine oxidase because of NADPH oxidase activation of a redox-sensitive pathway (19). The vasoconstrictive response to ANG II is largely due to increased ROS formation especially H2O2 (4, 43, 52), and NO scavenging through the binding of ANG II to NADPH and xanthine oxidases producing peroxynitrite (28, 43). With AT1Rs blocked, ANG II caused NO-independent vasodilation in control subjects, and infusion of the antioxidant sodium ascorbate, delivered through microdialysis catheters, increased the NO-dependent heat plateau in healthy subjects. This suggested a role for reactive oxygen species in the control of the local heat response (41).
In normal volunteers, we showed that the addition of ANG II is capable of attenuating the response to local heat in the skin (39–41). ANG II significantly attenuated baseline (unheated) blood flow as well decreasing the response to local heat by decreasing the magnitude of the first thermal peak, the nadir, and the final heat plateau. While there were no statistical differences between the values for baseline blood flow measured with and without drugs, apocynin and allopurinol resulted in noticeably higher values of %CVC compared with Ringer's. If substantiated in future determinations, these findings might suggest a role for ROS in cutaneous thermoregulation under normothermic conditions.
Interestingly, of the inhibitors tested, only allopurinol resulted in a significant increase in all three phases of the heat response, but had no significant effect on baseline blood flow. Ebselen caused a significant decrease in plateau blood flow compared with the heat response measured in the absence of drugs. While all of these inhibitors seemed able to partially mitigate the vasoconstrictive effects of ANG II, ebselen was the least effective. Perhaps this is because ebselen alone decreased the plateau heat response itself, and therefore was unable to effectively reverse the attenuated heat response caused by ANG II. It is possible, therefore, that H2O2 could increase the response to local heat. This is consistent with recent studies that have shown that H2O2 can cause NO-independent relaxation of the vasculature. These responses have been shown capable of being modified by the presence of antioxidants (8).
Since ANG II is thought to work through AT1R-dependent activation of NADPH oxidase and xanthine oxidase causing increased production of ROS (7, 11, 19), these oxygen species likely play a role in the regulation of the local heat response. Modulation of these products of oxidative stress may cause alterations in the response of skin to local heat and could be responsible for abnormalities of skin blood flow such as those described in variants of POTS (39–41). In addition to vasodilatation, the vasoconstrictor response of skin to local cooling has also been shown to involve ROS and stimulation of the RhoA-Rho kinase pathway (42).
The response to local skin heating is characterized by its biphasic nature, the first heat peak being due to C-fiber nociceptor-mediated axon reflex (21, 30, 35), which results in vasodilatation presumed to occur through the local release of calcitonin gene-related peptide (CGRP) (30, 45), substance P (3, 45), and neuropeptide Y (13). The second increase in skin blood flow from local heat, characterized by the heat plateau, has been shown to be largely dependent (∼70%) on NO (16, 24, 25). This is in contrast to the cutaneous vasodilatory response to whole body heat, which is thought to be due to cotransmission of acetylcholine and as yet unidentified neurotransmitter(s) mediated through sympathetic stimulation (17). However, even in whole body heating, NO is required to produce maximal cutaneous vasodilatation in young subjects, is thought to be responsible for ∼30% of maximal flow, and acts in conjunction with histamine and vasoactive intestinal peptide to afford active vasodilatation (14, 38, 47, 49).
NO serves to regulate skin blood flow in a variety of situations. The bioavailability of NO in the skin is a function of the balance between its synthesis and degradation. Increased ROS has been shown to decrease available cutaneous NO either directly (2, 43) or indirectly (14, 18), possibly influencing the ability of skin to vasodilate in response to local heat (14, 41). In the present studies, apocynin and allopurinol were able to partially reverse the ANG II-induced suppression of the response to local heat. These agents are inhibitors of NADPH oxidase and xanthine oxidase, respectively, and therefore can result in decreased local production of ROS, likely resulting in increased availability of NO which can potentiate vasodilatation.
The finding that allopurinol increased the heat plateau above that measured in the absence of drug suggests that local ROS, produced by xanthine oxidase, may play a normal regulatory role in cutaneous vasoregulation. This observation is unique as it is thought that the response to ANG II elicits its effects largely through direct effects on NADPH oxidase (4, 11, 12, 44). However, some support for the role of xanthine oxidase is suggested by studies which have shown that ANG II can elicit an effect by causing NADPH-dependent changes in redox potential that result in increased local xanthine oxidase activity and augmented superoxide production (19).
Ebselen, a glutathione peroxidase mimetic, can result in lowered oxidative stress by decreasing local production of H2O2, which as above, may increase the availability of local NO, thus reversing the ANG II-induced suppression of the local heat response. Ebselen alone decreased the plateau heat value compared with the response in the absence of drug; the reason for this may be due to its additional actions as a lipoxygenase inhibitor and modulation of vasodilatory prostaglandins (34).
Tempol is also capable of reversing this response to local heat but is likely mediated by its ability as an SOD mimetic to decrease local ROS availability by promoting catalysis to other species (46). Superoxide decreases NO directly, or through production of peroxynitrite can uncouple enzymatic production of NO through oxidation of BH4 (2), possibly causing decreased NO and a blunted response to heat. Superoxide dismutase can also generate hydroxyl radicals and H2O2 (7), which themselves may be capable of affording effects. Therefore the effect of Tempol may be due to many factors.
A recent study has shown that TRPV-1 channel activation contributes to the axon reflex component (first heat peak) of local cutaneous heating. These investigators also showed that TRPV-1 channel activation directly contributes to the plateau phase as well (48). These data suggest activation of TRPV-1 channels, which are putative channels primarily located on cutaneous sensory nerves, may constitute a mechanism by which cutaneous sensory nerves are depolarized during local heating of the skin, and thus act as a local heat sensor. Numerous studies have demonstrated the effects of ROS on ion channel activity (5, 15, 37) and its effect on transmembrane potential (32, 37). Modification of ROS can result in alteration of vascular responsiveness and of blood pressure mediated centrally (7, 20, 29), directly within the kidney (9, 51), and at the level of local vasculature (7, 31, 33). Therefore, ROS may possibly have a direct effect on the TRPV-1 channel activity as well, and thus modulate the response to local heat. These findings are consistent with several recent studies which have shown that exogenous antioxidants can reverse the effects of aging on the response to both local heat (14) and cooling (50).
Thus, by showing that the response of skin to local heat, which is largely influenced by NO, can be influenced by products of oxidative stress, we suggest that endogenous ROS may play a regulatory role in local thermoregulation. This is based on our current findings, as well as those which have shown that locally administered ascorbate (14, 41, 50) can modify local cutaneous vasodilation. This control may be afforded through two different mechanisms: the first through local vascular modification of NO by ROS (7, 31, 33), and the second through modulation of the response of local ion channels to heat by ROS. Together, these intermediaries of oxidative metabolism may subserve the function of controlling local vascular reactivity. Studies extending these observations in subjects with altered cutaneous responses, such as those with low-flow POTS are warranted, to evaluate which of these control mechanisms may be involved in altered hemodynamics measured in these subjects.
Limitations.
We studied women without regard to menstrual cycle. The phase of the menstrual cycle can affect NO-dependent mechanisms and may also exert influence on ROS-dependent functions (36).
We used exogenous ANG II; however, endogenous angiotensin was not measured. While many tissues produce ANG II, there are no data from skin. However, data from skeletal muscle microvessels suggest local concentrations on the order of 100 pmol/l (41), this is far less than the lowest dose of ANG II administered in current experiments.
Microdialysis is invasive and alters the interstitial milieu. The work of Anderson et al. suggests that flow responses return to baseline levels within ∼1 h (1). In pilot experiments, we measured baseline flows, removed the LDF probes, instrumented the same site with microdialysis catheters, replaced the probes, waited ∼1 h, and repeated the LDF measurements with similar results (on average). The present studies utilized our heat-reheat protocol, which yields reproducible results (40, 41). Other investigators however have reported that repeated heating of the skin can affect its responses (6). This discrepancy is likely due to the numerous methodological differences between their study design and ours, which included use of a different anatomic site and a dissimilar source of heat and laser measuring device.
We were also concerned that the values for %CVCmax recorded in these studies during the nadir phase were lower than those reported by other investigators. While this may be due in part to variability among subjects, it may also be related to our use of the lower leg as the site for investigation, compared with the forearm that is more commonly used (14, 17, 24, 25).
One additional limitation of these studies is that they only inform on the vasoregulatory response of skin to the application of local heat. Therefore any speculation regarding the role of ROS in modulation or influencing skin blood flow should be considered within this context and may not be applicable to whole body response to heat.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants 1-RO1-HL-074873, 1-RO1-HL-087803 and 1-F30-HL-097380 and a grant from the Chronic Fatigue and Immune Dysfunction Syndrome Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol 102: 807–811, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 9: 836–844, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Brain SD, Williams TJ. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature 335: 73–75, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res 97: 772–780, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci USA 94: 9932–9937, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciplak M, Pasche A, Heim A, Haeberli C, Waeber B, Liaudet L, Feihl F, Engelberger R. The vasodilatory response of skin microcirculation to local heating is subject to desensitization. Microcirculation 16: 265–275, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 56: 325–330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garry A, Edwards DH, Fallis IF, Jenkins RL, Griffith TM. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc Res 84: 218–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 91: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 4: 899–914, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst 4: 51–61, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hsieh CP. Redox modulation of A-type K+ currents in pain-sensing dorsal root ganglion neurons. Biochem Biophys Res Commun 370: 445–449, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fischer D, Manes C, Mueller M, Drexler H. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol 27: 943–948, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 55: 277–83, 6p, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol 497: 837–848, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation 15: 569–579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, Hahn SM. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res 10: 6411–6417, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Obata T. Allopurinol suppresses 2-bromoethylamine and 1-methyl-4-phenylpyridinium ion [MPP(+)]-induced hydroxyl radical generation in rat striatum. Toxicology 218: 75–79, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension 54: 1106–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sann H, Pierau FK. Efferent functions of C-fiber nociceptors. Z Rheumatol 57, Suppl 2: 8–13, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Sartorio CL, Fraccarollo D, Galuppo P, Leutke M, Ertl G, Stefanon I, Bauersachs J. Mineralocorticoid receptor blockade improves vasomotor dysfunction and vascular oxidative stress early after myocardial infarction. Hypertension 50: 919–925, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Sausbier U, Sausbier M, Sailer CA, Arntz C, Knaus HG, Neuhuber W, Ruth P. Ca2+ -activated K+ channels of the BK-type in the mouse brain. Histochem Cell Biol 125: 725–741, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension 51: 432–439, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Schewe C, Schewe T, Wendel A. Strong inhibition of mammalian lipoxygenases by the antiinflammatory seleno-organic compound ebselen in the absence of glutathione. Biochem Pharmacol 48: 65–74, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Schmelz M, Michael K, Weidner C, Schmidt R, Torebjork HE, Handwerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport 11: 645–648, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Serviddio G, Loverro G, Vicino M, Prigigallo F, Grattagliano I, Altomare E, Vendemiale G. Modulation of endometrial redox balance during the menstrual cycle: relation with sex hormones. J Clin Endocrinol Metab 87: 2843–2848, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Sesti F, Liu S, Cai SQ. Oxidation of potassium channels by ROS: a general mechanism of aging and neurodegeneration? Trends Cell Biol 20: 45–51, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1–7) production in postural tachycardia syndrome. Hypertension 53: 767–774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stewart JM, Taneja I, Glover J, Medow MS. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 294: H466–H473, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stewart JM, Taneja I, Raghunath N, Clarke D, Medow MS. Intradermal angiotensin II administration attenuates the local cutaneous vasodilator heating response. Am J Physiol Heart Circ Physiol 295: H327–H334, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Rho kinase-mediated local cold-induced cutaneous vasoconstriction is augmented in aged human skin. Am J Physiol Heart Circ Physiol 293: H30–H36, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Toda N, Ayajiki K, Okamura T. Interaction of endothelial nitric oxide and angiotensin in the circulation. Pharmacol Rev 59: 54–87, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Tsai MH, Jiang MJ. Reactive oxygen species are involved in regulating alpha1-adrenoceptor-activated vascular smooth muscle contraction. J Biomed Sci 17: 67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wallengren J, Hakanson R. Effects of substance P, neurokinin A and calcitonin gene-related peptide in human skin and their involvement in sensory nerve-mediated responses. Eur J Pharmacol 143: 267–273, 1987 [DOI] [PubMed] [Google Scholar]
- 46. Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126: 119–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol 97: 1291–1298, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 588: 4317–4326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamazaki F. Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J Appl Physiol 108: 328–333, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension 51: 500–506, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Yu Y, Zhong MK, Li J, Sun XL, Xie GQ, Wang W, Zhu GQ. Endogenous hydrogen peroxide in paraventricular nucleus mediating cardiac sympathetic afferent reflex and regulating sympathetic activity. Pflügers Arch 454: 551–557, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Zhang Y, Chan MM, Andrews MC, Mori TA, Croft KD, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am J Hypertens 18: 910–916, 2005 [DOI] [PubMed] [Google Scholar]